The incidence of bronchial hyperreactivity has increased to one-third of the population in developed countries, which requires the adoption of preventive and therapeutic measures. The objective of the present study was to assess the effects of a traditional Mediterranean diet on patients diagnosed with childhood asthma and determine if there is a beneficial effect from this dietary intervention.
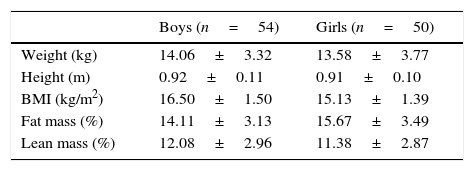
MethodsProspective before–after comparison study of 50 girls and 54 boys aged 1–5 years, who were enrolled in the 1-year programme “Learning to Eat from the Mediterranean”, designed to promote the adoption of a traditional Mediterranean diet. We studied the clinical and therapeutic variables and anthropometric measurements.
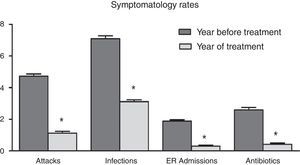
ResultsAll studied symptomatic indicators (number and intensity of asthmatic attack, infections and hospital admissions) showed a positive and statistically significant evolution of bronchial hyperreactivity from the first weeks of the intervention onwards. Throughout the treatment, 32.2% of patients remained free of crisis, 35.3% of the patients only had one attack throughout the year and 24.9% had two episodes, compared to 4.73 episodes on average in the previous year. The use of inhaled corticosteroids markedly decreased from 3.92±1.61 to 1.11±1.09 times per patient per year (P<0.001) and that of inhaled bronchodilators decreased from 4.14±1.61 to 1.12±1.40 (P<0.001). As a result, the families involved in the programme reported a high level of satisfaction.
ConclusionsThe adoption of a traditional Mediterranean diet could contribute significantly to the improvement of patients diagnosed with childhood asthma.
The incidence of childhood asthma and bronchial hyperreactivity (BHR) has been progressively increasing in developed countries, reaching nearly one-third of the population.1 This disease causes educational, economic and health problems for patients, their families, and society, which requires the adoption of preventive and therapeutic measures. The degree of asthma varies from patients who experience only a few episodes of wheezing, coughing with mild bronchospasm to patients with repeated episodes and persistent symptoms. The diagnosis of BHR in children is primarily clinical and can be complicated due to the subjective symptoms, which are sometimes difficult for their parents to assess.2 There is no universal test to determine the exact diagnosis. It has been demonstrated that the fundamental pathophysiological basis is bronchial inflammation, which triggers hypersensitivity to a variety of stimuli such as upper respiratory tract infections, air pollution, tobacco, smoke, exercise, central heating, seasonal pollination and obesity.
Current treatment is based on the use of bronchodilator β2-agonists (which reduce bronchial muscle spasticity), anti-inflammatory corticosteroids and anti-leukotrienes (which reduce BHR and clinical symptoms). All are effective upon acute application, but prolonged use is more questionable due to the limited evidence supporting efficacy and the emergence of adverse effects such as abnormal growth in children.3 It has been suggested that dietary interventions could be of potential use based on a number of publications that have linked the disease with the loss of traditional dietary patterns. Some of these results, however, are controversial.4–7 One of our recent studies on overweight and obese children supports this idea because patients with BHR improved considerably after controlling the quality of their diet.8 We therefore hypothesise that restoring traditional, healthy diets such as the Mediterranean diet could help decrease bronchial hyperreactivity significantly. In accordance with this idea, a meta-analysis has recently been published supporting the positive influence of Mediterranean diets on respiratory outcomes in children.9 The present study aims to extend knowledge in this direction by studying the effects of a traditional Mediterranean diet (TMD), on patients diagnosed with childhood asthma through a food re-education programme developed in the family sphere. The study diet was based on the Decalogue proposed by the Mediterranean Diet Foundation.10 The Mediterranean diet was proclaimed an Intangible Cultural Heritage of Humanity by UNESCO in 2010 and is characterised by a high intake of unrefined plant-based foods such as fruits and fresh vegetables, whole grains, legumes, olive oil and nuts; low to moderate consumption of foods of animal origin such as fermented milk, fish, eggs and lean meats; and by a low intake of sugar, refined flour and fast food.
MethodsStudy designThe study was approved by the Research and Ethics Committees of the General University Hospital of Ciudad Real, Spain. The research design was an analytical, before–after comparison study, which consecutively enrolled patients 1–5 years of age who met childhood asthma criteria11 and who attended a consultation at a primary care practice between May 2009 and September 2013. It was considered necessary to include the medical history of at least 1 year prior to the study and informed consent signed by their parents or guardians. The intervention focused on dietary re-education based on the TMD by a nutritional education programme named “Learning to Eat from the Mediterranean”. This programme is based on a series of visits to the nutritionist and paediatrician that are designed to assist the entire family. The visits were monthly during the first 4 months and bimonthly the rest of the year. To start the programme a 7-day recall questionnaire was required. On the first visit, we evaluated the food choices of each child and family. Based on the problems identified, we proposed dietary changes by providing dietary patterns, recipes, sample menus, etc. An anthropometric assessment was also performed and key health issues were explained, such as the importance of a good breakfast, variability in menus, the balance between food consumption and energy expenditure, the quality of fats, proteins and carbohydrates, understanding labels and creating a healthy shopping list. The patients were followed-up for 1 year to evaluate growth, clinical evolution, treatment needs, adherence to the TMD and the degree of family satisfaction. The conventional treatment remained initially unchanged and the drugs were reduced only when clinical improvements were observed.
Study variablesClinical parameters and treatmentPrimary end point: The number of BHR episodes and childhood asthma attacks per person per year (relapses or exacerbations). The Third International Pediatric Consensus has defined childhood asthma as a condition in which there are three or more episodes of wheezing and/or coughing in a clinical setting in which the diagnosis of asthma is the most likely after excluding other less common conditions. This conceptually strategic definition is still in use (2008 PRACTALL consensus) because it includes the expression of the disease (wheezing, coughing), recurrent episodes (three or more) and the absence of other conditions (wheezing and coughing unrelated to asthma).12
Secondary end points: Intensity of attacks, evaluated according to the protocols of the Spanish Pediatrics Association (1 – mild, 2 – moderate, 3 – severe),13 upper respiratory tract infections (URTIs), bacterial complications, emergency room visits, hospital admissions, drugs administered (inhaled corticosteroids, oral corticosteroids, short-acting bronchodilators, antibiotics and symptomatic treatment).
Clinical and therapeutic evaluation indexTo evaluate the patients’ clinical outcome, in addition to clinical assessment made by the paediatrician, we have developed a questionnaire given to the parents or guardians that assessed symptoms related to childhood asthma. Ten questions concerning the past 4 weeks were evaluated and scored from a minimum of 0 (poor control) to a maximum of 30 (asymptomatic). It was considered that the patient was well controlled when the sum of the 10 items was greater than 20.
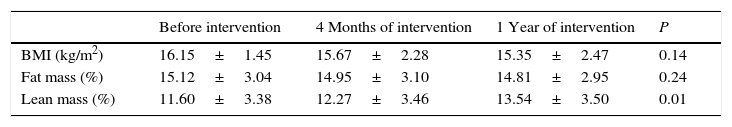
Weight and height evolution parametersTaking into account that the intervention performed involved a limitation of certain types of high-energy food, it was considered necessary to carefully evaluate the patients’ anthropometric development. Thus, we collected anthropometric data such as weight, height, skin folds and circumferences of the arm, abdomen and waist to calculate the body mass index (BMI), lean mass and fat mass following the procedures routinely used by our group.8
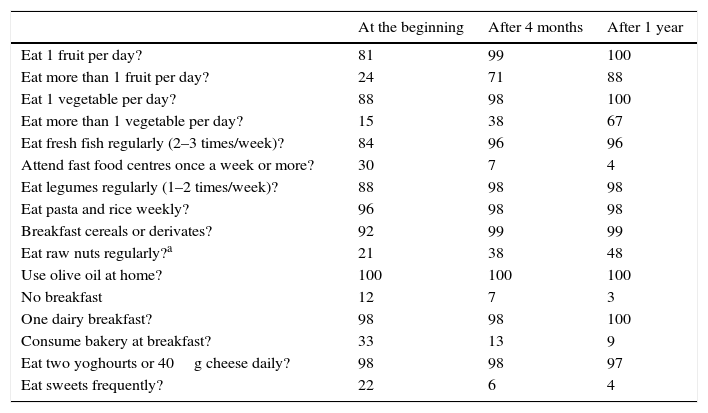
Parameters of adherence to the TMDAll participants received identical nutritional information intended for the whole family and whose characteristics are condensed in the Decalogue of the Mediterranean diet.10 To evaluate the new habits acquired by the patients and their families, we used the KIDMED test, which assesses the degree of adherence to the Mediterranean dietary pattern among children and adolescents.14
The 10 basic Mediterranean diet recommendations are: (1) use olive oil as your main source of added fat. (2) Eat plenty of fruits and vegetables; fruits, vegetables, legumes and nuts. (3) Bread and other grain products (pasta, rice, and whole grains) should be a part of your everyday diet. (4) Foods that have undergone minimal processing, that is fresh and locally produced are best. (5) Consume dairy products on a daily basis, mainly yoghourt and cheese. (6) Red meat should be consumed in moderation and if possible as a part of stews and other recipes. (7) Consume fish abundantly and eggs in moderation. (8) Fresh fruit should be your everyday dessert and, sweets, cakes and dairy desserts should be consumed only on occasion. (9) Water is the beverage par excellence in the Mediterranean diet. (10) Be physically active every day, since it is just as important as eating well.
For the sample size calculation, a significance level of 0.05 and a power of 85% were used, assuming a decrease in the mean number of childhood asthma attacks of one unit per patient per year, with a standard deviation of four units, adjusted for a 25% loss. The resulting sample size was 115 patients. For the analysis of the results, SPSS 15.0 was employed. We performed a descriptive statistical analysis of central tendency and dispersion for quantitative variables and absolute and relative frequencies for qualitative variables. After checking for normality with the Shapiro–Wilk test, the results of the different variables before and after treatment intervention were compared with Student's t-test for paired data, when the variables followed a normal distribution, and Wilcoxon's test otherwise.
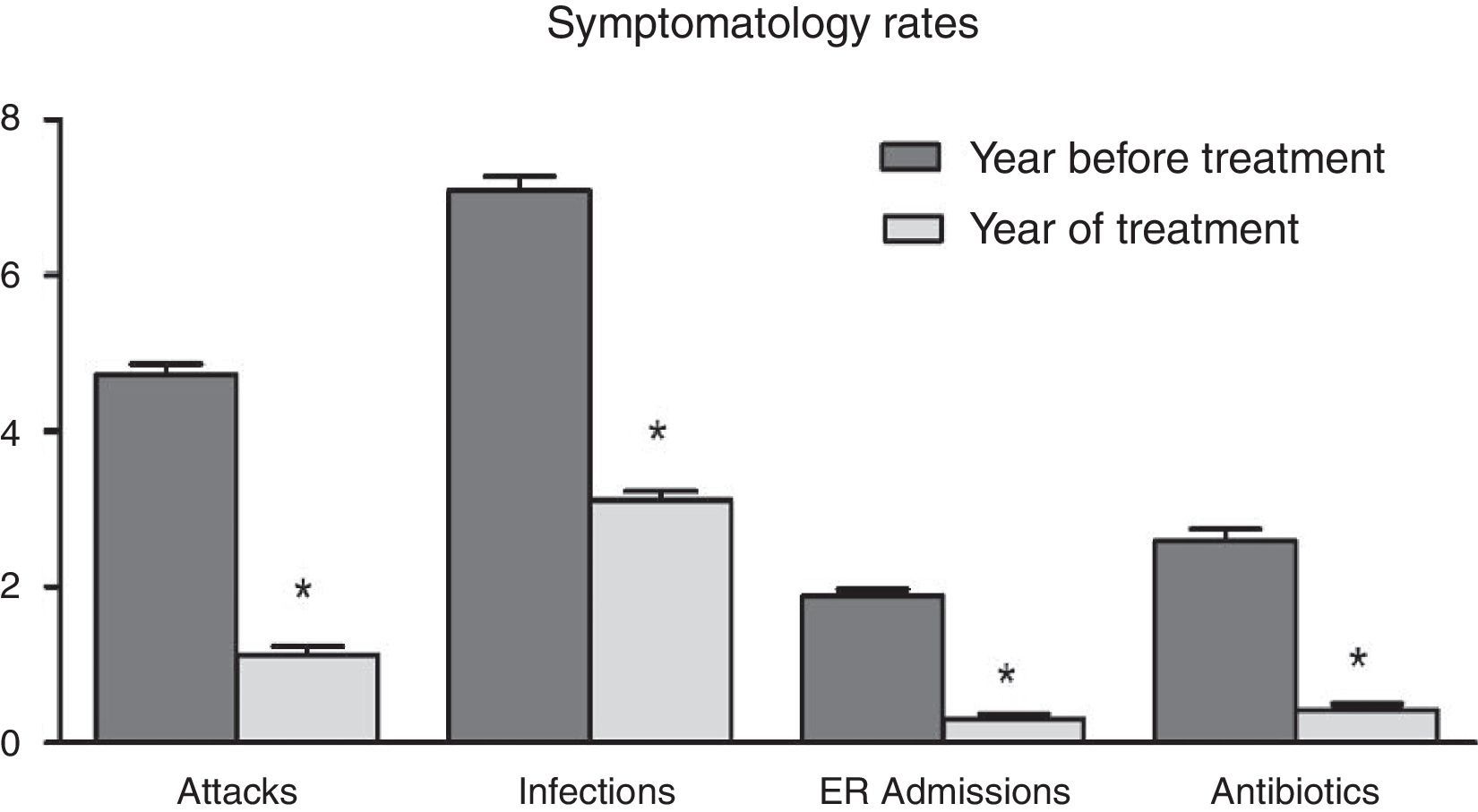
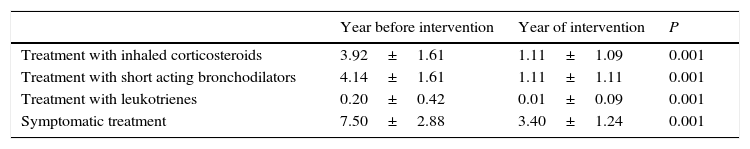
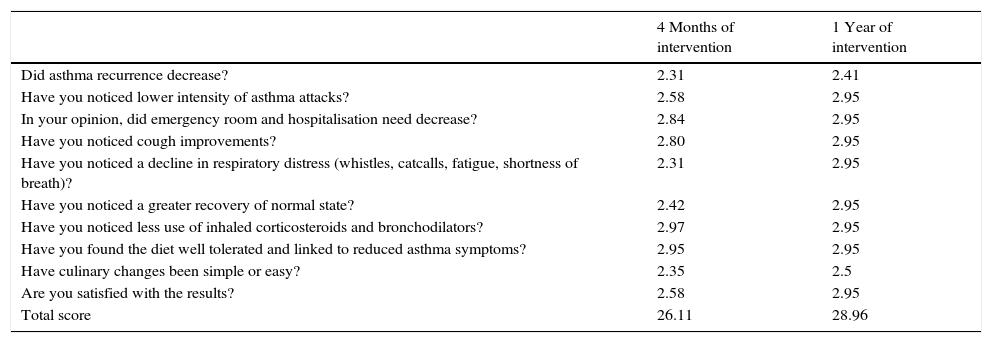
ResultsAccording to the sample size calculation, the families of the 137 patients who met the inclusion criteria were invited to participate in the programme “Learning to Eat from the Mediterranean”, of whom 24 families refused to participate. Of the 113 patients initially enrolled in the programme, 6 gave up the project after the first session and 3 after the fifth session. Five of these patients left due to social or personal difficulties in implementing the diet and four due to disagreement with the limitation of certain foods. Therefore, a total of 104 patients completed the study, of whom 50 were girls and 54 were boys, with a mean age of 2.74 years (Table 1). The results were similar for both sexes and are therefore presented together. Fig. 1 shows the evolution of the indicators studied in symptomatic patients (number and severity of attacks, respiratory infections, emergency and hospital admissions). All of them showed a positive and statistically significant change in the BHR from the first weeks of the intervention onwards. This clinical improvement was maintained until the end of the treatment year. There were no episodes of BHR in 32.2% of children during the year of treatment, 35.3% had only one attack during the year and 24.9% had two episodes, compared to 4.73 episodes on average in the previous year. The intensity of the crisis was assessed by the paediatrician and decreased from mild-moderate to low-mild. This positive evolution of symptoms was accompanied by a significant decrease in the URTIs, bacterial complications, ER admissions and emergency assistance. Moreover, the need for anti-asthma therapy, antibiotic use and other symptomatic medications was also dramatically reduced (Table 2). In the year of study, none of the patients required treatment with anti-leukotrienes, or oral corticosteroids. The degree of family satisfaction was very high, as seen in the questionnaire regarding the improvement observed (Table 3). Air pollution and smoke snuff was not object of study, so we interpret that the results are mainly due to nutritional changes. The anthropometric variables measured before the intervention, at 4 months, and after 1 year revealed an adequate evolution of parameters related to growth and development, with a significant increase of lean mass (Table 4). The patient's dietary habits had also improved in the entire sample by the end of the programme (Table 5). As a result, there were an increased number of patients who had breakfast and consumed fruits, vegetables, fish, whole grains and fermented milk, while the proportion of those who ate sweets daily and bakery products for breakfast decreased. According to these data, the KIDMED index positively evolved from an upper-middle score at the beginning of the programme to a good-optimal value by the end.
Evolution of drug treatments.
| Year before intervention | Year of intervention | P | |
|---|---|---|---|
| Treatment with inhaled corticosteroids | 3.92±1.61 | 1.11±1.09 | 0.001 |
| Treatment with short acting bronchodilators | 4.14±1.61 | 1.11±1.11 | 0.001 |
| Treatment with leukotrienes | 0.20±0.42 | 0.01±0.09 | 0.001 |
| Symptomatic treatment | 7.50±2.88 | 3.40±1.24 | 0.001 |
Questionnaire answers from parents or guardians about improvements in bronchial hyperreactivity. (3) A lot; (2) considerably; (1), a bit; (0) nothing.
| 4 Months of intervention | 1 Year of intervention | |
|---|---|---|
| Did asthma recurrence decrease? | 2.31 | 2.41 |
| Have you noticed lower intensity of asthma attacks? | 2.58 | 2.95 |
| In your opinion, did emergency room and hospitalisation need decrease? | 2.84 | 2.95 |
| Have you noticed cough improvements? | 2.80 | 2.95 |
| Have you noticed a decline in respiratory distress (whistles, catcalls, fatigue, shortness of breath)? | 2.31 | 2.95 |
| Have you noticed a greater recovery of normal state? | 2.42 | 2.95 |
| Have you noticed less use of inhaled corticosteroids and bronchodilators? | 2.97 | 2.95 |
| Have you found the diet well tolerated and linked to reduced asthma symptoms? | 2.95 | 2.95 |
| Have culinary changes been simple or easy? | 2.35 | 2.5 |
| Are you satisfied with the results? | 2.58 | 2.95 |
| Total score | 26.11 | 28.96 |
KIDMED test (percentage).
| At the beginning | After 4 months | After 1 year | |
|---|---|---|---|
| Eat 1 fruit per day? | 81 | 99 | 100 |
| Eat more than 1 fruit per day? | 24 | 71 | 88 |
| Eat 1 vegetable per day? | 88 | 98 | 100 |
| Eat more than 1 vegetable per day? | 15 | 38 | 67 |
| Eat fresh fish regularly (2–3 times/week)? | 84 | 96 | 96 |
| Attend fast food centres once a week or more? | 30 | 7 | 4 |
| Eat legumes regularly (1–2 times/week)? | 88 | 98 | 98 |
| Eat pasta and rice weekly? | 96 | 98 | 98 |
| Breakfast cereals or derivates? | 92 | 99 | 99 |
| Eat raw nuts regularly?a | 21 | 38 | 48 |
| Use olive oil at home? | 100 | 100 | 100 |
| No breakfast | 12 | 7 | 3 |
| One dairy breakfast? | 98 | 98 | 100 |
| Consume bakery at breakfast? | 33 | 13 | 9 |
| Eat two yoghourts or 40g cheese daily? | 98 | 98 | 97 |
| Eat sweets frequently? | 22 | 6 | 4 |
The overall results reflect a fast and positive evolution of patients enrolled in the programme, as shown by the various study variables. Thus, the number of attacks had notably decreased by the conclusion of the study, as had their intensity and associated complications. There was a very favourable start within weeks of dietary treatment, even in younger children and in those with more intense involvement response. Of particular importance is the marked decrease observed in the need for bronchodilators and inhaled corticosteroids, given that one of the main goals of the treatment according to the Global Initiative for Asthma is the prevention of exacerbations and the avoidance of adverse effects of antiasthma medication.15–17 There was a significant decrease in symptomatic drugs as a result of fewer illnesses and their lesser intensity degree. It is important to note that the use of anti-leukotrienes, and oral corticosteroids was not necessary during the study period. Significant improvements with respect to the previous year's situation were observed within the first 4 months of the intervention, which led to a high adherence to the programme and greatly enhanced monitoring. There was good tolerance of the proposed diet, with an easy adaptation and a lack of relevant culinary difficulties. The evolution of the height and weight of the patients was also satisfactory. There was a slight decrease in BMI and fat mass, and a significant increase in lean mass which happened in a similar way in our previous study of childhood obesity.8 The clinical outcome was assessed by a paediatrician when there was disease and by periodic inspections. The lack of a control group is the major weakness in our study and prevents us from categorically affirming that the dietary intervention was the direct cause of the observed improvements. However, the clinical history of the patients included in the study did not permit to expect a spontaneous favourable evolution of the symptoms18 and therefore the therapeutic efficacy of the diet seems obvious. Furthermore, our results agree with those of other studies that tend to show the beneficial effects of adherence to the TMD and the increased consumption of fruits, vegetables, legumes, oily fish and whole grains.4,6,19,20 In contrast, the consumption of fast food and sugar has been associated with childhood asthma and counteracts the protective effect of breastfeeding. Junk food21 and excessive salt consumption22 have also been linked with asthma. Despite these findings, a number of other studies do not support the idea that healthy food intake and/or avoidance of certain foods can play an important role in the control of childhood asthma.23 Thus, a recent review and meta-analysis concluded that nutritional factors responsible for the increase in these diseases are unclear.24,25 Of note is the cross-sectional study of 14,700 Spanish children that found no protective effect of the TMD on the prevalence and severity of asthma.5 Among the possible causes that could explain the lack of effectiveness of these studies related to the dietary intervention, we highlight the lack of an exhaustive follow up and inadequate diet control. We consider that the beneficial effect of our intervention is due to the implementation of a very specific and well-controlled TDM. The positive results of interventions such as ours on childhood asthma could depend on the effects of the TMD; we highlight the implementation of a very specific and well-controlled TMD on inflammation and the immune processes underlying the disease. This was previously proposed after a prospective study of children with asthma in Mexico City in which the strict adherence to the TMD appeared to have a beneficial effect on airway inflammation and lung function.7 In a recent clinical trial in adults with asthma, consistent improvements were seen in the clinic and spirometry in those who adhered to the TMD.26 In patients with mild asthma, it has been shown that inflammation remains asymptomatic for long periods from the start of the disease.27 The bronchial mucus inflammation will condition bronchial hyperresponse to stimuli, causing a major airflow limitation due to smooth muscle contraction, mucous oedema and mucus hypersecretion. The putative anti-inflammatory properties of the TMD could be partially attributed to a proper intake of carbohydrates. By limiting the intake of white bread, refined flour, sugar and bakery products, our dietary intervention decreases the amount of carbohydrates with a high glycaemic index and glycaemic load, which could prevent an excess of circulating insulin from interfering with the proper formation of eicosanoids. It is known that hyperinsulinaemia stimulates the delta-6-desaturase enzyme, which favours the formation of arachidonic acid and type II eicosanoids, which are strongly proinflammatory.28
In addition to limiting simple carbohydrates, reducing the intake of unhealthy fats appears to be a highly beneficial aspect of the TMD and could significantly contribute to limiting inflammation. Before participating in the study, most of our patients ate little in the way of vegetable fats and consumed significant amounts of animal or industrial fats. By limiting the intake of meat and animal derivatives, fatty and unfermented dairy, butter, margarine, bakery products and precooked meals, patients can reduce their intake of saturated fats, trans-fats and arachidonic acid, as well as reduce certain sensitising proteins29 that could interfere with the proper function of cell membranes that are more hyperreactive to triggers. The TMD also provides a correct balance of omega-3 and omega-6 fatty acids, which can decrease the levels of inflammatory cytokines (already high in patients with asthma)30 and affect the differentiation of T helper cells.4
Fresh and seasonal foods contain numerous vitamins, minerals, enzymes, yeasts and antioxidants, and many of these are important co-factors in the formation of the eicosanoids involved in bronchial inflammation.31 We have encouraged the use of raw and steamed foods and foods heated at low temperatures so as to avoid modification by excess heat. By promoting fresh, seasonal foods, many of the additives in processed foods have been eliminated, additives that might be involved in the enzymatic block of bronchial inflammatory mediators. The TMD is rich in antioxidants that can counteract the reduced intake of these substances, which have been associated with increased asthma prevalence.31,32 Additionally, plant foods, acting as symbiotic, favour intestinal flora fermentation, which has co-evolved with the human race since ancient times.33 Intestinal microbiota depends on the food we eat and have a critical role in modulating the immune system. The immune response also depends on the consumption of significant amounts of fibre,34 which is present in the TMD. The significant decrease of URTIs and their complications are remarkable in our study, which suggests a protective immune effect. Intestinal microbiota is essential for initiating individual IgA production in children younger than 1 year and influence the development of the entire IgA system.35 IgA is abundant in most mucous membranes, where harmless antigens are neutralised and prevented from entering the epithelium.36 Therefore, low levels of IgA in infancy might be the cause of the lack of a significant response to innocuous antigens, namely the induction of oral tolerance. This could be important in the prevention of allergic diseases. Microbiota have a symbiotic relationship with the host, and if this relationship is not adequate, it can cause immune dysregulation and pathological response to environmental antigens (atopy).37
It is noteworthy that the patients in our study showed a high score in the KIDMED test at the beginning of the intervention, before they had started undergoing dietary changes. In our opinion, the KIDMED test, which measures the quality of the Mediterranean diet, is not sensitive to all the nutritional factors proposed in the Decalogue of the Mediterranean diet.38,39 Thus, it does not take into account whether the cereals are whole grain or refined grain, the percentage of fresh or processed food, the kind of dairy products or the proportion of meat and sausages. A reformulation of this tool could be useful. In addition to the lack of a randomised control group, our study has a number of other limitations that should be mentioned. In the course of the study, it was impossible to make a clinical classification as proposed by the GEMA40 because the intervention began from the third crisis of BHR, and therefore the possible evolution of the patients could not be known. No additional systematic tests were conducted, which is why we have no asthma predictive index values. We did not use the Asthma Control Questionnaire (ACQ) in children proposed by the Spanish consensus because it does not include nutritional questions.
In conclusion, our study supports the hypothesis that a diet of high nutritional quality such as the Traditional Mediterranean Diet can contribute to a better control of asthma in children, possibly impacting the underlying immune and inflammatory pathophysiology. The study protocol presented here is easily reproducible in any primary care clinic and can significantly contribute to a better understanding of the therapeutic effects of diet on respiratory diseases, being thus complementary to the use of modern technologies in cross-sectional studies and clinical trials that also address childhood asthma but require much larger budgets.
Ethical disclosuresProtection of human subjects and animalsThe authors state that the procedures followed were in accordance with the regulations of the Ethics Committee for Clinical Research responsibly and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocol of the workplace in the publication of patient data and all patients included in the study have received sufficient information and gave written informed consent to participate in this study.
Right to privacy and informed consentThe authors declare that there is no patient data displayed on this article.
Conflict of interestThe authors have no conflict of interest to declare.