Drug allergy is the third most common reason for allergy consultations. There is a tendency to call any adverse drug reaction (ADR) allergic, even without confirmatory allergy study.
Objectives(1) Evaluate time of resolution allergy to beta-lactam's study in a sample of 100 patients. (2) Analyse cost-effectiveness of current diagnostic study (skin tests, specific IgE and drug provocation test (DPT)). (3) Describe type and frequency of ADRs in adult/paediatric patients. (4) Compare cost of complete study with DPT. (5) Assess the need to restructure current study methodology according to results obtained.
The study is part of a strategic plan of the allergy department (2005–2010). Patients with suspected allergy to beta-lactams were included. Procedures performed: medical history, specific IgE, skin tests and DPT. Cost/patient analysis. Cost of protocol analysis for current diagnostic/direct DPT.
Results100 patients were studied, 52 females/48 males; 43 children/57 adults. Symptoms: 89 cutaneous, 4 anaphylaxis, 3 vasovagal reactions, 6 non-specific symptoms and 4 not recalled. Allergy was confirmed in six patients (only one child). Complete-study cost: 149.3 Euros/patient. DPT-study cost: 97.19 Euros/patient (34.9% less). Resolution time 9–13 months, absenteeism 28.04%.
ConclusionsIn the series studied, diagnosis of allergy to beta-lactams was confirmed in 6% of patients (2.3% of paediatric patients). After analysing results and cost of the study we believe that we should propose a specific diagnostic algorithm in those paediatric patients without suspected IgE-mediated ADR, and for those patients direct DPT should be conducted. This will reduce cost/patient (−34.9%), time of resolution and absenteeism.
Suspected drug allergy is the third most common reason for first visits to allergy specialists. According to data from the Spanish “Alergológica-2005” observational epidemiological study (study on epidemiological, clinical and socioeconomic aspects of allergic diseases in Spain), drug allergy is the third leading reason for consultation after rhinoconjunctivitis and bronchial asthma.1 Thus, in 2005, of all patients seen in allergy clinics in Spain, 14.7% were referred for study of drug allergy (the percentage was lower, 9.8%, in the case of paediatric patients).
In general terms, most adverse drug reactions are attributed to allergy (especially when skin symptoms predominate) with between 5 and 10% of cases confirmed as such.
The group of drugs known as beta-lactams consists of natural and semi-synthetic antibiotics, which inhibit the synthesis of bacterial cell wall. They act as haptens and their attachment to a carrier allows them to be recognised by the body's immune system, enabling the triggering of hypersensitivity reactions.
Beta-lactam antibiotics are classified according to their chemical structure: penicillins, cephalosporins, monobactam, carbapenems, oxacephems and clavams. They are formed by a common ring (beta-lactam ring) which identifies them as a group and, among the subgroups, there are other chemical structures (thiazolidine rings, side chains) that allow differentiation and are responsible for the presence or absence of cross-reactivity between antibiotics of the same group.
An adverse drug reaction (ADR) is defined as that noxious and unintended response that occurs upon administration of a suitable drug dose in order to obtain a therapeutic, prophylactic or diagnostic benefit.2 ADRs are classified into predictable and unpredictable. Predictable reactions are the most common (over 80%), are dose-dependent and are explained by the pharmacological action itself. The unpredictable are unexpected, independent of the dose and do not form part of the pharmacological actions (hypersensitivity reactions included).
Type I hypersensitivity reactions manifest as urticaria, angio-oedema and/or anaphylaxis. The current beta-lactam allergy study protocol is based on clinical history, specific IgE measurement, immediate-reading and delayed-reading skin tests and challenge test. This protocol is valid for the study of reactions mediated by IgE antibodies and is not useful in diagnosing other delayed type reactions.3
ObjectiveThe study of drug allergy involves a long and complex diagnosis (patient risk, economic cost) a fact that highlights the need for a specific cost/benefit analysis.
The objectives of this work are:
- 1.
To assess the time of resolution for the study of allergy to beta-lactam type drugs (penicillin group).
- 2.
To analyse the cost-effectiveness of diagnostic tests for allergy to penicillin (study results, economic cost) according to the current study algorithm.
- 3.
To describe types and frequency of ADRs in patients according to age (younger and older than 14 years).
- 4.
To compare the cost of the current complete study with conducting direct DPT (gold standard).
- 5.
To assess the need to restructure the study methodology according to the results obtained.
The study is part of the strategic plan of the allergy department of the Althaia Foundation in the period 2005–2010.4
This is a retrospective descriptive analysis of patients referred to the allergy department suspected of allergy to beta-lactam drugs, from January 2009 to May 2010.
Data on health care activity during the study were obtained from the centre's Management Control Service and the allergy department itself. The Department of Biological Diagnosis provided data on specific IgE measurements.
The economic study was based on data provided by the Management Control Service. Calculating the cost of the study took into account costs including health intervention and resources consumed by the patient (staff time spent and cost of diagnostic extracts used). This study does not include analysis of indirect costs, due to a lack of sufficient data because it is a retrospective study.
The patients studied were divided according to age (younger or older than 14 years) and type of reaction presented (immediate or delayed).
The methodology of the study was as follows:
- 1.
Detailed clinical allergy study
- a.
drug(s) involved
- b.
dosage and means of administration
- c.
reason for prescription
- d.
symptoms, and time between drug intake and onset of symptoms (immediate/delayed reactions)
- e.
prior drug tolerance
- f.
subsequently tolerated drugs
- g.
time interval between allergic reaction and allergy study
- a.
- 2.
Inclusion/exclusion criteria (see Table 1).
Table 1.Inclusion and exclusion criteria for the study of allergic ADR.
Inclusion criteria Exclusion criteria Suspected allergy to beta-lactams.Informed consent signed (if under 16 it is signed by parents or legal guardians). No clinical history suggestive of allergic ADRConcomitant treatment with antihistamines or immunosuppressive agentsTreatment with beta-blockersUncontrolled asthmaPathologies that contraindicate the use of epinephrineInformed consent not signed - 3.
Measurement of total IgE and specific IgE (CAP EIA (Pharmacia)) to penicillin G, penicillin V, amoxicillin, ampicillin and/or clavulanic acid (variable according to each patient's medical history)
- 4.
Prick test and intradermal reaction (IDR)): PPL (5×10−5mmol/L, Diater laboratories), MDM (2.5×10−5mmol/L, Diater laboratories), amoxicillin (prick and IDR 20mg/mL), cefuroxime (prick and IDR 25mg/mL). Depending on the drug involved, in some cases skin tests are expanded to include amoxicillin-clavulanate (prick and IDR 20mg/mL), penicillin G (prick 250,000U, IDR 10,000U) or ampicillin (prick and IDR 20mg/mL). In cases of anaphylactic reaction, skin tests are initially performed at higher dilutions (1/100 and 1/10). The immediate result of a prick is considered positive if after 20min there is a wheal with a mean diameter equal to or greater than 3mm with respect to the negative control (saline) and with positive histamine (positive control, performed only in prick test). An immediate IDR response was considered positive when there was an increase of 3mm in wheal diameter after 20min. If any of the allergens in the prick test yielded a positive result, the IDR was not performed.
- 5.
Drug provocation test (DPT) with the drug suspected of causing the reaction (if points 3 and 4 are negative). In patients with a history of ADR over two years of evolution and negative results in points 3 and 4, a second DPT (re-exposure) was performed at 30 days
- 6.
DPT with alternative drug (cefuroxime, provided that the said drug skin tests were negative) if positive on point 3 or 4.
- 7.
Final visit 15 days after the DPT. Assessment of possible late responses.
- 8.
Analysis of the economic cost of the study per patient. This included calculating the time spent by staff, laboratory expense, cost of performing skin tests and DPT (Table 3)
- 9.
Analysis of the economic cost of the studies conducted according to the usual protocol compared with direct controlled DPT.
The classification used in this paper is a variant of that proposed by Levine5,6 based on the time between drug administration and the onset of symptoms. Levine classified reactions to penicillins as immediate, accelerated and delayed.
Immediate reactions (IR) are defined as those that appear with a latency period of <1h; these require the presence of IgE-specific antibodies against the drug. Said drug binds to specific-IgE molecules on the surface of basophils and mast cells to produce a cellular activation and release of mediators responsible for the allergic symptoms (urticaria, angio-oedema, bronchospasm or anaphylaxis). Accelerated reactions are those occurring more than 2h but less than 72h after drug intake. Reactions that are not immediate or are delayed type reactions (DR) are usually T cell-mediated and normally occur at skin level in varying degrees of severity (from exanthematous reaction to Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis), appearing after 72h. In this work, accelerated reactions are included in delayed or non-immediate reactions.
Depending on the latency time between taking the medication and the onset of symptoms, patients were classified into those who had an immediate type reaction (IR), those with a delayed reaction (DR) and those who did not recollect.
Regarding the time elapsed from the reaction that motivated the allergy study to performing such study, patients were divided into those who presented reaction <2 years prior to the visit, those who presented reaction ≥2 years before the study and those who did not recall these data.
To calculate the time of diagnostic resolution (from the visit request to confirming the diagnosis) required taking into account the waiting time for the first visit, the time to perform skin tests and challenge tests and the need for a re-challenge test in patients with reactions occurring more than two years before the study (Table 5).
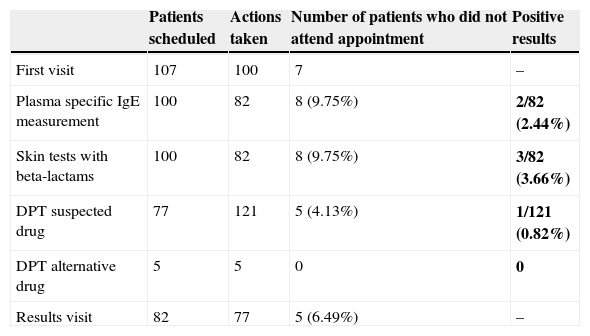
ResultsFrom January 2009 to May 2010 a total of 107 visits were scheduled with the presenting complaint being allergy to penicillins. A total of 100 patients attended the first visit (93.45%), of whom 52 were female and 48 male, 43 children and 57 adults, with a mean age of 27.32 years (range: 19 months–77 years).
Depending on the latency time between taking the medication and the onset of symptoms, 38 had an immediate type reaction (IR), 33 a delayed reaction (DR) and 29 patients did not recall these data.
The presenting symptoms prompting referral for the study were: 89/100 patients with cutaneous symptoms (37/89 urticaria, 20/89 maculopapular rash, 17/89 angio-oedema, 15/89 non-specific skin reaction); 4/100 anaphylactic reactions (4%), 3/100 type vasovagal reactions, 6/100 specific symptoms (general malaise, headache, dyspnoea, etc.), and 4/100 did not remember or did not know the symptoms that prompted the study.
Regarding the time between the reaction and the allergy study: 26 patients presented reaction <2 years before the visit, 60 patients ≥2 years before the study and 14 patients did not remember when the reaction had occurred.
Table 2 shows the activity scheduled and performed in these patients.
Record of activity and results.
| Patients scheduled | Actions taken | Number of patients who did not attend appointment | Positive results | |
|---|---|---|---|---|
| First visit | 107 | 100 | 7 | – |
| Plasma specific IgE measurement | 100 | 82 | 8 (9.75%) | 2/82 (2.44%) |
| Skin tests with beta-lactams | 100 | 82 | 8 (9.75%) | 3/82 (3.66%) |
| DPT suspected drug | 77 | 121 | 5 (4.13%) | 1/121 (0.82%) |
| DPT alternative drug | 5 | 5 | 0 | 0 |
| Results visit | 82 | 77 | 5 (6.49%) | – |
DPT: drug provocation test.
Bold values highlight positive results in this column.
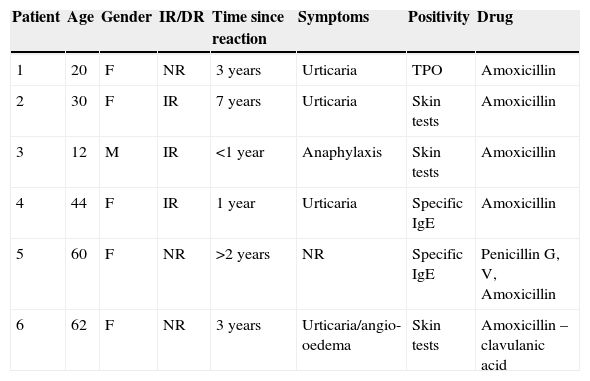
In the present series only six patients were diagnosed with allergy to penicillin (5 females/1 male, 5 adults/1 child). In 5/6 patients the diagnosis was made without the need for oral food challenge. All patients with confirmed penicillin allergy presented IgE-mediated symptoms (Table 4).
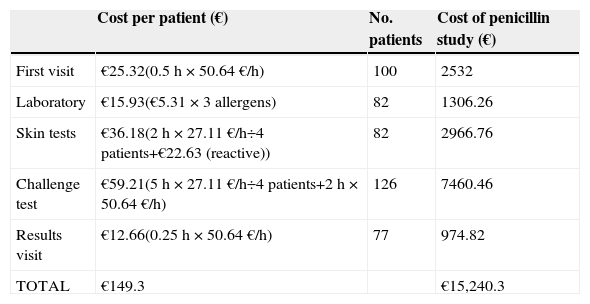
The average cost of a complete study was 149.3 Euros/patient with the cost of all patients amounting to 15,240.3 Euros. Table 3 shows the cost of each test in detail.
Economic analysis of standard study of allergy to beta-lactam.
| Cost per patient (€) | No. patients | Cost of penicillin study (€) | |
|---|---|---|---|
| First visit | €25.32(0.5h×50.64€/h) | 100 | 2532 |
| Laboratory | €15.93(€5.31×3 allergens) | 82 | 1306.26 |
| Skin tests | €36.18(2h×27.11€/h÷4 patients+€22.63 (reactive)) | 82 | 2966.76 |
| Challenge test | €59.21(5h×27.11€/h÷4 patients+2h×50.64€/h) | 126 | 7460.46 |
| Results visit | €12.66(0.25h×50.64€/h) | 77 | 974.82 |
| TOTAL | €149.3 | €15,240.3 |
Physician cost: 50.64€/h; nurse cost: 27.11€/h; cost of specific IgE measurement: 5.31€/allergen. Reagents cost: MDM-PPL kit €181 (8 patients), equals 22.63€/patient.
Characteristics of patients with positive study.
| Patient | Age | Gender | IR/DR | Time since reaction | Symptoms | Positivity | Drug |
|---|---|---|---|---|---|---|---|
| 1 | 20 | F | NR | 3 years | Urticaria | TPO | Amoxicillin |
| 2 | 30 | F | IR | 7 years | Urticaria | Skin tests | Amoxicillin |
| 3 | 12 | M | IR | <1 year | Anaphylaxis | Skin tests | Amoxicillin |
| 4 | 44 | F | IR | 1 year | Urticaria | Specific IgE | Amoxicillin |
| 5 | 60 | F | NR | >2 years | NR | Specific IgE | Penicillin G, V, Amoxicillin |
| 6 | 62 | F | NR | 3 years | Urticaria/angio-oedema | Skin tests | Amoxicillin –clavulanic acid |
IR, immediate reaction; DR, delayed reaction; NR, no recollection.
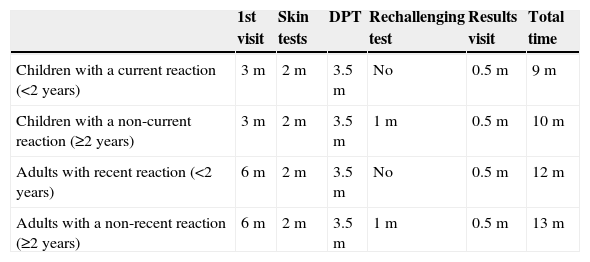
Time of diagnostic resolution in the study of allergy to penicillin.
| 1st visit | Skin tests | DPT | Rechallenging test | Results visit | Total time | |
|---|---|---|---|---|---|---|
| Children with a current reaction (<2 years) | 3m | 2m | 3.5m | No | 0.5m | 9m |
| Children with a non-current reaction (≥2 years) | 3m | 2m | 3.5m | 1m | 0.5m | 10m |
| Adults with recent reaction (<2 years) | 6m | 2m | 3.5m | No | 0.5m | 12m |
| Adults with a non-recent reaction (≥2 years) | 6m | 2m | 3.5m | 1m | 0.5m | 13m |
DPT, drug provocation test; m, months.
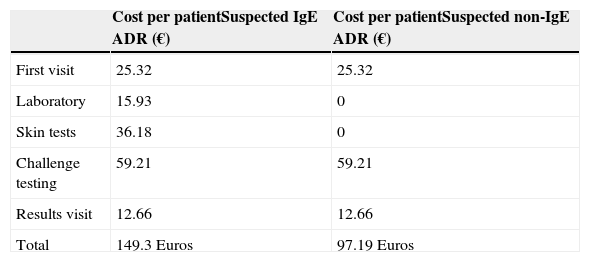
Comparative economic analysis of the penicillin study depending on risk.
| Cost per patientSuspected IgE ADR (€) | Cost per patientSuspected non-IgE ADR (€) | |
|---|---|---|
| First visit | 25.32 | 25.32 |
| Laboratory | 15.93 | 0 |
| Skin tests | 36.18 | 0 |
| Challenge testing | 59.21 | 59.21 |
| Results visit | 12.66 | 12.66 |
| Total | 149.3 Euros | 97.19 Euros |
Physician cost: 5.64€/h; nurse cost: 27.11€/h; specific IgE measurement test: 5.31€/allergen. Reagents cost: MDM-PPL kit €181 (8 patients), equals 22.63€/patient.
Subsequently, the economic analysis was performed comparing the cost of a full standard study (149.3 Euros/patient) with the cost of direct DPT without specific-IgE measurement or skin tests. In this case the cost of the study would be 97.19 Euros, representing a reduction of 34.9% (52.11 Euros) in the current cost per patient who performed a complete study (Table 6).
Time of diagnostic resolution, from when the study was requested to confirming diagnosis, varied between 9 and 13 months depending on the need for re-exposure (Table 5).
DiscussionThe series presented confirmed a low number of allergies to penicillin (only 6 of the 100 patients who started the study). The results obtained are similar to those found in the literature.7,8 Noteworthy is the low number of cases referred with severe reactions (4%) secondary to taking penicillin. The highest percentage of patients studied was due to skin reactions (89%). This could in part explain the low confirmation of cases of allergy to beta-lactams. Another factor that should be considered is the absence of a specific code in ICD-10 for anaphylaxis; there is a unique code for anaphylactic shock, a fact that could hinder the discharge diagnosis of a serious patient.
Moreover, in many cases a cutaneous skin reaction is considered by the physician as an allergic reaction. This is most evident in the paediatric population when it often coincides with the presence of an infection or febrile illness with antibiotic or anti-inflammatory treatment and the appearance of a generalised maculopapular rash.9–12 This means that many patients are “labelled” as allergic to a certain drug for many months, even years, when they are not, with the potential harm this implies (avoiding treatments of choice, depending on the germ involved, emergence of bacterial resistance, etc.).
In the history it is essential to describe if it is an immediate type skin reaction (urticaria, angio-oedema) or delayed (maculopapular rash, dermatitis) since in the latter case, studies of IgE would not be useful.
The latency time between taking the medication and the reaction does not seem to be a good indicator to differentiate hypersensitivity from Type I reactions because this is unknown in a significant number of patients studied. In those cases where data about the reaction produced is unknown (type of lesion, latency time, etc.) they would have to be studied with a complete study because performing a direct DPT would not be free of risks.
The division among patients with recent reactions (<2 years before the visit) or historical (>2 years) was not helpful in the group of patients studied since 14 of them were unaware and were considered as historical reactions, which possibly caused more DPT than necessary to be performed. It would be necessary to increase the number of patients studied and assess the need for change as a defining criterion for re-challenge.
The sensitivity and specificity of CAP EIA (Pharmacia) in beta-lactam allergy varies according to different studies (sensitivity between 43 and 62.5%, specificity 71.4 and 75%).13 There is a study that has linked higher sensitivity, specificity and positive predictive value to cases of severe anaphylaxis.14
Of the six positive results of the study, only one was in the challenge test. In 5/6 patients, it was not necessary to have a DPT because we had previously confirmed, the diagnosis by determining specific IgE in vivo or in vitro. It is noteworthy that in the four patients referred for possible anaphylaxis, the diagnosis was confirmed in only one of them, so although the clinical history is important, diagnostic confirmation is determined by the full allergy study.
The Althaia Foundation has had computerised medical records since 2007 and each patient record contains a separate section in which the doctor or nurse may record an adverse drug reaction. This section differentiates between allergic and non-allergic ADRs. According to data obtained from the centre's pharmacy and information service, in the period between November 2007 and November 2008, there were 2789 ADR-type alarms created. Of these, 94.2% (2629) were labelled as allergic ADR and the rest (160, 5.8%) as non-allergic ADRs.15 These are only notifications of suspected ADR that in most cases have not been studied by an allergist. Comparing this information, including reactions with different groups of drugs (beta-lactams, NSAIDs, sulphonamides, quinolones), with the results of the present study reveals a significant difference. There is probably a tendency to define any reaction as allergy when a patient has taken a drug, when in fact the percentage of drug allergy is much less than the supposedly expected. According to several studies published in the medical literature, allergic reactions to drugs account for between 10 and 20% of all ADRs.1,15,16
Of the total patients studied in this work only six had a positive result, i.e., allergy to penicillin was confirmed (5.6% of all initial patients, 7.3% if we count only those patients who completed the study). If the results are analysed according to age, it can be seen that of the 43 children studied only one 12-year-old patient had a positive result (2.3%). In this patient the reaction that prompted the study was anaphylaxis, unlike most children referred who had only cutaneous symptoms, mostly maculopapular rashes.
According to results of the Spanish “Alergológica-2005” study, drug allergy is confirmed in 12% of the children studied,1 it is ruled out in 58% and there is a suspected diagnosis based on clinical history in 30%. In the adult population drug allergy was confirmed in 29%, in 35% it was ruled out, and there was a suspected diagnosis in 36%.
The fact that this work has shown allergy to beta-lactam drugs in a low number of patients may be due to the fact that cases with more severe reactions or a clear cause-effect relation are not referred for an allergy study (due to a high diagnostic suspicion). Another factor to consider is the fact that many patients (especially elderly and pluripathological ones) consult for having presented ADRs with multiple drugs of different families and the allergist decides which drug(s) it would be most useful to study, with the least risk for the patient. In many cases, in assessing the risk-benefit ratio, tolerance testing is chosen rather than challenge. Therefore, it is possible that some patients would be left without a confirmed diagnosis and the percentage of positive reactions is lower than in other published works.
Given the cost-effectiveness results obtained in the studied series, and consistent with other published studies16–18 that proposed new faster diagnostic algorithms in studies of penicillin allergy in paediatric patients considered low risk, we considered the possibility of changing the study protocol, performing DPT directly in children with suspected non-IgE-mediated reaction to penicillin (children with compatible symptoms who presented with maculopapular rash).
Although most patients report amoxicillin as the suspected reaction agent, in the current diagnostic protocol specific IgE and skin tests were performed for various penicillins, since in case of positivity to amoxicillin the study could be completed with other penicillins that do not share the same side-chain. It is for this reason that we have not suggested reducing the number of allergens tested as a cost saving measure.
The implementation of these changes would only be possible in paediatric patients where the history does not suggest a type I hypersensitivity reaction (IgE-mediated). In such cases, conducting a direct DPT should be considered without measurement of specific IgE in peripheral blood or skin tests.
There are currently no published works on the real cost of drug allergy study. This paper presents a cost analysis study performed in our centre and a small group of patients so there could be variations in the cost if applied in another centre with a different organisational structure. The aim is not to highlight the cost of one type of study, but the savings that would occur if changes were made in the current diagnostic algorithm, given the low percentage of positive studies.
The cost of a complete study in a child with suspected IgE-mediated reaction is 149.3 Euros while the study in children with suspected IgE-mediated reactions in the DPT would be 97.19 Euros, which would represent a saving of 34.9% per patient (Table 6).
Another point to consider is the rate of patients who do not attend the visit. Of the 107 patients scheduled to visit, 77 completed the entire study (71.9%), while 28.04% of patients did not finish it. Not considering the final visit of the study 82 patients completed the study (representing 76.6%, with 23.36% absenteeism.) The patient is informed at the first visit that the study of allergy to penicillins is long and complex (Table 5), and that there are risks/benefits inherent in incomplete studies. Simplifying the study as proposed herein could reduce the level of absenteeism. The improvement in the waiting time for the first visit may also help to optimise the data and avoid, in some cases, re-challenge testing (interval between reaction and study <2 years), a fact that would favourably affect cost.19–21
The application of a shorter protocol in a given group of paediatric patients, and generating a lower cost per patient, could reduce the time of diagnostic resolution, since the number of patients undergoing skin tests would be lower, and in these challenges would be direct, reducing the entire study duration by about two months.
Conclusions- •
The diagnosis of allergy to beta-lactams was confirmed in 6% of patients studied, 2.3% in the paediatric population.
- •
After analysing the results in this group of patients and the cost of a study of allergy to penicillin we believe that a shorter study algorithm could be applied in those paediatric patients with low suspicion of allergic type I ADR. They should undergo direct DPT, which would likely reduce the cost per patient and the time of resolution and absenteeism. A prospective study with a larger number of patients is warranted to confirm these data and make changes to the current study of allergy to beta-lactams in the paediatric population.
The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.