Heart failure is a primary health concern in North and South America, with hospitalizations for heart failure as the primary diagnosis continuing to rise. There is a positive relationship between the prevalence of heart failure and age. However, mortality from heart failure is on the decline due to medical advancements, pharmacotherapy and nonpharmacological interventions. One of these nonpharmacological interventions is physical training or exercise. Physical training or exercise is becoming widely accepted by the medical community as a viable option in the medical management of stable heart failure patients. Both aerobic and resistance type exercise have been shown to be efficacious in stable heart failure patients. Evidence now exists not only supporting exercise to improve or maintain physical function in heart failure patients, but also quality of life. Many studies have shown that utilizing exercise in this population improves mood and overall self-reported well being. While the myocardial benefits from exercise may be minimal in heart failure patients, the peripheral benefits leading to improved physical function and preservation of independence are indispensible. Based on the research that has been conducted in the area of exercise and heart failure to this point, clinicians working with this population have the data necessary to prescribe evidence-based exercise prescriptions that can be utilized as part of a comprehensive medical management approach. Currently, several medical position statements endorse exercise as a safe and effective modality in heart failure patients.
Heart failure (HF) has become a mounting health care concern and economic burden in North and South America. The cost for treatment of HF is escalating due to the increased number of people living with this problem. Costs related to HF are predicted to reach $44.6 billion in the United States (U.S.) by 2015 (1). HF is defined by the presentation of multifaceted clinical symptoms that are due to a functional or structural cardiac disorder that impairs the ability of the heart to fill or eject blood. The subjective complaints of HF are dyspnea and fatigue, which in turn, attenuate exercise tolerance and may cause fluid retention. This ultimately leads to pulmonary or peripheral edema (2). HF may be caused by impaired left ventricular function due to coronary artery disease, dilated cardiomyopathy, hypertension, structural congenital heart disease, myocarditis, valvular heart disease, rheumatic heart disease, myocardial infarction, Chagas disease, abnormal heart rhythms or idiopathic cardiomyopathy.
These numbers are augmented due to an aging population and improved survival following critical cardiac events. Due to survival of early cardiac events, the incidence of HF is increasing, but mortality related to HF is decreasing slowly due to improved intervention strategies. In addition to advanced medical technology and pharmacotherapy, exercise is becoming an important part of managing HF patients with viable left ventricular function.
Many exercise studies have demonstrated improvements in functional capacity (aerobic fitness) in HF patients similar to those seen in healthy populations. Exercise training studies in HF patients have demonstrated improvements of 18 to 25% in peak oxygen consumption and 18 to 34% in peak exercise duration with symptoms, functional class and quality of life measures also improving (3). These data and other resulted in the American College of Cardiology and the American Heart Association to recognize exercise training in HF patients as a beneficial intervention (4). Exercise training is being used to treat people with HF because it has been shown to impart physiological and psychological benefits (5-7).
Incidence and prevalenceMany countries disclose escalating numbers of people with heart disease and HF. In the U.S., it is estimated that more than 550,000 new HF diagnoses are made each year and more than five million people are currently living with HF (8). In the U.S., between 1990 and 1999, hospitalizations with HF as a primary diagnosis have gone from 810,000 to more than one million. Furthermore, these numbers reached 3.6million when HF was a primary or secondary diagnosis (9). HF affects 2.4% of the adult population and the incidence is increasing with age to over 7% in people above 75 years of age. The lifetime risk of developing HF at age 40 is 20% in females and 21% in males. A decrease in mortality with HF is leading to an increase in prevalence. Age-adjusted increase in HF noted in one community based study (1996-2000) reveals 29 per 10,000 in women and 38 per 10,000 for men (8). HF mortality has decreased from 57% between 1979 and 1984 to 48% between 1996 and 2000 (10). The largest influencing factor for HF prevalence and incidence is the growing elderly population. The number of persons diagnosed with HF is predicted to double from 2000-2030 (9).
Trends in countries such as those in South America (SA) reveal the number of people with HF is steadily reaching the number in North America and the Western World. Coronary artery disease in the presence of hypertension and diabetes mellitus is the most common cause of HF in SA (11). In 2002,1,524 hospital admissions in Brazil were due to HF, which reflects approximately 3% of all admissions (12). In 2002, 6.9% of all hospital mortality in SA was due to HF. Furthermore, the prevalence of HF in SA is estimated to be approximately 4% in those over 65. In 2003, the World Heart Failure Society reported approximately 30,000 deaths due to HF with the majority of the mortality occurring in those >75 years of age (13).
HF is the leading cause of hospitalizations in SA due to coronary artery disease, valvular heart disease, idiopathic dilated cardiomyopathy and chagastic symptoms. HF is responsible for 6.3% of all deaths in SA. Chagas heart disease is a cause of heart failure in many Latin American countries and has become a serious health and economic crisis and mortality from Chagas disease related HF is high. Chagas disease is usually acquired during childhood as the host is infected with Trypanosoma cruzi. This parasitic disease is transmitted to humans through the feces of infected insects found in endemic areas such as poor rural housing (14). Focusing on prevention and early treatment of Chagas disease will likely reduce the incidence of HF in SA countries (15).
Aerobic exercise training in heart failureIn addition to traditional and advanced medical management, physical activity and exercise are well-recognized therapies for HF and have been shown to improve cardiac function, quality of life and reduce symptoms related to HF. In people with normal heart function, aerobic exercise training results in improved cardiovascular function and oxygen consumption and a lower resting heart rate as well as an improved rate-pressure product. The improvement in oxygen consumption results from increased cardiac output and oxygen extraction by working muscles. The increased cardiac output results from an increase in ventricular filling and higher stroke volume. There is an increase in the size of the coronary vascular bed and a greater capacity for the coronary arteries to dilate. Blood volume increases and the blood is less viscous which decreases resistance to flow and improves the delivery of oxygen.
Aerobic exercise training for HF patients is generally prescribed at 70 to 80% of the heart rate achieved on the exercise test. This is considered a moderate to high intensity range. Some patients cannot exercise at this level and will benefit from a lower intensity (16, 17). As with people with normal left ventricular function, patients with HF are advised to exercise 30minutes or more five or more days weekly. This can be continuous or divided into shorter intervals. One study compared the effect of aerobic interval training (AIT) with moderate continuous exercise of 60 to 70% peak heart rate in HF patients (18). The AIT group performed three to four minute intervals of high intensity exercise of 90 to 95% peak heart rate. The AIT group showed higher oxygen consumption, more reverse left ventricular remodeling or increase in ejection fraction, and decreased left ventricular volume. Endothelial and mitochondrial function were also more favorably impacted with AIT. These changes are important due to both endothelial and mitochondrial function diminishing with age.
Muscle wasting also occurs in normal aging as well in HF patients. This contributes to exercise intolerance and morbidity and mortality. A recent study demonstrated exercise turns off the muscle wasting pathways and turns on the pathways involved in muscle growth in both HF patient groups of those less than 55 years of age and those greater than 65 years of age. Before and after the exercise intervention vastus lateralis muscle biopsies were obtained. Specifically, the exercise program of four weeks at 70% of peak oxygen consumption reduced levels of muscle ring finger 1 (MuRF-1) by 32.8% in patients younger than 55 (P=0.02) and by 37% (P<0.05) in those 65 and over. The authors conclude, MuRF-1, a component of the ubiquitin-proteasome system involved in muscle proteolysis, is increased in the skeletal muscle of patients with HF. Exercise training results in reduced MuRF-1 levels, suggesting that it blocks ubiquitin-proteasome system activation and does so in both younger and older HF patients. There was also a marked improvement in oxygen consumption following training in both groups (19).
The recent HF-ACTION trial (20) enrolled 2331 patients at 82 study sites in the US, Canada, and France. This was a multi-center, randomized controlled trial designed to measure the effects of exercise on clinical outcomes in medically optimized and stable patients with systolic HF (left ventricular ejection fraction less than 35%). Patients either received usual care or usual care plus an exercise-training program. The training was initially supervised then transitioned to a home-based exercise program. The mean left ventricular ejection fraction (LVEF) was 25%, the average age of the patients was 59, and one-third were women. The exercise program gradually progressed, with a goal of three 30-minute exercise sessions three times a week. The exercise group worked at a moderate intensity, which was 60 to 70% of their heart rate reserve. Following 18 sessions they transitioned to the home-based exercise program, with the goal being 40minutes four days a week and the protocols being a stationery bike or treadmill. Patients were followed for an average of 2.5 years. Investigators found that exercise resulted in an 11% reduction in risk of hospitalization or mortality (p=.03). When examining a secondary end point of this study, the exercise group had a 15% lower risk of mortality from cardiovascular disease and hospitalization due to complications of HF (p=.03).
The loss of physical function in heart failure is well documented and contributes to difficulties in performing both leisure time and activities of daily living (ADLs). In part due to an attenuated tolerance to physical exertion (21), HF patients have difficulty performing normal ADLs, which generally employ more upper than lower extremity activity. The difficulty in performing ADLs is a major factor reducing quality of life (QOL) in those with HF. In individuals with HF, exercise training can increase physical endurance (22) and has been shown in some but not all studies to improve health-related QOL (17,23-27). The reasons for incongruity between studies may be related to how subjects train (upper extremity vs. lower extremity protocols) and intensities of the exercise programs.
Upper extremity exercise trainingResearch has shown exercise training in subjects with left ventricular dysfunction to be safe and efficacious. The functional benefits relate to skeletal muscle improvement as well as changes in cardiac dynamics (5,6). However, the majority of previous research studies utilized exercise programs of short duration (three to 12 weeks) and involved predominantly lower extremity exercise training. There are fewer studies supporting improved left ventricular function when the exercise-training program is of longer duration or greater intensity. Additionally, lower extremity exercises have been more commonly prescribed compared with upper extremity exercises in many studies.
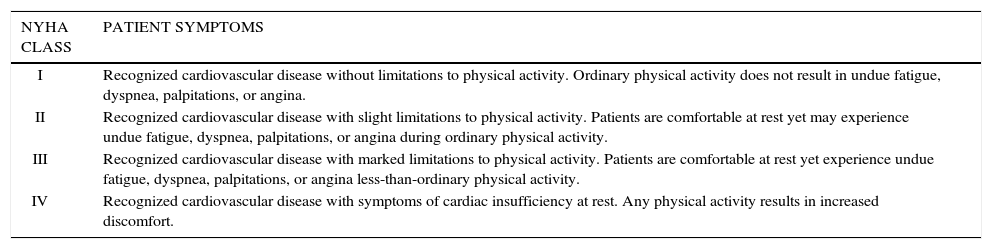
Based on this previous research, a pilot study was done utilizing an upper body ergometer(UBE) in HF patients (28).The investigators hypothesized that UBE training should be beneficial in reducing exercise intolerance during activities that require sustained arm work. Since most ADLs involve upper extremities, training on an UBE should be beneficial in this group. Subjects were more than three months post myocardial infarction, were stable and on pharmacotherapy. Ten subjects with mean LVEF of 30 +/- 6 (New York Heart Association (NYHA) Class II- III) were recruited (Table 1)(29).
New york heart association functional classification of heart failure
| NYHA CLASS | PATIENT SYMPTOMS |
|---|---|
| I | Recognized cardiovascular disease without limitations to physical activity. Ordinary physical activity does not result in undue fatigue, dyspnea, palpitations, or angina. |
| II | Recognized cardiovascular disease with slight limitations to physical activity. Patients are comfortable at rest yet may experience undue fatigue, dyspnea, palpitations, or angina during ordinary physical activity. |
| III | Recognized cardiovascular disease with marked limitations to physical activity. Patients are comfortable at rest yet experience undue fatigue, dyspnea, palpitations, or angina less-than-ordinary physical activity. |
| IV | Recognized cardiovascular disease with symptoms of cardiac insufficiency at rest. Any physical activity results in increased discomfort. |
Subjects were administered the Minnesota Living with Heart Failure Questionnaire, the Medical Outcomes Study Short Form-36 and had symptom-limited UBE cardiopulmonary testing with echocardiography. Following UBE testing subjects were enrolled in cardiac rehabilitation for 12 to 15 weeks. Upper extremity training was performed for 40minutes three times a week, using a UBE, the upper extremity handles of a NuStep® device and an Airdyne® stationery cycle. Target heart rate was 75 -85% of peak heart rate on baseline exercise testing. Follow-up testing was conducted at the end of the exercise-training period.
Six men and one woman, with a mean age of 68 +/- 4 years completed the study. Three subjects dropped out because of health problems unrelated to the exercise training protocol or worsening of HF. UBE training increased arm exercise test duration by approximated 22% (p=.008), Cohen‘s d=1.5)). Echocardiogram results showed resting LVEF was not changed. Maximal exercise LVEF mean values were not statistically different (p=.16) but Cohen‘s d=0.53 indicated a moderate improved change.
The mean Minnesota Living with Heart Failure Questionnaire total score was improved following the intervention (p=.02, Cohen‘s d=1.1), and six out of seven participants exhibited a clinically significant difference of greater than five points. The Medical Outcomes Study Short Form-36 total score showed five subjects had a change in scores ranging from three to 13 points on the 100-point scale (p=.05, Cohen‘s d=.5). Significant improvements were present for role limitation due to improved physical functioning (P=.04, Cohen‘s d=1.0). The improvements in overall QOL and exercise duration without a deleterious change in LVEF are encouraging for the use of upper extremity exercise training in HF. Continued research is warranted for specific duration and mode of exercise training in HF patients.
Resistance exerciseThe hallmark symptoms of HF are dyspnea, fatigue and exercise intolerance. There is a growing body of evidence supporting the role of skeletal muscle dysfunction (reductions in skeletal muscle mass, strength, and endurance) contributing to the increased fatigue and exercise intolerance exhibited in HF patients. Additionally, the degree of skeletal muscle dysfunction in HF negatively impacts functional mobility, which further diminishes the ability to complete ADLs, reported QOL, and disease prognosis.
Skeletal muscle mass and strength are significantly correlated with exercise capacity in HF and can independently predict survival in patients with severe HF (30-34). Harrington et al. (32) categorized 100 HF patients by severity of their exercise limitations (as measured with peak VO2 in ml/kg/min); mild 24.7ml/kg/min, moderate 17.4ml/kg/min, and severe 12.2ml/kg/min. Muscle strength, quadriceps cross-sectional area, and total thigh cross-sectional area were attenuated with increasing severity of exercise intolerance. Izawa et al. (33) found significant decreases in VO2peak in HF patients as NYHA functional class progressed from class I to class III (23.4, 19.6, and 14.7ml/kg/min respectively) (Table 1). Importantly, both upper body and lower body muscular strength were found to predict VO2peak in these patients. Hulsmann et al. (31) reported that knee flexor strength was superior to VO2peak and workload as a predictor of mortality in severe HF patients (LVEF 21 %), highlighting the importance of muscular strengthening activities (MSA).
QOL measures in HF patients decrease significantly by NYHA functional class and are significantly reduced when compared to a healthy reference group (35). Changes in QOL in HF patients can be better explained by exercise capacity (six-minute walk test and V02peak) than LVEF (35). Additionally, ADLs and QOL can independently predict mortality and HF-related hospitalizations (36). As mentioned above, engaging in MSA, plays a primary role in optimizing exercise capacity in HF and therefore should positively impact ADLs, QOL, mortality, and HF-related hospitalizations.
Resistance training (RT) has traditionally been the exercise modality of choice to slow the loss of both skeletal muscle mass and strength and facilitate muscle hypertrophy, muscular strength, and anaerobic fitness in both clinical and healthy populations. Therefore, RT is gaining acceptance as an adjunct therapy to aerobic exercise in stable HF patients that have been prescribed exercise therapy. Unfortunately, early reports of a considerable pressure load being placed on the heart of young healthy subjects completing maximal or near maximal static or dynamic RT discouraged many clinicians from considering this intervention in the rehabilitation programs of HF patients (37). The initial concerns were that RT would further deteriorate left ventricular structure and function.
Further investigation identified several factors in RT exercise prescription which influence the severity of the pressure load (38):
- 1)
The magnitude of relative load to a one repetition maximum (1-RM). The pressure load increases as the load increases toward the maximal weight an individual can lift for only one repetition. Example: If the 1-RM of a patient is 100 lbs, the pressure load will be greater when he lifts 100 lbs one time than when he lifts a submaximal weight (60 lbs) one time.
- 2)
The number of repetitions relative to a multiple repetition maximum. The pressure load increases as the lifter approaches volitional fatigue with multiple repetitions. Example: If a patient can lift 60 lbs 20 consecutive times before fatigue, the pressure load will be greater during the 20th repetition than during the 15th repetition.
- 3)
The volume of contracting muscle mass. Bilateral limb RT exercises create a greater pressure load than unilateral limb RT exercises. Two muscles contracting at the same time create a greater pressure load than one muscle contracting at the same relative intensity.
- 4)
Duration of the muscle contraction relative to the rest between repetitions and sets. When rest between sets is short, subsequent sets of the same relative workload will increase the pressure load. Therefore, rest between sets is recommended to have a minimum of 1:2 work to rest ratio. Example: If a set of 10-repetitions takes one minute to complete, the rest period before a subsequent RT exercise is performed must be at least two-minutes.
More recently, clinicians with RT program design expertise have developed low or moderate resistance exercise protocols, which minimize the pressure load and have been well tolerated by patients diagnosed with HF (38-40). Clearly the implementation of exercise programs in the treatment and rehabilitation of HF patients is an evolving practice. Indeed, in the past 10 years professional guidelines recommended by the American College of Sports Medicine (41), American Heart Association (42), Heart Failure Association and the European Society of Cardiology (43), and Exercise and Sports Science Australia (44) have endorsed the implementation of RT exercises in the treatment and rehabilitation of HF patients. Specific position statements have been published detailing RT exercise prescriptions in HF (43,44).
Resistance training interventions in HFThe most important recent research findings are that properly designed RT programs in HF patients do not have a negative impact on LV function or structure. Furthermore, RT is well tolerated by patients with HF (43-45). Following an eight-week structured RT protocol involving 10 NYHA class II or III (Table 1) male patients performing two sets of 12 RT exercises at 60% 1-RM three days per week, significant improvements in LVEF (pre 32% vs. post 37%) and stroke volume (pre 46ml/beat vs. 53ml/beat) were observed (45). A review by Smart and Marwick45 of 81 exercise studies in patients with HF reported no exercise related deaths in over 60,000 patient-hours of exercise (30% of these studies included RT either separately or in combination with aerobic exercise).
Consistent improvements in muscular strength and endurance, often exceeding 20 to 30%, have been reported with RT interventions in HF (5,6). RT has consistently attenuated age or disease related muscle loss in HF patients and evidence exists that shows increases in lean mass. Feiereisen et al. (47) randomized 45 male and female NYHA class II and III (Table 1) HF patients with LVEF <35% into either a RT group that performed four sets of 10 exercises at 60 to 70% 1-RM, an aerobic training group that performed bicycle and treadmill exercise at 60 to 75% VO2peak, or a combination of RT and aerobic training. All subjects performed 40 exercise sessions lasting 40minutes each. Fifteen HF patients who were unable to attend exercise sessions due to geographic constraints were used as controls. Significant strength gains were only recorded among the RT and combination groups. Interestingly, quadriceps muscle mass (measured by CT scan), VO2peak, LVEF, left ventricular end-systolic volume, left ventricular end-diastolic volume, and QOL significantly improved in all three training groups. No change in these parameters was observed in the controls.
RT interventions in HF have resulted in significant improvements in patient QOL. Edelmann et al. (48) studied 64 male and female HF patients NYHA functional class II and III (Table 1) with a mean age of 65. Forty-four patients were randomized in to either a 32-session exercise-training group that included both RT (one set if 15 repetitions at 60-65% 1-RM of six different upper and lower body exercises twice a week) and aerobic training (cycling two to three days a week at 50 to 70% VO2peak) or a usual care control group. Significant improvements were reported using both the Medical Outcomes Study Short Form-36 and the Minnesota Living with Heart Failure Questionnaire. No improvement in QOL was found in the control group.
While the primary role of aerobic exercise in HF cannot be discounted, the inclusion of dynamic resistance exercise as a secondary and complimentary exercise modality is now broadly recognized in HF treatment guidelines. Although, specific precautions should be taken when adopting RT exercise in HF:
- 1)
Patients must be clinically stable and have demonstrated aerobic exercise tolerance before beginning RT.
- 2)
RT intensity should be individualized.
- 3)
Start slow and progress slowly.
- 4)
Monitor ECG, BP, perceived exertion, and body weight.
- 5)
Be cognizant of possible indications that the severity of HF may have changed.
- 6)
RT is usually contraindicated for NYHA Class IV HF patients (Table 1).
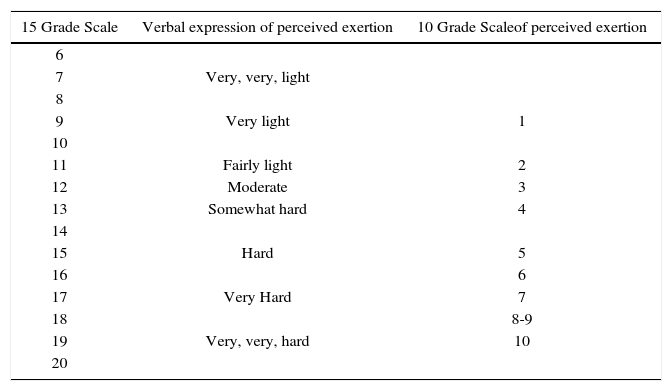
Generally initial RT loads should be light, with patients completing 6 to 15 repetitions without strain (10 to 13 on the 6 to 20 Borg scale of perceived exercise) of exercises that address both upper and lower body muscles one to two days a week. Exercise pace should be slow, allowing at least twice the time for rest/recovery phases as compared to work/contraction phases. Beginning with unilateral exercises can increase RT exercise tolerance. Load and frequency can be slowly progressed over time to an exertion level of 11 to 15 (on the 6 to 20 Borg scale) and two to three days per week. For more detailed exercise prescription criteria, please refer to the publication by Braith et al. (38). An example integrating the 15 grade and 10 grade Borg Ratings of Perceived Exertion can be found in Table 2 (49).
Borg 15 grade and 10 grade rating of perceived exertion (RPE) scales
| 15 Grade Scale | Verbal expression of perceived exertion | 10 Grade Scaleof perceived exertion |
|---|---|---|
| 6 | ||
| 7 | Very, very, light | |
| 8 | ||
| 9 | Very light | 1 |
| 10 | ||
| 11 | Fairly light | 2 |
| 12 | Moderate | 3 |
| 13 | Somewhat hard | 4 |
| 14 | ||
| 15 | Hard | 5 |
| 16 | 6 | |
| 17 | Very Hard | 7 |
| 18 | 8-9 | |
| 19 | Very, very, hard | 10 |
| 20 |
Finally, clinicians also need to be cognizant of rate-pressure product (RPP) (also known as double-product). This unitless indicator of myocardial oxygen demand is the product of systolic blood pressure and heart rate. RPP is typically utilized in a clinical or rehabilitative setting when prescribing exercise. Any combination of systolic blood pressure and heart rate resulting in an RPP that brings on patient symptoms should be avoided. For example, our case patient reported angina at an RPP of 24000 (160mmHg×150bpm). This same patient will also experience angina when his systolic blood pressure and heart rate responses are 136mmHg and 177bpm respectively. This is important because various modes of exercise produce different hemodynamic responses. RPP is also a good indicator of exercise training progress. A lower RPP at a similar previous workload indicates improved functional capacity or fitness. A greater RPP could indicate declining functional status or when dealing with ischemic heart disease progression of disease. RPP is an excellent non-invasive clinical tool.
The authors have provided the following clinical case reportRT in a 59-year-old male from Santiago, Chile. He previously worked as a butcher in a local grocery store. He had a myocardial infarction in May, 2012 resulting in HF with a left ventricular ejection fraction of 32% (Class II NYHA). Now, in August, he is stable with some fatigue and dyspnea during ADLs. He reports taking his cardiovascular medications on a regular basis. He Is doing some walking In his neighborhood, but wants to Increase his physical activity level and hopefully return to work. His physician agrees, but wants an exercise test to more accurately prescribe his exercise. The modified Bruce protocol treadmill test with oxygen consumption was done and the following data were obtained:
Time on treadmill - 11minutes
Resting heart-rate (RHR)=68 beats per minute (bpm)
Heart rate - 68 to 150bpm
Exercising blood pressure response -130/80 to 160/90mmHg
RPE-16
VO2peak-18ml/kg/min
METs − 5.1 (3.5ml/kg/min=1 MET)
RPP − 24000 (at peak exercise)
End points were shortness of breath (SOB) with no angina. ECG showed slight non-significant (non-diagnostic) ST depression.
Based on these results, his physician and healthcare team prescribed the following aerobic exercise program. The exercise was to be performed In cardiac rehabilitation three days weekly with additional walking at home two to three days weekly.
Cardiac rehabilitation exercise programAerobic exerciseExercise Intensity can be prescribed based on HR (55 to 80% heart-rate reserve (HRR), RPE, and VO2 or MET level (40 to 75% VO2peak).
- •
Calculation of HRR=Peak HR - RHR=150-68=82bpm Determining exercise Intensity based on HRR (HRR × training level + RHR)
- •
Calculation of “low end” of exercise Intensity using HRR: (82×55%+68)=113bpm
- •
Calculation of “high end” of exercise Intensity using HRR: (82×.80 + 68)=134bpm
- •
Prescribed exercise Intensity based on
HRR=Obtain and maintain exercise HR between 113 to 134bpm. Alternatively, RPE, or MET level can be used for prescribing exercise Intensity In patients where HRR is either Inappropriate or Inadequate (e.g., beta-blockers or atrial fibrillation)
RPE of 11 to 14 (fairly light to somewhat hard)
- •
Calculation of Exercise Intensity based on VO2peak or MET level:
40 to 75% VO2 peak=7 to 13ml/kg/min or approximately 2 to 4 METs. Prescribed aerobic exercise Intensity: 113 to 134bpm, RPE 11 to 14, or 2 to 4 METS. Workloads will be adjusted periodically to maintain prescribed Intensity (as the patient becomes more conditioned, previous workloads will result in lower exercise HR and perceived exertion).
WARM UP - Five minutes slow walking In facility
Exercise Duration - Initially 10 to 20minutes progressing gradually as patient demonstrates tolerance to 40 to 60minutes.
Mode - Treadmill walking, stationary cycling, elliptical, UBE, rowing ergometer, etc. Patient is encouraged to divide his aerobic exercise time between multiple exercise modes. He Is also advised to use modes that Incorporate arm exercise to better prepare him for return to work.
Cool Down - Five minutes slow walking in facility Aerobic exercise may be done continuously or with short breaks depending upon symptoms and staying within target ranges.
Home Walk Program
Week 1 - 2 - Walk ½ mile in 10minutes twice daily*
Week 3 - 4 - Walk 1mile in 20minutes twice daily*
Week 5 - 6-Walk 1.5miles in 30minutes twice daily*
Week 7 - 8 - Walk 2miles in 40minutes*
week 9 - on - Continue walking 30 - 60minutes two to three days per week at a 20-minute per mile pace or faster If tolerated (can perform Intervals or continuous bouts of walking)*
*lf dizziness, SOB, angina, or Irregular heart beat occur, stop the exercise and report symptoms to your health care team. Walking should be done on level ground If possible.
Resistance exerciseOnce aerobic exercise tolerance has been demonstrated, resistance exercise can be Initiated in three progressive steps. Resistance exercise should be performed in addition to aerobic exercise.
- 1)
Instruction period (one to two weeks): Patient Is shown two to three dynamic RT exercises segmented for upper or lower body muscle groups (four to six exercises total) and Is Instructed to complete five to 10 repetitions. Exercise Intensity should be light with an RPE of about 10 on the 15-grade scale (<30% 1 RM) (Table 2). These exercises should be performed two to three days per week. The focus should be on enhancing coordination of movement and checking for any orthopedic limitations throughout the range of motion for RT exercises.
- 2)
Resistance/muscle endurance period (two to four weeks): Load is gradually Increased from light (RPE of 10) to moderate (RPE of 12) and then to somewhat hard (RPE of 13) as tolerated (Table 2). Repetitions can range from eight to 15 per exercise. Repetitions should be performed in a controlled fashion (one to three seconds concentric, one to three seconds eccentric). Allow for at least a 1:2 work to rest ratio (If the patient completes 10 repetitions of dynamic RT in one minute allow at least two minutes for recovery before moving on to the next exercise). These exercises should be performed two to three days per week. At no time should the patient be straining to complete a repetition at this workload.
- 3)
Resistance/muscle strength period: Load is gradually increased from somewhat hard (RPE of 13) to hard (RPE of 15) as tolerated (Table 2). Repetitions can range from eight to 15 per exercise. Repetitions should be performed in a controlled fashion (one to three seconds concentric, one to three seconds eccentric). Allow for at least a 1:2 work to rest ratio. An additional exercise or set can be added for upper or lower body muscle groups as tolerated (six to eight total exercises). These exercises should be performed two to three days per week. At no time should the patient be straining to complete a repetition at this workload. Clinician should initially check blood pressure response to RT to assure hemodynamic stability to this mode of exercise. Follow-up blood pressure checks should be done periodically.
Stretching exercises for the major muscle groups should be performed two to three days per week for five to 10minutes per session as tolerated.
Following four months of program adherence, the patient was referred to complete a follow-up modified Bruce treadmill test to evaluate his progress. The following data were obtained:
RHR - 64bpm
Time on treadmill -14minutes
Heart Rate - 64 to 145beats per minute
Exercising blood pressure response -124/80 to 160/90mmHg
RPE - 17
VO2peak - 21ml/kg/min
METs - 6 (3.5ml/kg/min=1 MET)
RPP – 23200
End points were SOB, no reported angina; ST changes were similar to initial exercise test. His physician was pleased with his progress and advised him to continue his exercise program and return to work at a limited schedule of four hours three to four days a week.
Authors would like to thank Michael Ryan Richardson for his assistance in formatting all citations and bibliography for this manuscript.
The authors have no conflicts of interest with this article.