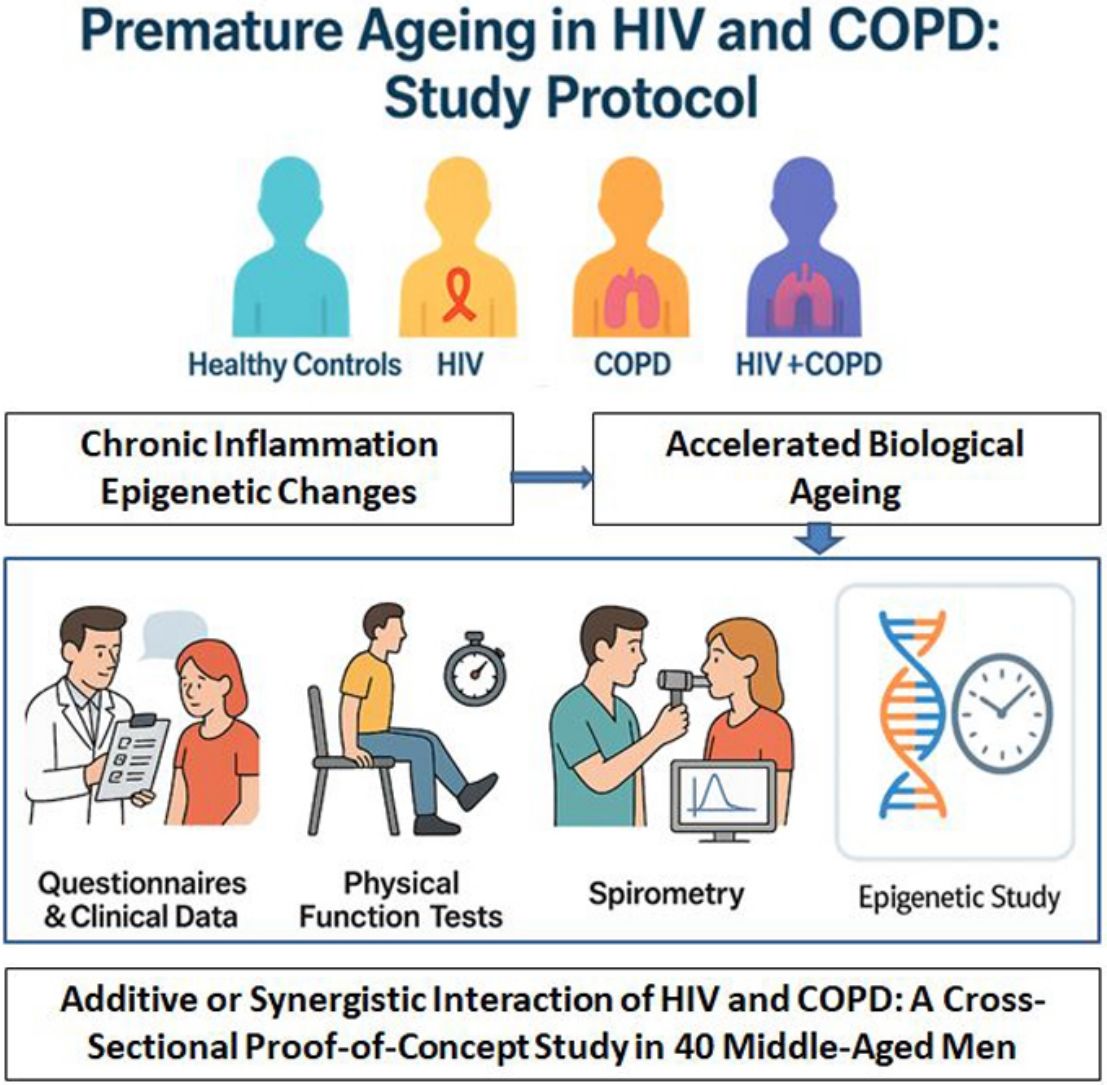
Our project aims to study premature ageing in people living with HIV and COPD. We hypothesize that the chronic inflammation associated with both conditions accelerates the ageing process. HIV infection has undergone a significant paradigm shift in recent years, transitioning from a rapidly fatal disease to a chronic condition. People living with HIV experience more comorbidities (cardiovascular events, osteoporosis, cancer, neurodegenerative diseases, …) at a younger age. This phenomenon, often referred to as ‘premature ageing,’ is associated with chronic inflammation and epigenetic changes. Epigenetic clocks, composite markers based on DNA methylation alterations, have emerged as valuable tools for predicting biological age as they predict mortality better than chronological age. These alterations are described both systemically and at the pulmonary level and are related to a higher prevalence of chronic obstructive pulmonary disease (COPD) and worsened respiratory function.
Material and methodsTo achieve this, we will perform a case–control study analysing epigenetic clocks and comparing four different groups: healthy control subjects, patients living with HIV without COPD, COPD patients without HIV, and patients with both HIV and COPD.
ConclusionsWe hypothesise that patients with both COPD and HIV will exceed the cumulative ageing effect of each condition separately, which suggests a multiplicative effect of ageing between HIV and COPD. Should our hypothesis be supported, it could justify a re-evaluation and potential modification of current screening protocols for COPD in HIV patients or the implementation of case-finding strategies.
Nuestro proyecto pretende estudiar el envejecimiento prematuro en personas que viven con virus de la Iinmunodeficiencia humana (VIH) y enfermedad pulmonar obstructiva crónica (EPOC). Nuestra hipótesis es que la inflamación crónica asociada a ambas enfermedades acelera el proceso de envejecimiento. La infección por VIH ha experimentado un cambio de paradigma significativo en los últimos años, pasando de ser una enfermedad rápidamente mortal a una enfermedad crónica. Las personas que viven con VIH experimentan más comorbilidades (eventos cardiovasculares, osteoporosis, cáncer, enfermedades neurodegenerativas, …) a una edad más temprana. Este fenómeno, a menudo denominado «envejecimiento prematuro», está asociado con la inflamación crónica y los cambios epigenéticos. Los relojes epigenéticos, marcadores compuestos basados en alteraciones de la metilación del ADN, han surgido como herramientas valiosas para predecir la edad biológica, ya que predicen la mortalidad mejor que la edad cronológica. Estas alteraciones se describen tanto a nivel sistémico como pulmonar y están relacionadas con una mayor prevalencia de EPOC y un empeoramiento de la función respiratoria.
Material y métodosPara lograr esto, realizaremos un estudio de casos y controles analizando los relojes epigenéticos y comparando cuatro grupos diferentes: sujetos de control sanos, pacientes que viven con VIH sin EPOC, pacientes con EPOC sin VIH y pacientes con VIH y EPOC.
ConclusionesNuestra hipótesis es que los pacientes con EPOC y VIH superarán el efecto de envejecimiento acumulativo de cada condición por separado, lo que sugiere un efecto multiplicativo del envejecimiento entre el VIH y la EPOC. Si nuestra hipótesis se confirma, podría justificar una reevaluación y una posible modificación de los protocolos actuales de detección de EPOC en pacientes con VIH o la implementación de estrategias de detección de casos.
Since the introduction of effective antiretroviral treatment, HIV infection has transformed from a fatal condition to a chronic one. However, non-AIDS-related comorbidities have become the leading cause of death among HIV patients in developed countries. Their greater prevalence in this population contributes to the persistence of the life expectancy gap compared to the general population.1,2 These events include cardiovascular disease, cancer, bone metabolism disorders, respiratory health issues, etc.
The impact of HIV on ageing is complex: it involves epigenetic changes and chronic inflammation, largely influenced by immunomodulation.3–5 When considering the effects of HIV, it is also advisable to acknowledge the potential role of antiretroviral therapy in modulating immune activation and inflammation, as these factors may also influence epigenetic changes.
Epigenetics studies the mechanisms regulating chromatin structure, which affects gene expression and genome stability. DNA methylation is protective in genome stability and directly correlates with chronological age.6–8 DNA methylation provides a more accurate estimation of biological age compared to chronological age. Epigenetic clocks measure methylation patterns in specific CpG sequences that are strongly correlated with ageing.9–11
Studies have demonstrated accelerated ageing in patients with HIV and COPD using these epigenetic clocks12–14 but have not previously compared its effect between both pathologies in blood samples. In the lungs of HIV patients, hypomethylation is associated with COPD and worsening of pulmonary function. These associations have been observed both in blood cells as well as respiratory samples15–17 though it is important to clarify that the consistency of these findings across different tissues is an area of active investigation.
GOLD guidelines recognise the higher risk of COPD in HIV patients due to the methylation disruptions in the airway epithelium.18 It has been clearly demonstrated that COPD is particularly problematic in people living with HIV as they have higher COPD prevalence, with earlier onset and worse pulmonary function.19–22
Our study aims to assess the interaction between HIV and COPD in the context of ageing using epigenetic clocks. We hypothesize that patients with both HIV and COPD experience accelerated ageing beyond what would be expected from the sum of the ageing effects of each condition individually. This suggests a synergistic or multiplicative effect between HIV and COPD, potentially driven by a convergence of inflammatory and oxidative stress pathways common to both diseases, leading to an amplified, non-additive state of cellular dysfunction. To measure ageing, we will employ epigenetic clocks, alongside with functional markers such as body composition, handgrip strength, and walking speed.
Materials and methodsStudy objectivesOur aims are the following:
- -
To evaluate epigenetic clock scores (PhenoAge and Horvath), functional and physical parameters (grip strength, walking speed and body composition) in people living with HIV, COPD, and individuals affected by both conditions, as well as in healthy control group.
- -
To assess the interaction of epigenetic markers between HIV and COPD.
- -
To investigate the relationship between the results of epigenetic markers and functional tests, to establish potential associations between epigenetic ageing and functional decline.
This study will be an observational proof-of-concept study with four groups of 10 patients in each study group (HIV, HIV–COPD, COPD, and healthy control group). It will be conducted at the Hospital Universitari Son Espases, Mallorca, Spain.
Sample size calculationSample size was calculated using Granmo software (version 7.12), considering an alpha error of 0.05, a beta error of 0.2 and an effect size of 7 residual years. This effect size has been extracted from a study that evaluates methylation in bronchial epithelial brushings of HIV and COPD patients.15 Although our study will use peripheral blood samples, this value was chosen as the best available estimate for a proof-of-concept study, given the lack of directly comparable data in blood. It should therefore be considered an approximation. The results of this pilot study will be crucial for calculating a more precise sample size for a future large-scale study. The total number of participants will be 40, 10 in each group.
Characteristics of participantsInclusion and exclusion criteriaWith the aim of obtaining the most homogenous possible sample, all patients will be males in an age range between 40 and 60 years old. Patient inclusion criteria for each group will be as follows:
- -
Group 1: people living with HIV without COPD. They will have a positive serology for HIV and spirometry results indicating a post-bronchodilator FEV1/FVC ratio ≥70% with normal FVC and FEV1 values (e.g., ≥80% of their predicted value).
- -
Group 2: patients with COPD without HIV. They will have spirometry results indicating FEV1/FVC less than 70% and a negative HIV serology.
- -
Group 3: people living with HIV and COPD. They will have positive HIV serology, and spirometry results indicating FEV1/FVC ratio less than 70%.
- -
Group 4: healthy controls without COPD or HIV. They will have a negative HIV serology HIV and spirometry results indicating a post-bronchodilator FEV1/FVC ratio ≥70% with normal FVC and FEV1 values (e.g., ≥80% of their predicted value).
Confirmation of HIV serological status will be performed as follows:
- •
For patients in the groups of people living with HIV (Groups 1 and 3), a previously confirmed diagnosis according to current clinical protocols, which require a second confirmatory assay, will be mandatory.
- •
Additionally, at the study inclusion visit, all participants will undergo HIV serology testing using a fourth-generation immunoassay (ELISA). The purpose of this test is to rule out potential acute or previously undiagnosed infections among participants in the seronegative groups (Groups 2 and 4), thereby ensuring accurate group assignment.
Patients in the groups of people living with HIV (Groups 1 and 3) will have maintained an undetectable viral load for at least 12 months and a optimal adherence to antiretroviral therapy (defined as an intake of >90% of medication doses, based on pharmacy records).
Patients will be excluded if they have other diseases that may affect ageing or prognosis: cancer, cardiovascular disease, or other chronic inflammatory diseases such as rheumatologic diseases, inflammatory bowel disease, psoriasis, etc or being under immunosuppressive treatment.
Patient enrolmentPeople living with HIV and with both COPD and HIV will be recruited from the HIV clinic at the Hospital Universitari Son Espases. COPD patients will be recruited from the COPD clinic in the same hospital, and their COPD diagnosis will be based on spirometry results, following the current GOLD guidelines.18 Control subjects will be enrolled from voluntary personnel from Internal Medicine and Infectious Disease department, Pneumology department and Emergency Room department.
VariablesThe presence or absence of the study variables, as well as the fulfillment of exclusion criteria, will be determined based on a review of the patient's medical record, a clinical interview, and the verification of any targeted treatments. The variables to be collected include: identification number, age (years), body mass index (BMI, kg/m2), smoking habit (Yes/No), smoking load (pack-years), and presence of high blood pressure, diabetes, or cardiovascular disease (Yes/No). Additionally, we will record the results of the Horvath and PhenoAge epigenetic clocks (calibrated in units of year), handgrip strength (kg), walking speed (m/s), and body composition analysis (e.g., body fat %, skeletal muscle mass kg). Lung function will be assessed via post-bronchodilator FEV1 (L) and the FEV1/FVC ratio (%). HIV-specific markers include HIV status, CD4 count (cells/μL), and the CD4/CD8 ratio. Information on other comorbidities listed in the exclusion criteria (e.g., cancer) will be recorded during the screening process to confirm patient eligibility and to describe the final cohort.
Smoking habit will be included as it plays a role as a confounding variable, keeping in mind that smoking is the main risk factor for COPD in developed countries.
Description of the testsFirst, a medical interview will be conducted to determine smoking habit and the tobacco load as well as the comorbidities previously described (high blood pressure, diabetes, cardiovascular disease).
The following tests will be conducted:
- -
Body measurements: height and weight for BMI calculation.
- -
Blood analysis: determination of variables necessary for PhenoAge clock (albumin, creatinine, glucose, C-reactive protein, lymphocytes, MCV (mean cell volume), RDW (red cell distribution width), alkaline phosphatase, WBC (white blood cells), HIV serology, CD4 count and CD4/CD8 ratio.
- -
Spirometry: post-bronchodilator FEV1 and post-bronchodilator FEV1/FCV will be obtained as per by GOLD spirometry guideline.24
- -
Horvath clock: we will need 0.250mL peripheral blood in EDTA.
- -
Handgrip strength test
- -
Walking speed test
- -
Body composition analysis (InBody)
Peripheral blood samples will be collected in EDTA from the antecubital vein between 08:00am and 09:00amh after an overnight fasting period. Participants will be instructed to avoid moderate physical activity in the previous 24h. The collected blood samples will be analyzed and stored at a temperature of 4°C and centrifuged within 4h. Subsequently, the samples will be divided into smaller portions (0.250mL), and send frozen at −80°C for analysis at The Clock Development Foundation Epigenetics Laboratory (Torrance, CA).
Physical function testsGait speed will be assessed with the 4-meter walking test at the usual and fast pace. Participants will be instructed to walk at their usual or fast pace in a 4-meter distance on the floor at the Clinical Research Unit. The tests will be repeated three times.
Muscle strength will be evaluated using the grip strength test using a Kern hand dynamometer. Participants will be instructed to keep their arms in a relaxed and stationary position. Three maximum grip measurements will be taken with both hands and the highest value, measured in kilograms (kg), will be used for the analysis.
Body composition analysisTo assess body composition, we will use the InBody 770, a bioelectrical impedance analysis (BIA) machine (InBody Co. Ltd. Korea). Participants will be asked to fast for a minimum of 12h, and to avoid drinking any kind of liquid for at least 2h before the test. During the assessment, participants will be standing barefoot on the back foot electrode, keeping their arms away from the sides of their body while holding the hand electrodes and no wearing any metal or electronic devices. Participants will maintain this position for approximately 2min until the test is completed.
Study outcomesThe primary outcomes will be the biological age (using the Horvath clock and PhenoAge clock) and the functional tests in each of the four groups previously described. The secondary outcome will be the correlation between epigenetic and the functional tests.
Data managementParticipation in this study is completely voluntary. Written consent will be obtained from every patient and control. Patients will be able to terminate their participation in the study at any time. The IP will ensure that the data will remain confidential, as the patients will be identified by a code. The correlation between the codes and the patients’ identification as well as the consent forms will be stored in a file in a single folder dedicated to this study and located in IdISBa (Institut d’Investigació Sanitaria Illes Balears).
Data analysisDescriptive characteristics will be summarized as means and standard error of the mean (SEM) or standard deviation (SD) or as numbers and percentages (%). One-way analysis of variance (ANOVA), Chi-square tests (χ2), and/or Fisher's exact test will be used to assess differences across groups for continuous and categorical variables respectively. If statistical assumptions are met, post hoc analyses will be conducted using Tukey's HSD test to pinpoint specific group differences. Potential baseline differences between groups will be assessed, and multivariate regression models will be used to control for key confounders, particularly age and smoking history (pack-years). No prior matching of groups is planned; adjustments will be handled statistically. Non-normally distributed continuous data will be compared using the Mann–Whitney–Wilcoxon test or the Kruskal–Wallis H-test. The relationships between the variables will be studied using Spearman or Pearson correlations and partial correlations. The epigenetic clock algorithm will be applied to our DNA methylation data to predict the biological age of our participants.25 This algorithm calculates a “DNA methylation age” based on the weighted average of methylation levels at the selected CpG sites. The predicted DNA methylation age will be compared to the individual's actual chronological age. The difference between predicted and actual age will be referred as the “epigenetic age acceleration” and will be used to identify individuals who appear to be aging faster or slower than expected based on their DNA methylation patterns. The PhenoAge algorithm,26 will be used to measure phenotypic age. To minimize technical variability in methylation data, standard bioinformatic pipelines for quality control, normalization, and batch effect correction will be applied. All analyses and plots will be performed using RStudio 3.5.3. (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS statistics 25.
Ethical considerations and declarationsThe protocol was approved by Ethics Research Committee of the Balearic Islands with the code IB 5182/23 PI (Date: 26-07-2023) and Son Espases Hospital Research Committee (Ref: CI-767-23; Date: 24-04-2023). All included patients will sign a consent form.
Timeline of the studyAll participants will be recruited from month 1 to 3. The clinical interviews, blood test analysis and functional tests will be carried out from month 4 to 7. The Clinical Data Registry is expected to be completed by month 8. The data analysis will be performed in months 9 and 10 and the research article will be completed by month 12.
DiscussionOur hypothesis is that patients with both COPD and HIV will exceed the cumulative ageing effect of each condition separately, which suggests a multiplicative effect of ageing between HIV and COPD. To our knowledge, this will be the first study utilizing an epigenetic clock on blood samples to compare patients with each pathology separately and combined.
People living with HIV have a higher prevalence of COPD, even adjusting for smoking habits and CD4 count, which suggests that other factors play a role in its pathogenesis. The relationship between HIV and emphysema is widely described. Many research groups have demonstrated the impact of chronically active immune system, changes in lung microbiome, oxidative stress and protease-antiprotease imbalance on DLCO and emphysema.19,20,23 Recently, studies have also focused on epigenetic changes. Hernández-Cordero et al. described methylation age acceleration in the airway epithelium of PLHIV with COPD and related DNA methylation with airflow obstruction.15–17
This is a proof-of-concept study that aims to provide preliminary evidence supporting our hypothesis. If we obtain positive results, it will pave the way to a larger-scale study to establish statistical significance and further validate our hypothesis. Should our hypothesis be supported, it could justify a re-evaluation and potential modification of current screening protocols for COPD in HIV patients or implementation of case-finding strategies. Three studies have already approached case-finding programmes with questionnaires or peak-flow measurements, the most successful strategy being same-day spirometry testing.19
Our study has certain limitations:
First, as a proof-of-concept study, the sample size is small, which limits the statistical power to detect smaller effects and increases the risk of a Type II error. Positive results will therefore require validation in a larger-scale study to establish statistical significance.
Second, to obtain a homogeneous sample and reduce potential confounders, we have restricted inclusion to males within a specific age range (40–60 years). While methodologically sound for internal validity, this means our findings may not be directly generalizable to females or other age groups, a point that should be explored in future research.
Third, the cross-sectional design of the study will allow us to identify associations between variables, but it precludes any inference of causality.
Finally, despite our efforts to control for known confounders, the presence of unknown or unmeasured variables that could impact biological age cannot be entirely ruled out.
In conclusion, our proof-of-concept study aims to test the hypothesis that there is a synergistic effect of ageing between HIV and COPD, likely driven by epigenetic changes in the airways of people living with HIV.
Ethical approvalThis study has been approved by the Ethics Committee of the Balearic Islands under code IB 5182/23 PI. Written informed consent was obtained from all participating patients.
Declaration of generative AI and AI-assisted technologies in the writing processNo article material has been produced with the help of any artificial intelligence software or tool.
FundingThe funding for our study comes from:
- -
Project Pilot 2024 from the Investigation Commission from Hospital Universitari Son Espases.
- -
Mapfre Foundation. Ignacio H. de Larramendi Research Grant 2024.
MGM, RR and MGF participated in writing the protocol. FF supervised the protocol. FF and MR will contribute to the enrolment of HIV and both HIV and COPD patients. ES and BGC will help in the enrolment of COPD patients. MGM and RR will contribute to the recruiting of control patients. JLV will contribute by performing spirometries.
MGM, RR and FF will conduct the medical interviews. MGF will obtain the results from the epigenetic clocks and functional tests. MGM and RR will collect the data, do the statistical analysis and write the article with the help of FF.
Conflicts of interestThe authors declare that they have no conflict of interest.
The authors would like to acknowledge Xavier Capo, Ana Ortega-Moral and Cayetano Navas-Enamorado from Translational Research in Aging and Longevity (TRIAL) group from Institut d’Investigació Sanitària Illes Balears (IdISBA).