COPD is a respiratory condition characterized by chronic airflow limitation. Exacerbations are an acute worsening of the symptoms. The objective of this study was to achieve a consensus on the management of COPD exacerbation syndrome in inpatient and outpatient settings.
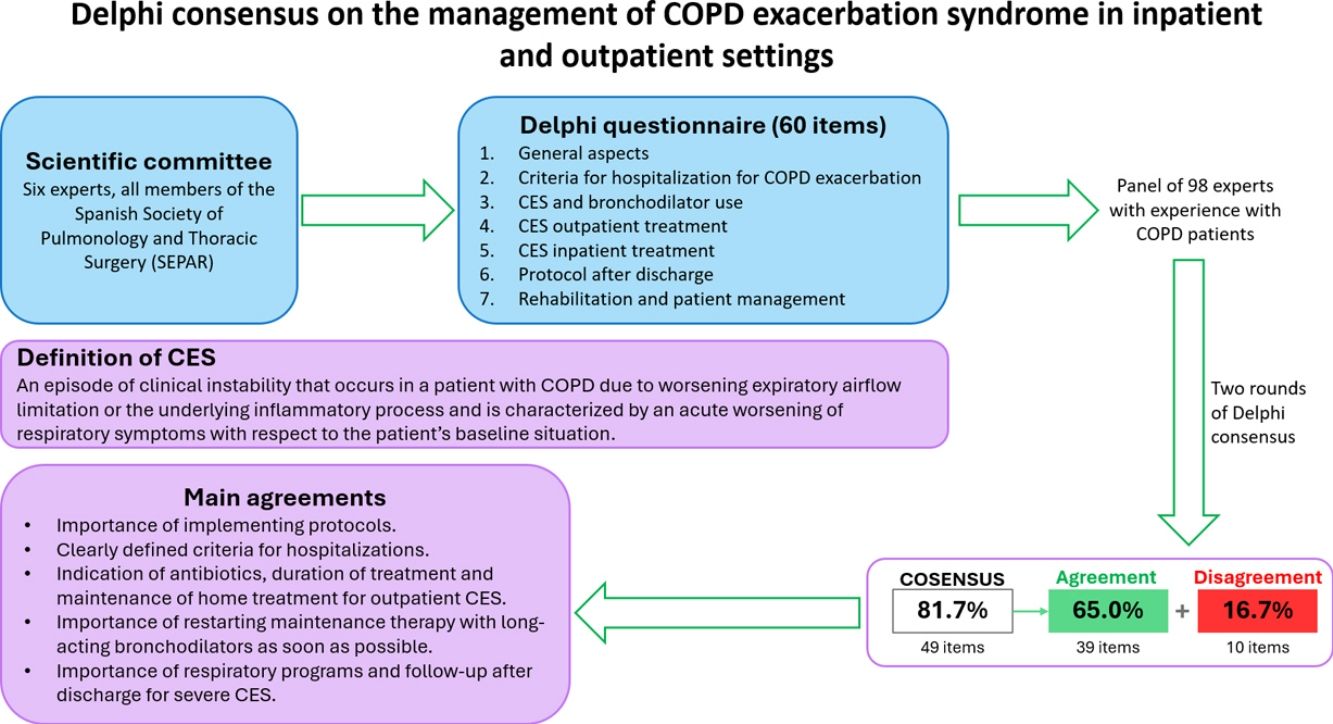
Material and methodsA committee of experts developed a 60-item questionnaire to be agreed by a panel of experts, categorized into seven sections.
ResultsAfter two rounds, consensus was reached on 81.7% of the items. Strong consensus (more than 85%) was reached on the importance of implementing protocols to help patients with exacerbations in both outpatient (92.7%) and inpatient (94.3%) settings. Regarding the criteria for hospitalization due to an exacerbation, respondents agreed that they are clearly defined (75.5%). Regarding bronchodilator use for CES, the only statement that did not achieve agreement was whether there are clinical differences between the use of nebulized rescue bronchodilators and pressurized metered-dose inhalers (pMDIs) with a spacer. Regarding CES treatment in the outpatient setting, consensus was reached for almost all statements, in contrast to what was found for inpatient treatment. Respondents disagreed with the statement that the use of SABA should be accompanied by the discontinuation of LAMAs or LABAs, with or without corticosteroids (74.8%). In the context of a COPD exacerbation requiring hospitalization, inhaled triple therapy should be prescribed (regardless of prior treatment) in the absence of contraindications. Regarding post-discharge protocols and rehabilitation, respondents reached consensus on all statements.
ConclusionsThis Delphi consensus study provides valuable insights into the current management of CES, highlighting several areas where consensus remains elusive.
La EPOC es una enfermedad respiratoria caracterizada por la limitación crónica del flujo aéreo. Las exacerbaciones representan un empeoramiento agudo de los síntomas. El objetivo de este estudio fue establecer un consenso sobre el manejo del síndrome de exacerbación de la EPOC en entornos hospitalarios y ambulatorios.
Material y métodosUn comité desarrolló un cuestionario de 60 ítems, estructurado en 7 secciones, para alcanzar consenso entre expertos.
ResultadosSe logró consenso en el 81,7% de los ítems tras 2 rondas. Se destacó el fuerte consenso sobre la importancia de implementar protocolos para pacientes con exacerbaciones tanto en atención ambulatoria (92,7%) como hospitalaria (94,3%). Se acordó que los criterios de hospitalización están claramente definidos (75,5%). En cuanto al uso de broncodilatadores para el síndrome de exacerbación de la EPOC, no se logró consenso respecto a las diferencias clínicas entre nebulizadores e inhaladores presurizados con espaciador. Se alcanzó consenso sobre la mayoría de los aspectos del tratamiento ambulatorio, pero hubo discrepancias en el ámbito hospitalario. Los encuestados no estuvieron de acuerdo con la afirmación de que el uso de SABA debería ir acompañado de la suspensión de LAMA o LABA, con o sin corticosteroides (74,8%). En el contexto de las exacerbaciones que requieren hospitalización, se recomendó la prescripción de triple terapia al alta (independientemente del tratamiento previo) en caso de no existir contraindicaciones. En cuanto a aspectos como los protocolos tras el alta y el uso de rehabilitación, los encuestados llegaron a un consenso en todas las afirmaciones.
ConclusionesEste estudio Delphi proporciona información sobre el manejo actual del síndrome de exacerbación de la EPOC, resaltando algunas áreas con falta de consenso.
Chronic obstructive pulmonary disease (COPD) is a respiratory condition characterized by chronic airflow limitation, which results from long-term lung damage, typically caused by smoking or exposure to pollutants.1,2 COPD is one of the leading causes of morbidity and mortality worldwide, with 3.2 million deaths recorded globally in 2021. In Spain, the prevalence of COPD is 11.8% among individuals aged 40 years or older.2 As reflected in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) report, patients’ symptoms include dyspnea, wheezing, chest tightness, fatigue, activity limitation and/or cough with or without sputum.3
Within the clinical expression of COPD, its chronic and often progressive clinical course is frequently altered by an acute worsening of the symptoms, which has been defined as an exacerbation. The intensity, duration and frequency of exacerbations vary greatly from patient to patient and even within the same patient, making it difficult to pinpoint their consequences. However, several studies have shown that exacerbations lead to a deterioration in health-related quality of life, greater decline of pulmonary function and higher costs affecting the multidimensional progression of the disease and increasing the risk of death.4,5
From the pathophysiological point of view, an exacerbation is a complex, heterogeneous event that includes a set of diverse alterations,6 which either in isolation or more frequently in combination, are clinically expressed in a similar way in patients with COPD. Taking these aspects into account, the syndromic approach can better encompass the heterogeneity of this acute episode. With this approach, GesEPOC (the Spanish guidelines of COPD) in its latest version introduces the concept of COPD exacerbation syndrome (CES).7 CES is defined as an episode of clinical instability that occurs in a patient with COPD as a consequence of worsening expiratory airflow limitation or the underlying inflammatory process and is characterized by an acute worsening of respiratory symptoms with respect to the patient's baseline situation.7 Factors such as the patient's clinical situation, baseline situation or burden of comorbidities among others would influence which location should receive treatment (outpatient or inpatient). Despite improved knowledge of the mechanisms involved in COPD exacerbation, the failure rate is high.8 The lack of identification of the underlying biological mechanism, the heterogeneity of the condition or the lack of specificity of the symptoms are some of the reasons why there are still important deficiencies in the management of exacerbations. For this reason, societies such as the European Respiratory Society and the American Thoracic Society,9 as well as GesEPOC5 or GOLD10 have tried to establish the best course of action,4 with the aim of reducing this uncertainty.
Despite such recommendations, and as reported by López-Campos et al.,11 there is a low degree of consensus among experts on how to treat CES, reflecting the complexity of the disease. Indeed, it was shown that COPD patients, particularly those at high risk, are a very heterogeneous and complicated cohort. Therefore, the choice of treatment sequence might not be clear.11 In line with the above, another Delphi consensus12 highlighted the relevance of achieving diagnostic and therapeutic recommendations for all kinds of patients with exacerbations. The dynamic nature of the field of COPD showcases the importance of assessing how patient care protocols have evolved in the last few years as well as their level of implementation.
The main objective of this Delphi consensus was to identify areas of improvement in both the management and treatment of outpatient and inpatient CES.
Material and methodsStudy designThis was a nationwide exploratory study conducted by a panel of experts using the Delphi methodology. The study consisted of two rounds of participation, carried out between November 2024 and January 2025.
A scientific committee composed of six experts, all members of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), was involved in the design and development of the study. In addition, a panel of 98 experts showed their degree of agreement or disagreement with the items proposed in the questionnaire. All participating respondents were anonymous members of SEPAR's COPD assembly and were initially proposed on the basis of their experience, publications in recent years, participation in research projects on the subject, and geographic diversity.
The project was divided into three phases. In the first one, the scientific committee developed the study protocol following the Delphi methodology. The questions included in the questionnaire were also agreed upon based on the selected topics, resulting in a total of 60 statements. The second phase involved a two-stage online survey conducted from November 1 to November 29 and from December 20 to January 15. In the third phase, the scientific committee organized a meeting to discuss the results, analyze them, and draft the most relevant conclusions.
QuestionnaireThe questionnaire consisted of 60 statements grouped into seven sections: general aspects of the implementation of protocols and patient assessments, admission criteria for COPD exacerbation, COPD exacerbation syndrome (CES) and the use of bronchodilators, considerations in the treatment of outpatient CES, hospital treatment of COPD exacerbation, protocol after discharge and rehabilitation and patient management after admission for exacerbation of COPD.
The questionnaire was anonymous and non-compensated, and SEPAR distributed it to members of the COPD section via mail. Since the process was entirely anonymous, participants were instructed to participate in the second round only if they had taken part in the first. A total of 123 respondents participated in the first round, and 97 in the second.
Consensus determinationA five-point Likert scale was used to determine the degree of consensus for each statement: strongly disagree (1), disagree (2), neither agree nor disagree (3), agree (4), and strongly agree (5). A consensus in favor of agreement was established when more than 70% of participants selected “agree” or “strongly agree” for a given item. Conversely, a consensus in favor of disagreement was defined when more than 70% of respondents selected “disagree” or “strongly disagree.” If neither of these two consensus thresholds was met, no consensus was established for that item. Statements that did not reach a consensus in the first round were carried over to the second round, with the results from the previous round added to each item.
ResultsThis Delphi consensus study comprised two rounds of questionnaires designed to define the management of COPD exacerbation syndrome. A total of 60 statements, categorized into seven sections, were assessed using a threshold of at least 70% agreement or disagreement to establish consensus. Consensus was achieved for 49 statements (81.7%): 39 (65.0%) reached agreement, while 10 (16.7%) reached disagreement. The remaining 11 statements (18.3%) did not reach consensus. All the results and the degree of consensus are provided in Table 1 and the statements that did not reach consensus are highlighted in Table 2.
Results from the two rounds of the questionnaire.
| Statements | First round | Second round | Result | ||||
|---|---|---|---|---|---|---|---|
| % disagreement | % neutral | % agreement | % disagreement | % neutral | % agreement | ||
| Section 1: General aspects of the implementation of protocols and patient assessments | |||||||
| 1. Implementation of protocols for ambulatory exacerbation care improves health care during exacerbations. | 5.70% | 1.60% | 92.70% | – | – | – | Consensus on the agreement |
| 2. The implementation of protocols for acute hospitalization care improves health care during exacerbations. | 3.30% | 2.40% | 94.30% | – | – | – | Consensus on the agreement |
| 3. Assessment by nutrition and rehabilitation services is essential in outpatient exacerbations. | 16.30% | 35.00% | 48.80% | 19.40% | 32.70% | 48.00% | No consensus |
| 4. Assessment by nutrition and rehabilitation services is essential in hospitalizations for acute exacerbations | 4.10% | 11.40% | 84.60% | – | – | – | Consensus on the agreement |
| 5. The development of multidimensional and personalized self-management programs is useful during an exacerbation. | 3.30% | 11.40% | 85.40% | – | – | – | Consensus on the agreement |
| 6. The inclusion of mobile technology is useful during exacerbations. | 3.30% | 38.20% | 58.50% | 5.10% | 22.50% | 72.50% | Consensus on the agreement |
| Section 2: Aspects related to admission criteria for COPD exacerbation | |||||||
| 7. Admission criteria for COPD exacerbations are clearly defined. | 23.60% | 17.90% | 58.50% | 14.30% | 10.20% | 75.50% | Consensus on the agreement |
| 8. The criteria for admission for COPD exacerbations are sound from a scientific evidence point of view. | 25.20% | 31.70% | 43.10% | 18.40% | 35.70% | 45.90% | No consensus |
| Section 3: Aspects related to COPD exacerbation syndrome (CES) and the use of bronchodilators | |||||||
| 9. In the case of mild or moderate COPD exacerbation syndrome (CES) (according to GesEPOC), outpatient treatment will be initiated. | 2.40% | 2.40% | 95.10% | – | – | – | Consensus on the agreement |
| 10. In outpatient CES, the main intervention consists of optimizing short-acting bronchodilators, increasing their doses and/or the frequency of bronchodilators. | 19.50% | 12.20% | 68.30% | 9.20% | 5.10% | 85.70% | Consensus on the agreement |
| 11. There are no clinical differences between the administration of rescue bronchodilator drugs by nebulized or pMDI (metered dose devices) with spacer chamber. | 23.60% | 18.70% | 57.70% | 22.50% | 8.20% | 69.40% | No consensus |
| 12. In every patient with mild/moderate CES treated with DPI (dry powder inhaler) the peak inspiratory flow during the episode should be assessed to ensure correct delivery of inhaled medication. | 22.00% | 13.80% | 64.20% | 11.20% | 7.10% | 81.60% | Consensus on the agreement |
| 13. Long–acting bronchodilators (LABDs) should not be discontinued during outpatient treatment of CES. | 6.50% | 4.90% | 88.60% | – | – | – | Consensus on the agreement |
| Section 4: Considerations in the treatment of outpatient CES | |||||||
| 14. All ambulatory CES should receive antibiotic treatment. | 88.60% | 10.60% | 0.80% | – | – | – | Consensus in disagreement |
| 15. Only episodes of ambulatory CES with purulent expectoration should be treated with antibiotic therapy. | 20.30% | 12.20% | 67.50% | 13.30% | 2.00% | 84.70% | Consensus on the agreement |
| 16. In the antibiotic treatment of ambulatory CES, 5-day antibiotic treatment is not inferior in clinical efficacy to 7 days. | 5.70% | 13.00% | 81.30% | – | – | – | Consensus on the agreement |
| 17. During ambulatory CES it is not advisable to change the maintenance treatment for COPD. | 19.50% | 15.50% | 65.00% | 8.20% | 3.00% | 88.80% | Consensus on the agreement |
| 18. In outpatient CES there is no need for an evaluation of treatable features since all patients should be treated in the same way. | 91.90% | 6.50% | 1.60% | – | – | – | Consensus in disagreement |
| Considerations with the use of corticosteroids for the treatment of CES | |||||||
| 19. All mild CES should be treated with systemic corticosteroids. | 86.20% | 8.90% | 4.80% | – | – | – | Consensus in disagreement |
| 20. All moderate CES should be treated with systemic corticosteroids. | 34.20% | 19.50% | 46.30% | 29.60% | 7.10% | 63.30% | No consensus |
| 21. Systemic corticosteroids should be used exclusively in mild/moderate CES if the patient has an eosinophil count >2% in peripheral blood at the time of evaluation. | 54.50% | 31.70% | 13.80% | 80.60% | 10.20% | 9.20% | Consensus in disagreement |
| 22. The recommended dose of systemic corticosteroids in ambulatory CES is 0.5mg/kg/day of prednisone or equivalent. | 10.60% | 10.60% | 78.90% | – | – | – | Consensus on the agreement |
| 23. Treatment duration of 5 days with systemic corticosteroids in ambulatory CES is similar in clinical efficacy to the duration of >7 days. | 7.30% | 11.40% | 81.30% | – | – | – | Consensus on the agreement |
| Considerations related to the prevention of cardiovascular events | |||||||
| 24. In ambulatory CES, in which the patient remains bedridden or inactive for three or more days, the use of low molecular weight heparins (LMWH) at prophylactic doses of high risk of venous thromboembolic disease (VTE) is indicated. | 10.60% | 23.60% | 65.90% | 8.20% | 8.20% | 83.70% | Consensus on the agreement |
| 25. In outpatient CES, cardiovascular prevention strategies with antiplatelet agents should be carried out due to the increased risk of cardiovascular events. | 52.00% | 32.50% | 15.50% | 72.50% | 22.50% | 5.10% | Consensus in disagreement |
| 26. Cardiovascular prevention strategies with statins should be implemented in outpatient CES due to the increased risk of cardiovascular events. | 61.00% | 30.10% | 8.90% | 80.60% | 13.30% | 6.10% | Consensus in disagreement |
| 27. In outpatient CES, cardiovascular prevention strategies with iSGLT2 drugs should be implemented due to the increased risk of cardiovascular events. | 61.00% | 34.20% | 4.90% | 85.70% | 12.20% | 2.00% | Consensus in disagreement |
| 28. In active recommendation for physical exercise during the episode should be made in all patients with moderate CES. | 14.60% | 21.10% | 64.20% | 11.20% | 10.20% | 78.60% | Consensus on the agreement |
| Section 5: Aspects related to hospital treatment of COPD exacerbation | |||||||
| 29. The baseline condition of the COPD patient is a relevant aspect when deciding on the need for hospital admission in the context of an exacerbation. | 1.60% | 1.60% | 96.80% | – | – | – | Consensus on the agreement |
| 30. In COPD patients admitted for an exacerbation, proBNP and troponin levels should be determined at the beginning of the hospital stay. | 9.80% | 11.40% | 78.90% | – | – | – | Consensus on the agreement |
| 31. In COPD patients admitted for an exacerbation, transthoracic echocardiography should be performed if a recent study is not available. | 31.70% | 33.30% | 35.00% | 32.70% | 34.70% | 32.70% | No consensus |
| 32. Thoracic echocardiography is a useful tool in the initial assessment of severe exacerbation of COPD. | 9.80% | 28.50% | 61.80% | 9.20% | 25.50% | 65.30% | No consensus |
| Considerations regarding markers used for decision-making | |||||||
| 33. The presence of a change in sputum color is a useful marker to guide the administration of antibiotics in patients with severe exacerbation of COPD. | 1.60% | 4.10% | 94.30% | – | – | – | Consensus on the agreement |
| 34. Determination of C-reactive protein is a useful marker to guide antibiotic administration in patients with severe COPD exacerbation. | 8.10% | 15.50% | 76.40% | – | – | – | Consensus on the agreement |
| Medication withdrawal considerations and treatment decisions | |||||||
| 35. In COPD patients admitted for an exacerbation, oral intake should be avoided for the first 24–48h to reduce the risk of bronchoaspiration. | 8.10% | 15.50% | 76.40% | – | – | – | Consensus on the agreement |
| 36. In an exacerbation of COPD requiring hospital admission. Once treatment with short-acting bronchodilators is initiated. Maintenance inhaled therapy (LAMA. LABA±inhaled corticosteroids) should be discontinued. | 74.80% | 22.00% | 3.30% | – | – | – | Consensus in disagreement |
| COPD exacerbation considerations and treatment decisions | |||||||
| 37. Whenever the patient's clinical situation permits, in an exacerbation of COPD requiring hospital admission, the use of MDI devices with chamber should be prioritized over nebulization for the administration of short-acting bronchodilators. | 22.00% | 23.60% | 54.50% | 15.30% | 16.30% | 68.40% | No consensus |
| 38. In patients with COPD who are admitted for an exacerbation, priority should be given to the administration of short-acting bronchodilators by nebulized route. | 44.70% | 25.20% | 30.10% | 61.20% | 20.40% | 18.40% | No consensus |
| 39. In COPD patients admitted for an exacerbation, azithromycin should be discontinued at prophylactic doses while antibiotic treatment is administered. | 37.40% | 22.00% | 40.70% | 28.60% | 16.30% | 55.10% | No consensus |
| 40. In an exacerbation of COPD requiring hospital admission. Withdrawal of systemic corticosteroids should be performed progressively. | 48.80% | 22.00% | 29.30% | 73.50% | 10.20% | 16.30% | Consensus in disagreement |
| 41. In an exacerbation of COPD requiring hospital admission, inhaled corticosteroids should be associated with short-acting bronchodilators as part of the treatment. | 42.30% | 19.50% | 38.20% | 54.10% | 11.20% | 34.70% | No consensus |
| 42. In COPD patients admitted for an exacerbation, maintenance therapy with long-acting bronchodilators should be restarted as soon as possible during admission. | 5.70% | 7.30% | 87.00% | – | – | – | Consensus on the agreement |
| Considerations regarding the treatment of COPD exacerbation in hospitalized patients | |||||||
| 43. If there is no contraindication, in COPD patients admitted for an exacerbation, inhaled corticosteroids should be prescribed at discharge and their indication reevaluated when a period of stability is reached. | 26.00% | 18.70% | 55.30% | 15.30% | 5.10% | 79.60% | Consensus on the agreement |
| 44. Given the high morbimortality of an exacerbation of COPD requiring hospital admission, triple inhaled therapy (LAMA, LABA and inhaled corticosteroids) should be prescribed (regardless of previous treatment) if there are no contraindications. | 26.80% | 17.90% | 55.20% | 13.30% | 6.10% | 80.60% | Consensus on the agreement |
| 45. In patients admitted for suspected COPD exacerbation, diagnostic spirometry should be performed during admission. | 71.50% | 13.00% | 15.50% | – | – | – | Consensus in disagreement |
| 46. In patients admitted for a first episode of COPD decompensation, diagnostic spirometry should be deferred if a recent one is not available, one month after admission. | 9.80% | 8.10% | 82.10% | – | – | – | Consensus on the agreement |
| 47. In patients with COPD who are admitted for an exacerbation, systemic corticosteroids should be administered on a 24-h schedule if possible. | 10.60% | 14.60% | 74.80% | – | – | – | Consensus on the agreement |
| 48. In COPD patients admitted for an exacerbation, the administration of systemic corticosteroids should not exceed 15 days. | 2.40% | 4.90% | 92.70% | – | – | – | Consensus on the agreement |
| 49. During a mild–moderate exacerbation of COPD in a patient on triple therapy, the bronchodilator regimen should be increased at the expense of short-acting anticholinergics. | 42.30% | 31.70% | 26.00% | 66.30% | 23.50% | 10.20% | No consensus |
| COPD exacerbation considerations and evaluation of device use | |||||||
| 50. In COPD patients admitted for an exacerbation, the adequacy of the inhaled device (by measuring peak inspiratory flow) should be assessed prior to discharge. | 4.90% | 15.50% | 79.70% | – | – | – | Consensus on the agreement |
| 51. In patients with COPD who are admitted for an exacerbation, the technique of the device prescribed for home use prior to discharge should be evaluated. | 0.80% | 0.00% | 99.20% | – | – | – | Consensus on the agreement |
| Considerations regarding the treatment of COPD exacerbation and smoking | |||||||
| 52. In patients with COPD who are admitted for an exacerbation, smoking intervention should be performed during admission. | 0.80% | 1.60% | 97.60% | – | – | – | Consensus on the agreement |
| Section 6: Aspects related to the protocol after discharge | |||||||
| 53. Generally, the patient who has presented an exacerbation that has been managed in the outpatient setting should be evaluated in primary care consultations within 72h after discharge, either by telephone or in person. | 4.90% | 11.40% | 83.70% | – | – | – | Consensus on the agreement |
| 54. Generally, after discharge from the hospital, the patient who has presented an exacerbation should be evaluated in primary care consultations within 1 week after discharge, either by telephone or in person. | 8.90% | 4.10% | 87.00% | – | – | – | Consensus on the agreement |
| 55. Generally, after discharge from the hospital, the patient who has presented an exacerbation should be evaluated in specialized care consultations within a maximum of 3 months. | 5.70% | 0.80% | 93.50% | – | – | – | Consensus on the agreement |
| Section 7: Aspects related to rehabilitation and patient management after admission for exacerbation of COPD | |||||||
| 56. As a general rule, the patient who is hospitalized for an exacerbation of COPD should be included in a respiratory rehabilitation program early (within 3 weeks after discharge). | 6.50% | 18.70% | 74.80% | – | – | – | Consensus on the agreement |
| 57. Access to early respiratory rehabilitation (up to 3 weeks after hospital care) is widely established in clinical practice. | 74.80% | 13.00% | 12.20% | – | – | – | Consensus in disagreement |
| 58. The care and management of the patient with an exacerbation should be performed in a coordinated manner. Within the framework of an integrated care program between primary care and pulmonology. | 0.80% | 4.10% | 95.10% | – | – | – | Consensus on the agreement Consensus on the agreement |
| 59. Patients with an exacerbation should be subsequently monitored by pulmonology, always within integrated programs that include pulmonology and other clinical specialties (nutrition, rehabilitation, etc.). | 6.50% | 12.20% | 81.30% | – | – | – | Consensus on the agreement |
| 60. Home-centered care for the patient with an exacerbation is essential with the support of other available resources (monographic consultations, day hospital, Telemedicine, etc.). | 0.80% | 6.50% | 92.70% | – | – | – | Consensus on the agreement |
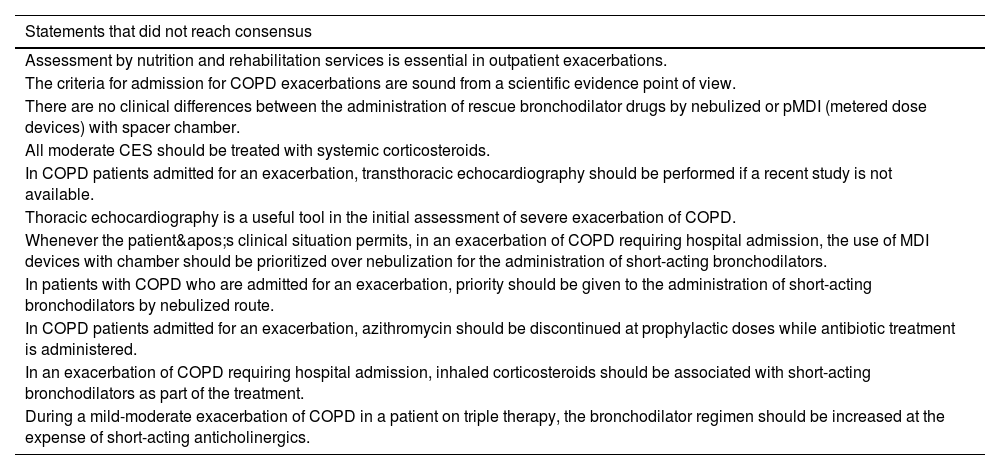
Statements that did not reach consensus after the two rounds of the questionnaire.
| Statements that did not reach consensus |
|---|
| Assessment by nutrition and rehabilitation services is essential in outpatient exacerbations. |
| The criteria for admission for COPD exacerbations are sound from a scientific evidence point of view. |
| There are no clinical differences between the administration of rescue bronchodilator drugs by nebulized or pMDI (metered dose devices) with spacer chamber. |
| All moderate CES should be treated with systemic corticosteroids. |
| In COPD patients admitted for an exacerbation, transthoracic echocardiography should be performed if a recent study is not available. |
| Thoracic echocardiography is a useful tool in the initial assessment of severe exacerbation of COPD. |
| Whenever the patient's clinical situation permits, in an exacerbation of COPD requiring hospital admission, the use of MDI devices with chamber should be prioritized over nebulization for the administration of short-acting bronchodilators. |
| In patients with COPD who are admitted for an exacerbation, priority should be given to the administration of short-acting bronchodilators by nebulized route. |
| In COPD patients admitted for an exacerbation, azithromycin should be discontinued at prophylactic doses while antibiotic treatment is administered. |
| In an exacerbation of COPD requiring hospital admission, inhaled corticosteroids should be associated with short-acting bronchodilators as part of the treatment. |
| During a mild-moderate exacerbation of COPD in a patient on triple therapy, the bronchodilator regimen should be increased at the expense of short-acting anticholinergics. |
In the section addressing general aspects, a strong consensus (over 85%) was reached on the importance of implementing protocols to assist patients with exacerbations in both outpatient (92.7%) and inpatient settings (94.3%). Strong agreement was also observed regarding the significance of nutrition and rehabilitation services during hospital admission (84.6%) and the utility of multidimensional and individualized programs (85.4%). Additionally, there was agreement on the importance and usefulness of incorporating remote monitoring (72.5%). However, no consensus was reached on the significance of nutrition and rehabilitation services in an ambulatory setting.
Criteria for hospitalizationRegarding the criteria for hospitalization due to an exacerbation, the respondents agreed that these criteria are clearly defined (75.5%). However, no consensus was reached on whether these criteria are supported by scientific evidence.
Bronchodilator useIn the context of bronchodilator use for CES, a strong consensus (over 85%) was reached in favor of almost all statements. The only statement that did not achieve agreement concerned whether there is a clinical difference between the use of nebulized rescue bronchodilator drugs and pressurized metered-dose inhalers (pMDI) with a spacer.
Outpatient treatmentRegarding the treatment of CES in the outpatient setting, consensus was reached for nearly all statements. The indication of antibiotics, the duration of treatment and the maintenance of home treatment were some of the aspects on which a high degree of consensus was reached. The only statement that did not reach consensus pertained to the use of systemic corticosteroids for moderate CES. Regarding considerations related to the prevention of cardiovascular events, there was a high level of agreement on aspects such as the use of low molecular weight heparins (LMWH) at prophylactic doses when the patient remains bedridden or inactive for three or more days, as well as on the recommendation of physical exercise during the episode.
Inpatient treatmentConversely, consensus on the treatment of CES in an inpatient setting was less consistent. Out of 24 statements, 7 did not achieve consensus. The respondents did not agree on the utility of thoracic echography for CES; however, they strongly agreed on the importance of considering the patient's baseline status for hospitalization (96.8%) and the necessity of measuring proBNP (brain natriuretic peptides) and troponins at the start of hospitalization (78.9%). Additionally, there was agreement on the relevance of specific biomarkers for decision-making, particularly sputum color changes (94.3%) and C-reactive protein (CRP) (76.4%).
Furthermore, respondents concurred that in patients hospitalized due to an exacerbation, oral intake should be avoided for the first 24–48h to reduce the risk of bronchoaspiration (76.5%). However, they disagreed with the statement suggesting that the use of short-acting bronchodilators should be accompanied by the discontinuation of long-acting muscarinic antagonist (LAMA) or long-acting beta-agonist (LABA), with or without inhaled corticosteroids (ICS) (74.8%).
CES considerations and treatment decisionsIn general, there is limited consensus on the topic CES considerations and treatment decision. However, two exceptions were noted: respondents disagreed on the progressive withdrawal of systemic corticosteroids (73.5%) and agreed on the importance of restarting maintenance therapy with long-acting bronchodilators as soon as possible (87.0%).
There is also a lack of consensus regarding whether the bronchodilator regimen should be intensified by increasing the use of short-acting anticholinergics in patients receiving triple therapy during mild-moderate CES. However, respondents agreed that, given the high morbidity and mortality of a COPD exacerbation requiring hospital admission, triple inhaled therapy should be prescribed (regardless of previous treatment) in the absence of contraindications.
Inhalation devicesRegarding the use of inhalation devices, respondents agreed that both the appropriateness of the inhaled device (79.7%) and the patient's inhalation technique (99.2%) should be assessed before discharge. Additionally, they strongly agreed that a smoking cessation intervention should be conducted during hospitalization (97.6%).
Post-discharge protocolsConcerning post-discharge protocols, respondents reached a consensus on all three statements: patients should be evaluated 72h after discharge (83.7%), assessed in primary care consultations within one week (87.0%), and seen in specialized care consultations within three months (93.5%).
Rehabilitation and patient managementFinally, regarding rehabilitation and patient management following hospitalization for CES, respondents reached a consensus on all statements. They agreed that patients who had been hospitalized should be enrolled in a respiratory rehabilitation program (74.8%), that the care and management of patients with exacerbations should be well-coordinated (95.1%), that CES patients should undergo follow-up with a pulmonologist (81.3%), and that home-centered care for patients with exacerbations is essential (92.7%). However, they disagreed with the statement that early respiratory rehabilitation is widely established in clinical practice.
DiscussionIn this Delphi document, a high degree of consensus was reached on various aspects of CES management, although significant gaps remain, especially in treatment regimens for exacerbations in a hospital setting. These results underscore the complexity of managing these patients and highlight areas where standardization in treatment protocols and further research are necessary.
One of the key findings of this study was a strong consensus across various sections of the questionnaire, in line with current guidelines. Regarding the use of bronchodilators for CES, the consensus achieved in most statements supports current recommendations, reinforcing their role in symptom relief management. Both GesEPOC and GOLD guidelines7,10 establish that, to address CES of any intensity, the first and primary intervention is the optimization of bronchodilation, either by increasing its dose or frequency. Similarly, and in accordance with the guidelines, a high degree of consensus has been reached on the use of systemic corticosteroids in CES cases requiring hospitalization. Systemic corticosteroids have demonstrated efficacy in expediting symptom recovery, enhancing pulmonary function, and reducing therapeutic failures. Several systematic reviews and meta-analyses have explored the comparative efficacy of systemic and inhaled corticosteroids in this context.13-16 However, their impact on mortality remains negligible.17 In the case of outpatient CES, the panelists agree that systemic corticosteroids should only be used if the patient has an eosinophil count greater than 2% in peripheral blood at the time of evaluation. GesEPOC guidelines recommend the use of oral corticosteroids in patients experiencing an acute exacerbation with eosinophil levels ≥300cells/μL. Although this agreement aligns with the recommendations provided in clinical practice guidelines, its practical implementation may significantly deviate from reality. This is particularly evident in outpatient settings, where laboratory analyses are often unavailable at the time of an exacerbation.
Surprisingly, diverging from the current recommendations outlined in the GOLD document, a high level of consensus was reached regarding the prescription of triple inhaled therapy (LAMA, LABA, and ICS) in COPD exacerbations requiring hospital admission, regardless of previous treatment, provided there are no contraindications. The goal of preventing COPD exacerbation is to significantly minimize all the negative impacts such as symptom burden, health status worsening, hospital admission and mortality. GOLD 2025 and Canadian COPD guidelines18 offer different approaches to initiating triple therapy. GOLD 2025 emphasizes a gradual, biomarker-driven process, starting with dual bronchodilation (LAMA plus LABA) and recommending triple therapy (LAMA, LABA and ICS) only if exacerbations persist, particularly when eosinophil levels are ≥300cells/μL. This approach seeks to balance efficacy with caution, minimizing unnecessary ICS use. In contrast, the Canadian guidelines advocate for initiating triple therapy in high-risk patients regardless of prior treatment or eosinophil count. Their strategy prioritizes rapid intervention to reduce exacerbation risks, placing less emphasis on biomarker thresholds and more on addressing the patient's immediate clinical needs. Based on the panelists’ responses, it seems that the Canadian guidelines are more aligned with routine clinical practice for this group of patients. Along the same lines, in a recently published Delphi consensus, carried out to assess the level of consensus among Greek experts on the use of triple therapy in COPD as an initial and follow-up treatment, the panelists agreed that COPD patients with a history of one severe exacerbation in the previous year should be initially treated with triple therapy in the case that they have a blood eosinophil count over 100cells/μL, a number which is lower compared to that suggested by the GOLD recommendations.19 This approach is shared in other COPD management documents endorsed by different scientific societies.20
This study also revealed a divergence in expert opinion regarding the criteria for hospitalization due to an exacerbation, with 75.5% of respondents agreeing that these criteria are clearly defined, but no consensus on whether they are adequately backed by scientific evidence. This can be justified by the fact that the current criteria are based on expert opinion. There are few studies that have compared the different proposals, finding divergent results.21,22 This reflects the ongoing uncertainty surrounding the optimal threshold for hospitalization, emphasizing the need for more robust, evidence-based guidelines to support clinical decision-making in this regard.
The management and treatment of CES in inpatient settings showed less consensus overall, with respondents disagreeing on the utility of thoracic echography and on whether diagnostic spirometry should be routinely performed during admission. A thoracic echography is used routinely in clinical practice. It is a safe and effective technique in both diagnosis and prognosis, and it is preferred over radiation-based techniques. Thoracic echography is also viewed as a key technique in emergency settings, helping in decision-making during exacerbations, respiratory failure or evaluation of response to therapy.17 In routine clinical practice, thoracic echography is likely to assume greater importance within emergency settings, where achieving an accurate diagnosis is paramount. Its application facilitates the exclusion of concomitant conditions such as heart failure, pneumonia consolidation, or pleural effusion, among others.23,24 This would plausibly justify the responses provided by the panelists who would not consider this test relevant once the exacerbation has been diagnosed. Conversely, there was a strong agreement on the use of biomarkers such as sputum color and CRP levels for decision-making. This supports their continued role in assessing exacerbation severity and guide treatment. As mentioned in the Spanish GesEPOC guidelines, the administration of antibiotics is indicated in COPD patients with a change in sputum color (from mucoid to purulent) and/or elevated CRP levels (≥20mg/dL), even if the appearance of the sputum is inconclusive. During a COPD exacerbation, NT-proBNP and troponin play essential roles in detecting associated cardiovascular complications. These complications are frequently observed in such patients and can substantially impact the course of treatment and clinical decision-making.25–27 These findings highlight the complexity of inpatient management and suggest that while some diagnostic tests and interventions may be beneficial, further research is needed to establish their routine use and clinical utility.
Moreover, lack of consensus in other areas of our study, such as whether nebulized rescue bronchodilator drugs differ clinically from metered-dose inhalers (pMDI) with a spacer, maintaining home-based inhaled therapy during a severe exacerbation of COPD or the application of rehabilitation and nutritional support in the outpatient setting, warrants further investigation. Several methods of administration are available in the acute setting, including wet nebulizers and pMDI with spacer device. Both methods have demonstrated equal efficacy in relieving acute airflow obstruction,28 with no significant difference in length of hospital stay.29 However, pMDI with spacers may offer theoretical advantages, including faster administration, improved cost-effectiveness and increased opportunity for inhaler technique education.30 Additionally, nebulizers are known to generate aerosols, which could contribute to infection transmission. Given that the familiarity of staff and patients with each delivery method may vary (patient factors including reduced level of consciousness or severe dyspnea) the choice of device must be carefully considered. In hospital settings, inhaled bronchodilators are often administered in nebulized formulations, which can be easier to use for older or more compromised patients or those with poor inspiratory effort.31 Despite these issues, respondents to our Delphi consensus indicated that there are no clinically significant differences between device types; the lack of consensus observed may be due to a lack of awareness of such distinctions. Indeed, current guidelines suggest that, when proper delivery technique is ensured, there are no significant differences in forced expiratory volume (FEV1) between devices.7 However, many healthcare professionals do not instruct their patients in proper inhalation technique, resulting in a high rate of errors in pMDI use.32 Therefore, educating patients and caregivers on the correct use of inhaler devices is essential to optimize their efficacy.
Regarding whether to maintain home-based respiratory therapy during a severe COPD exacerbation, no consensus was reached. Such medications may include LABA, LAMA, ICS, theophylline, or oral phosphodiesterase-4 inhibitors. While in outpatient CES we clearly see the need to keep chronic inhaled treatment, this is less evidence in the inpatient setting. Several studies that have evaluated the usefulness of long-acting bronchodilators for the treatment of patients during a COPD exacerbation have shown limited efficacy and increased costs.33 Thus, hospitalists should consider whether these treatments are appropriate in the acute setting. Treatment guidelines do not specify that this therapy should be maintained in severe exacerbation and further studies are needed to clarify this aspect.34
With respect to the application of rehabilitation and nutritional support in the outpatient setting, only 48.0% of the respondents agreed with its necessity. This signals a need for further exploration of these interventions in non-hospitalized patients. In the subsequent discussion following the questionnaire, the panel of experts noted that there is insufficient scientific evidence to support these measures; consequently, they are not implemented in clinical practice. However, it is worth noting that a recent study35 aimed to assess the effectiveness of nutritional support in malnourished COPD patients. It was found out that individualized dietary counselling improved quality of life as well as nutritional intake and body weight. This improvement, when combined with rehabilitation, has been linked to an increase in inspiratory and expiratory muscle strength, which have also a positive impact in expectoration, reducing mucus accumulation and risk of recurring exacerbations.35,36 The benefit from nutritional support and rehabilitation for outpatient COPD patients has been suggested to improve treatment results and lower the risk of exacerbations.35,36 This highlights the importance of further studies to understand the relevance of rehabilitation and nutritional support outside of the nosocomial setting. It is also crucial to include these findings in COPD guidelines.
Finally, despite the lack of consensus on certain aspects, the study showed broad agreement on the importance of post-discharge protocols, including follow-up consultations after discharge in primary care and in specialized care, which is consistent with other Delphi documents that have analyzed similar aspects.37 This emphasizes the critical role of continuity of care in reducing readmissions and improving long-term outcomes for CES patients. Moreover, the agreement on including patients in respiratory rehabilitation programs post-discharge, as well as ensuring coordinated care, aligns with current recommendations aimed at enhancing recovery and reducing the burden of exacerbation. The agreement on the utility of remote monitoring and multidimensional rehabilitation programs is particularly noteworthy, as the respondents recognize these elements as valuable tools in chronic disease management. In fact, a recent systematic review on this topic in COPD patients showed that the implementation of telemonitoring, tele-education and telerehabilitation had positive results, including better quality of life, improved exercise tolerance, and a lower frequency of exacerbations and dyspnea. This new technology seems to be widely accepted among patients, without detected adverse effects.38
ConclusionsOverall, this Delphi consensus study provides valuable information on the current management of CES, highlighting several areas where consensus remains elusive. While the clinical management of stable COPD is well defined, the treatment of CES presents significant variability. In most of the cases discussed here, the lack of consensus reflects the insufficient scientific evidence available. Therefore, further studies on best clinical practices for COPD patients with CES are needed to standardize treatment protocols. This is essential to improve patient management and improve patient outcomes and quality of life
Declaration of generative AI and AI-assisted technologies in the writing processNothing in the study was developed with the help of any artificial intelligence software or tool.
FundingThis research was supported by SEPAR (Sociedad Española de Neumología y Cirugía Torácica) COPD working group.
Authors’ contributionsAll authors were involved in the conception and design of the work, acquisition, analysis, interpretation of data, drafting the work, revising it critically for important intellectual content.
Conflicts of interestJMD is part of the Editorial board of Open Respiratory Archives and declare that he has remained outside the evaluation and decision-making process in relation to this article. On the other hand, he has received grants and honoraria from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Esteve, FAES, Ferrer, Gebro, GSK, Grifols, Janssen, Menarini, Novartis, Sanofi, Roche, Teva, Pfizer, and Zambón.
JMFG has received honoraria for speaking engagements and funding for conference at-tendance from Laboratories Esteve, MundiPharma, AstraZeneca, Boehringer Ingel-heim, Ferrer, Menarini, Rovi, GSK, Chiesi, Novartis, and Gebro Pharma.
CRG has received honoraria for speaking engagements and funding for conference at-tendance from Laboratories AstraZeneca, Menarini, GSK, Bial, Chiesi, CSL Behring and Grifols.
MB is part of the Editorial board of Open Respiratory Archives and declare that he has remained outside the evaluation and decision-making process in relation to this article. On the other hand, she has received speaker fees from Grifols, Menarini, CSL Behring, GSK, Chiesi, and AstraZeneca, and consulting fees from GSK, Bial, CSL Behring, Chiesi and Grifols.
CAAD has received speaker or consulting fees from Boehringer Ingelheim, Pfizer, AstraZeneca, Novartis, Chiesi, Faes Farma, Esteve, GSK and Bial.
BAN reports grants and personal fees from GSK, grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi, grants, personal fees and non-financial support from Laboratorios Menarini, personal fees from Bial, personal fees from Zambon, personal fees from MSD, and personal fees from Sanofi.
The authors would like to thank IMC for their help in the preparation of the manuscript.