Typhoid fever, commonly known as enteric fever, is a significant, resilient community-acquired systemic bacterial infection caused by Salmonella typhi.1 It is most prevalent in tropical countries like India, due to limited resources, overcrowding, poor sanitation, and major hygienic unawareness, resulting in remarkable morbidity and continues to be a public health challenge even in this era of antimicrobials.1 Neurological manifestation of typhoid fever is relatively rare,2 especially nowadays, when almost no case gets complicated thanks to the early institution of effective antibiotics therapy. Landry-Guillain-Barre syndrome and its variant, Miller Fisher syndrome, have been rarely reported.2–4
The authors herein report a case of typhoid fever with concurrent central and peripheral nervous system involvement manifesting as splenial lesions on neuroimaging (i.e., the boomerang sign) and Miller Fisher syndrome, respectively.
A 34-year-old previously healthy female from lower socio-economic strata of rural West Bengal (India) was brought to the emergency department with high-grade fever for the last seven days, unsteady gait, and recurrent falls in the last three days. It was associated with a history of recent onset progressive paresthesia involving bilateral lower extremities, ascending gradually from toes in a stocking pattern for the last five days. Initially, along with low-grade fever, she also had developed a diarrheal episode for two days, followed by generalized abdominal discomfort and headache, for which she has been prescribed some medicines from local quacks without any relief of symptoms. Clinical examination revealed that she was anxious, febrile, and had mild dysarthria, axial and appendicular ataxia, external ophthalmoplegia, gaze-evoked horizontal nystagmus, generalized hypo-to-areflexia, and reduced sensitivity sensation of all modalities below knee level. She also had relative bradycardia and mild soft splenomegaly. Signs of meningeal irritation were absent. Visual acuity, color vision/perception, pupillary reflexes, intraocular pressures, and fundoscopic examinations were non-contributory. Cognitive, motor strength, and autonomic functions revealed no abnormality. She tested negative for SARS-CoV-2 and was admitted with a provisional diagnosis of infection-associated Miller Fisher syndrome.
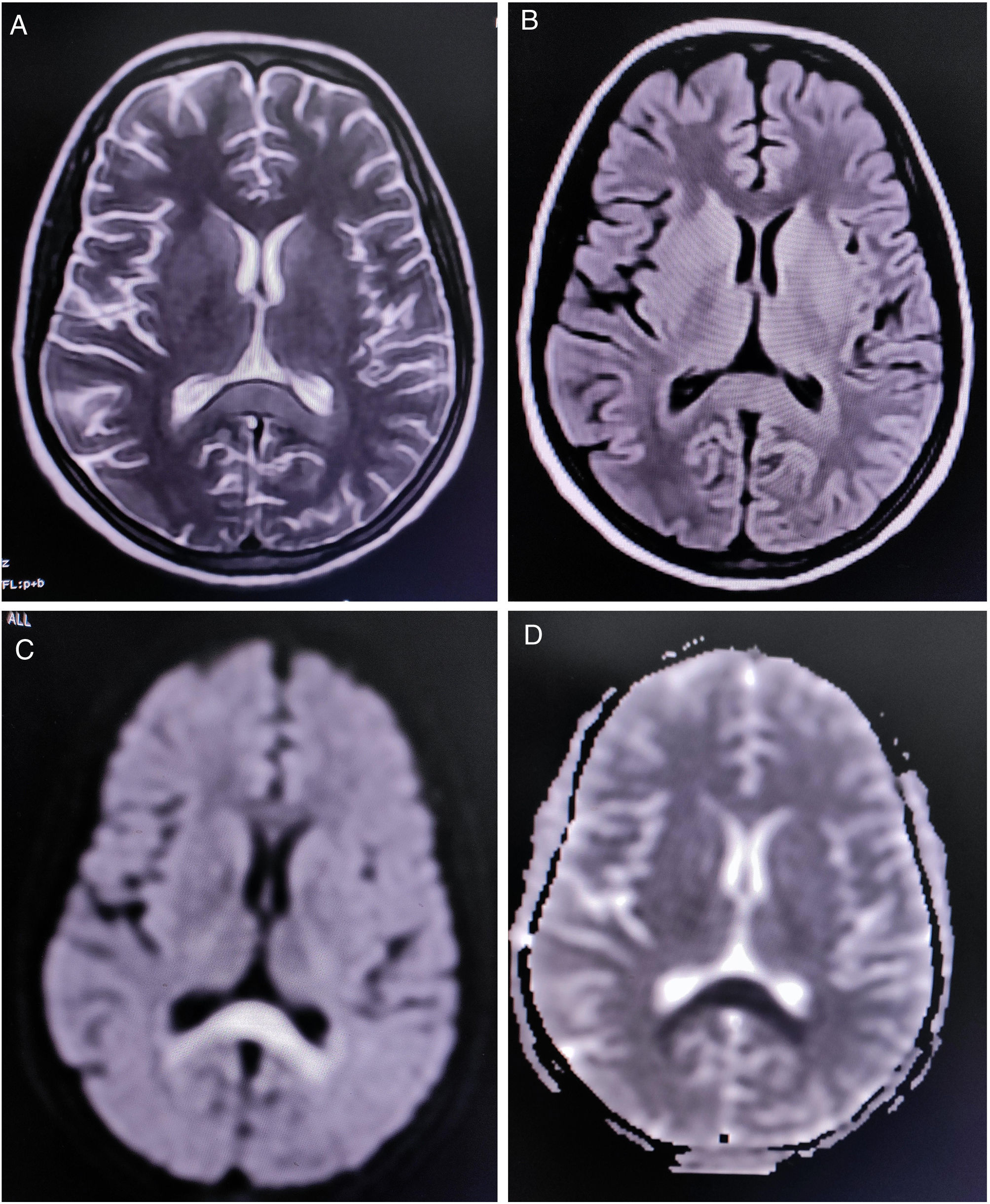
Complete blood cell count revealed mild anemia (hemoglobin 9.0g/dL), elevated erythrocyte sedimentation rate (80mm/h), relative lymphopenia, and thrombocytopenia (platelets count=60,000/μl (reference range 150,000–450,000). Liver function tests showed an approximately two-fold rise of the transaminases. Renal and thyroid function tests and blood glucose levels were within normal limits. Diagnostic tests were negative for endemic infections like malaria, dengue, Leptospira, Japanese encephalitis, and scrub typhus. However, Typhi Dot IgM (ELISA) was found to be positive. Magnetic resonance imaging (MRI) of the brain demonstrated a non-enhancing hypertense lesion on T2-weighted image and T2-FLAIR, with substantial diffusion restriction on diffusion-weighted imaging sequences, involving the splenium of the corpus callosum, suggestive of boomerang sign (Fig. 1). MR angiography of the brain was normal. Cerebrospinal fluid (CSF) studies revealed lymphocytic pleocytosis (10cells/μl, all lymphocytes), slightly low glucose levels (48mg/dL, normal range 50–80mg/dL), and normal protein levels (46mg/dL, normal range 15–50mg/dL) without any albuminocytologic dissociation. Oligoclonal bands, anti-aquaporin 4, and anti-myelin oligodendrocyte glycoprotein antibodies were not detected in the CSF and serum. However, serum GQ-1b-IgG antibodies were positive. CSF polymerase chain reaction for relevant neuroviruses and tuberculosis and tests for neurosarcoidosis and neuroborreliosis were negative. Tests for Mycoplasma, Campylobacter jejuni, influenza, HIV, and hepatitis viruses were also non-reactive. Sensory and motor nerve conduction studies revealed bilateral sensory-motor polyneuropathy, predominantly axonal; needle electromyography and repetitive nerve stimulation tests were unremarkable. Blood cultures resulted positive for Salmonella typhi.
As per institutional protocol on admission, she was put on empirical intravenous antibiotics (ceftriaxone) and other symptomatic therapies. When the Typhi Dot IgM report came positive (day three of admission), we added oral azithromycin (600mg/day for one week) to ceftriaxone (4g/day for two weeks). Symptoms of the immediate infective illness waned rapidly within seven days of the institution of antibiotic therapy. However, symptoms due to Miller Fisher syndrome did not respond until we prescribed intravenous immunoglobulins (0.4g/kg/day for five days, on day eight of admission). The anti-GQ-1b-IgG report came positive at the same time. Ophthalmoparesis and ataxia improved remarkably within five days of the institution of intravenous immunoglobulins therapy. After three weeks of in-hospital stay, the patient could be discharged with a minimal residual neurological deficit (only generalized hyporeflexia).
Miller Fisher syndrome is characterized by the acute onset of external ophthalmoplegia, ataxia, and loss of tendon reflexes.5–7 We have described one patient with typhoid fever who had a Miller Fisher syndrome and a unique neuro-radiological finding known as the boomerang sign.8
Neuroimaging in this patient showed an uncommon radiological finding, i.e. cytotoxic lesion of the corpus callosum (CLOCCs), popularly known as the “boomerang sign”.8 CLOCCs, especially involving the splenium, are seen in association with cerebrovascular events, demyelinating disorders, metabolic disorders, seizures, medications, infections, malignancies, trauma, high-altitude cerebral edema, preeclampsia toxicity, posterior reversible encephalopathy syndrome, autoimmune encephalitis, post-partum cerebral angiopathy, and vaccination, among others.9,10
Like other variants of Landry-Guillain-Barré syndrome, Miller Fisher syndrome has been shown to develop following an infectious illness, suggesting an ongoing para- or postinfectious immune-mediated process.5–7 The occurrence of Miller Fisher syndrome in this patient with the Salmonella typhi infection could be simply coincidental. However, considering the temporal relationship, we feel that Salmonella typhi might have been responsible for developing both splenial lesions and Miller Fisher syndrome. Further supporting this hypothesis was the recent publication of a single case report suggesting a possible association between Miller Fisher syndrome and boomerang sign with Salmonella typhi infection.4
The pathogenesis of Miller Fisher syndrome in Salmonella typhi infection may include immune mechanisms or direct viral neuropathogenic effects. We, however, feel that the primary mechanism was an aberrant immune response. First, serum GQ-1b-IgG antibodies were positive in the patient, supporting the hypothesis of immune-mediated injury rather than direct bacterial neurotropism. Finally, there was a significant recovery of the neurological deficit using intravenous immunoglobulin therapy.
We have described one patient with typhoid fever with Miller Fisher syndrome and a splenial lesion who had good outcomes. We suggest considering the presence of Salmonella typhi infection in those patients with Miller Fisher syndrome.
Authors’ contributionsAll authors contributed significantly to the creation of this manuscript; each fulfilled criteria as established by the ICMJE.
Ethics statementInformed written consent was obtained from the patient involved in this study.