Myasthenia gravis (MG) is a neuromuscular autoimmune disease that requires prompt diagnosis and management by neurologists. The challenges of MG management are associated with the diagnostic process and the selection of an effective form of therapy enabling long-term remission of the disease. The objective of this study was to assess the state of MG in Spain to identify unaddressed needs in the clinical progression, diagnosis, and treatment approaches.
MethodsA survey was distributed via email between 19 July and 25 October 2022 to neurologists belonging to the Spanish Society of Neurology. A comprehensive descriptive analysis was conducted on all collected data.
ResultsIn total, 69 completed surveys, representing 64 Spanish hospitals, were analyzed and included in the results. The study identified that economic difficulties, such as departmental budgets, have led to a lack of access to antibody testing during diagnosis. Furthermore, corticosteroids were found to achieve the best clinical response, despite also being associated with a higher frequency of adverse effects. The results show that 34% of patients with MG present active symptoms. Eighteen percent have symptoms that limit their daily activities. Additionally, 29% of patients have requested an unscheduled follow-up visit within the last year.
ConclusionsSeveral areas present unmet needs in terms of improving access to diagnostic assays, residual disease burden in treated patients, and the management and diagnosis of MG.
La miastenia gravis (MG) es una enfermedad neuromuscular autoinmune que requiere un diagnóstico y tratamiento rápido por parte de neurología. Los desafíos en su manejo están asociados al proceso diagnóstico y a la selección de una terapia eficiente para la remisión a largo plazo. El objetivo fue evaluar el estado de la MG en España, para identificar las necesidades no cubiertas en el ámbito de la progresión clínica, diagnóstico y enfoques terapéuticos.
MétodosSe distribuyó una encuesta entre el 19 de julio y el 25 de octubre de 2022 a los neurólogos miembros de la Sociedad Española de Neurología (SEN). Se realizó un análisis descriptivo exhaustivo de todos los datos recogidos.
ResultadosSe incluyeron los resultados de 69 encuestas en 64 hospitales. Este estudio identificó que existen razones económicas, como la capacidad financiera de los departamentos, que han provocado una falta de acceso a las pruebas de anticuerpos durante el diagnóstico. Además, los corticoides se identificaron como la terapia con la mejor respuesta clínica, a pesar de su asociación con una mayor frecuencia de efectos adversos. Se demostró que el 34% de los pacientes con MG experimentan síntomas activos. De ellos, un 18% presentan actividades diarias limitadas. Finalmente, un 29% de los pacientes han solicitado una visita de seguimiento no programada en el último año.
ConclusionesLa encuesta identificó áreas con necesidades no cubiertas en términos de mejorar el acceso diagnóstico a las pruebas, la carga residual de la enfermedad en los pacientes tratados, el manejo y el diagnóstico.
Myasthenia gravis (MG) is a rare, multifactorial, autoimmune disease with a prevalence of 15–20 cases/100000 population, and is characterized by complex pathophysiology, symptoms, diagnosis, and treatment.1,2 MG is caused by the failure of neuromuscular transmission resulting from the binding of IgG autoantibodies to signaling proteins, mostly the nicotinic acetylcholine receptor (AChR) at the neuromuscular junction.3 This induces muscle weakness that can differ between individual muscles and muscle groups. Extraocular muscles and muscles innervated by the cranial nerves are frequently affected, leading to intermittent ptosis, diplopia, reduced facial expression, speech, and swallowing weakness.4 In the most severe cases, the respiratory muscles are also affected, causing breathing problems.5 Approximately 10% of patients with MG have substantial symptoms and recurrent exacerbations that may necessitate hospitalization.6,7
MG requires prompt identification and treatment due to the high potential for improvement, and even remission in some cases. However, its diagnosis can be challenging and delayed because of the nature of muscle weakness and the overlap of signs and symptoms with other neuromuscular diseases.8 Diagnosis is based on the patient's signs and symptoms, electrophysiological study results suggesting impaired neuromuscular transmission, and positive tests for pathogenic IgG autoantibodies. These include antibodies against AChR, muscle tyrosine kinase (MuSK), and lipoprotein-related protein 4 (LRP4). However, 10%–15% of patients with MG are double-seronegative for AChR and MuSK antibodies.9
The standard treatment is based on patient-specific characteristics and typically involves the administration of acetylcholinesterase (AChE) inhibitors, immunosuppressive drugs, and thymectomy. MG crises are usually managed in an intensive care setting with ventilatory support and treatment with corticosteroids and intravenous immunoglobulins (IVIG) or plasma exchange.8 A range of immunomodulatory therapies have conventionally been used to achieve clinical remission (previously published estimates of clinical remission rates ranged from 6.5% to 45.9%). Although currently available therapies control clinical symptoms reasonably well in most patients, health-related quality of life remains lower than expected due to the chronic, disabling nature of the disease, treatment-related adverse events, tolerability problems, and even treatment resistance, all of which contribute to the significant burden of the disease.5,10–13 Additionally, different studies have shown that 8.5%–15% of patients with MG are unresponsive to conventional treatments.14–16 Furthermore, new therapies with new mechanisms are emerging in the market, such as agents targeting B cells and plasmablasts, complement inhibitors, and neonatal fragment crystallizable receptor (FcRn) antagonists17; it is important to understand which patients could benefit from these to improve disease management and patient outcomes.2 Due to the complex nature of MG, diagnosis and treatment are often limited to specialized neurologists.18
Management of the disease can present challenges including its diagnosis, treatment (resistance, adverse effects), coexisting immune conditions, lack of specialized medical care, and the lack of identification of unmet needs. Therefore, the objective of this study was to assess the state of MG management and unmet needs in Spain.
MethodologyThis paper reports data from a survey completed by neurologists from Spanish hospitals. Scientific endorsement was obtained from the Spanish Society of Neurology (SEN, for its Spanish initials).
Participant selection and recruitmentBetween 19 July and 25 October 2022, all neurologists belonging to the SEN (n=2000) were invited to participate. Participants were sent a link to the online survey, with information about the aim and the importance of the study. Participants received no incentives or funding.
Survey questionsThe online survey included 34 questions related to the number of patients observed, their symptoms, diagnostic approach, referrals, tools, and difficulty and disease management (the survey questions are provided in Supplementary Table 1). The questions were written and validated by the authors of this article.
Data analysisNo formal sample size calculation was performed. A descriptive analysis was conducted.
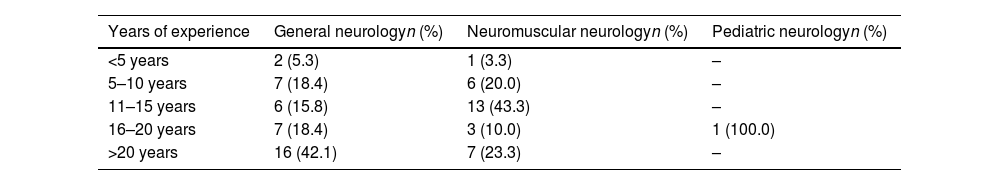
ResultsEighty-one responses were received; however, 12 surveys were incomplete and therefore excluded from the final analysis. The final data included 69 surveys completed by neurologists from 64 Spanish hospitals (54 public and 10 private). Most respondents were general neurologists (n=38, 55.1%) and neuromuscular neurologists (n=30, 43.5%). One survey was completed by a pediatric neurologist (1.4%). One-third of respondents had more than 20 years’ experience in clinical practice (n=23); 15.9%, 25.5%, 18.8%, and 3.3% had 16–20, 11–15, 5–10, and <5 years of experience, respectively. A greater number of years of experience was observed among general neurologists (Table 1).
Number of years of experience of the neurologists participating in the survey.
| Years of experience | General neurologyn (%) | Neuromuscular neurologyn (%) | Pediatric neurologyn (%) |
|---|---|---|---|
| <5 years | 2 (5.3) | 1 (3.3) | – |
| 5–10 years | 7 (18.4) | 6 (20.0) | – |
| 11–15 years | 6 (15.8) | 13 (43.3) | – |
| 16–20 years | 7 (18.4) | 3 (10.0) | 1 (100.0) |
| >20 years | 16 (42.1) | 7 (23.3) | – |
In the 64 hospitals included in this study, 890 patients with MG were estimated to be diagnosed each year (475 by neuromuscular neurology, 411 by general neurology, and 4 by pediatric neurology). Most patients were followed up by the neurology department (89.1%), by neuromuscular neurologists (61.34%), or by general neurologists (38.40%). On a global scale, most patients with MG (89.12%) are not referred to other medical departments, with only a minority (10.74%) being referred. However, when examining patient referrals by neurologist category, the data show a noteworthy contrast in referral rates across different areas of neurological expertise. Specifically, in pediatric neurology, a substantial proportion of patients (70.0%) were referred, whereas a considerably lower percentage of patients were referred by neuromuscular neurologists (3.27%) and general neurologists (15.8%).
The size of hospitals or centers that received patients was analyzed according to the number of beds at each institution. Most cases of MG were diagnosed at hospitals or centers with 501–1000 beds (57.6%), followed by those with more than 1000 beds (23.5%). The percentage of patients diagnosed in hospitals or centers with 200–500 beds or with fewer than 200 beds was 16.9% and 2.0%, respectively. The data indicated that a larger proportion of patients received follow-up care at hospitals with 501–1000 beds (46.1%), followed by those with more than 1000 beds (34.4%), 200–500 beds (18.6%), and fewer than 200 beds (0.9%). Furthermore, the analysis of patient referrals by hospital shows that a higher percentage of patients from hospitals with fewer than 200 beds are referred for further medical attention (44.3%), followed by hospitals with 200–500 beds (12.1%), hospitals with 501–1000 beds (4.1%), and finally, hospitals with more than 1000 beds (4.1%).
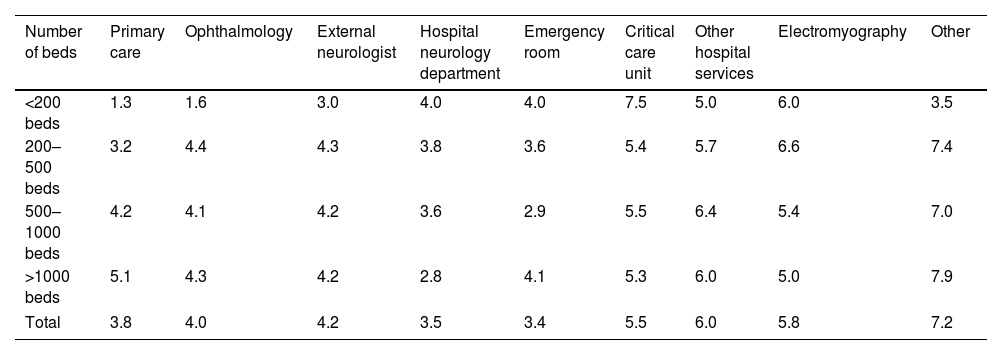
Patient referrals were described by order of importance (from 1, meaning the main source of referrals, to 9, residual) (Table 2). Overall, the most relevant units were the emergency room (scored as 3.4), the hospital neurology department (3.5), and primary care (3.8). In smaller hospitals (fewer than 200 beds) primary care stood as the most relevant unit (1.3), while for larger ones (more than 1000 beds) the neurology department was identified as the most important (2.8).
Patients referrals by order of importance (from 1, main source of referrals, to 9, residual).
| Number of beds | Primary care | Ophthalmology | External neurologist | Hospital neurology department | Emergency room | Critical care unit | Other hospital services | Electromyography | Other |
|---|---|---|---|---|---|---|---|---|---|
| <200 beds | 1.3 | 1.6 | 3.0 | 4.0 | 4.0 | 7.5 | 5.0 | 6.0 | 3.5 |
| 200–500 beds | 3.2 | 4.4 | 4.3 | 3.8 | 3.6 | 5.4 | 5.7 | 6.6 | 7.4 |
| 500–1000 beds | 4.2 | 4.1 | 4.2 | 3.6 | 2.9 | 5.5 | 6.4 | 5.4 | 7.0 |
| >1000 beds | 5.1 | 4.3 | 4.2 | 2.8 | 4.1 | 5.3 | 6.0 | 5.0 | 7.9 |
| Total | 3.8 | 4.0 | 4.2 | 3.5 | 3.4 | 5.5 | 6.0 | 5.8 | 7.2 |
According to our respondents’ experience, indicators of unstable disease in a patient with MG are (in order of importance): presence of persistent symptoms, need for rescue therapy, need for high-dose treatment, and occurrence of adverse effects. Moreover, 34.4% of patients presented active symptoms, with 18.2% presenting limiting symptoms with an impact on their daily lives. A total of 28.9% had requested at least one unscheduled visit per year. Scheduled visits were held every 3 months (13.0%) or every 6 months (18.8%), but in most cases there was no established frequency and/or it depended on the severity (68.1%).
Respondents reported that the most frequent comorbidities were other autoimmune diseases (73.9%), followed by diabetes (71.0%), hypertension (69.6%), anxiety/depression (55.1%), osteoporosis (40.6%), obesity (26.1%), infection (13.0%), cancer (10.1%), cataracts (8.7%), and liver or kidney alterations (7.2%).
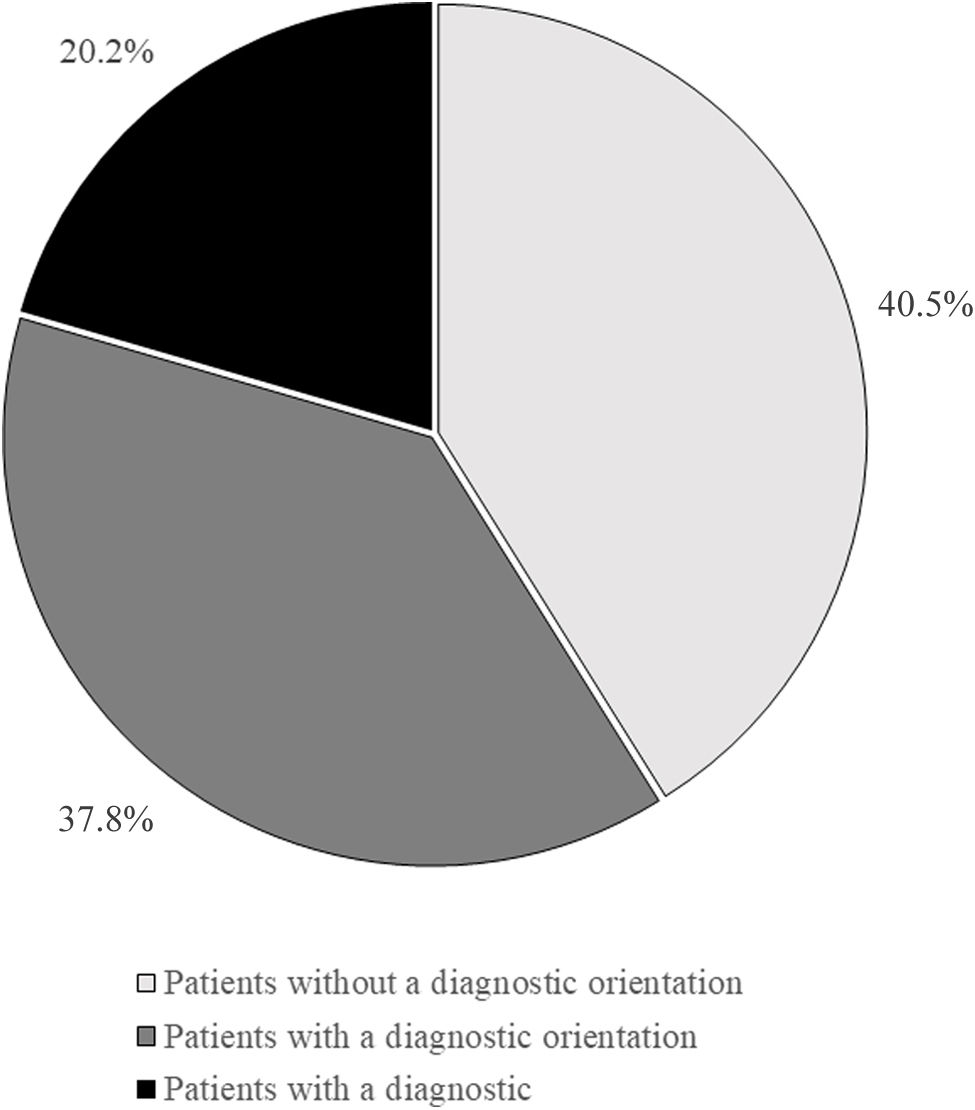
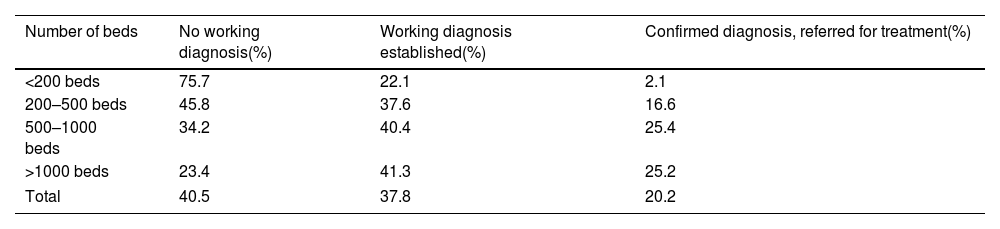
Diagnostic managementAround 40.5% of patients arrived at neurological departments without a working diagnosis, 37.8% were referred with a working diagnosis, and 20.2% arrived with a confirmed diagnosis for treatment (Fig. 1). These percentages vary according to the hospital (Table 3), with the highest number of patients without a working diagnosis observed at smaller hospitals (<200 beds or 200–500 beds). Similar patterns were noted in both general neurology and neuromuscular neurology, where 43.9% of patients were initially referred to the neurology department with a working diagnosis, 27.9% without a working diagnosis, and 24.9% had already received a diagnosis and were referred for treatment.
Working diagnoses of myasthenia gravis at first visit, by hospital size.
| Number of beds | No working diagnosis(%) | Working diagnosis established(%) | Confirmed diagnosis, referred for treatment(%) |
|---|---|---|---|
| <200 beds | 75.7 | 22.1 | 2.1 |
| 200–500 beds | 45.8 | 37.6 | 16.6 |
| 500–1000 beds | 34.2 | 40.4 | 25.4 |
| >1000 beds | 23.4 | 41.3 | 25.2 |
| Total | 40.5 | 37.8 | 20.2 |
Specialists rated the diagnostic difficulty of MG on a scale of 1–5 (where 1 is an easy and fast diagnosis and 5 is an extremely difficult diagnosis). On average, general neurologists rated the difficulty at 2.9 and neuromuscular neurologists rated it at 2.5.
Most neurologists requested antibody profiles (n=62, 89.8%), in a sequential approach (n=45, 72.6%). The protocol involves an initial analysis of anti-AChR antibodies, followed by anti-MuSK antibody testing in the event of negative results. If anti-MuSK antibody results are negative, further testing for anti-LRP4 antibodies is conducted. The remaining specialists who did not request these studies (n=7, 10.2%) attributed this to a lack of economic resources at their departments.
Treatment managementMore than one-third of patients (34.3%) underwent thymectomy in the past 2 years. Most were treated (72.7%) with immunosuppressants (30.5%), corticosteroids plus immunosuppressants (22.5%), or corticosteroids only (19.6%).
The treatment sequence followed by the specialists started with AChE inhibitors, followed by corticosteroids such as prednisone, and immunosuppressants such as azathioprine, tacrolimus, cyclosporine, cyclophosphamide, and rituximab.
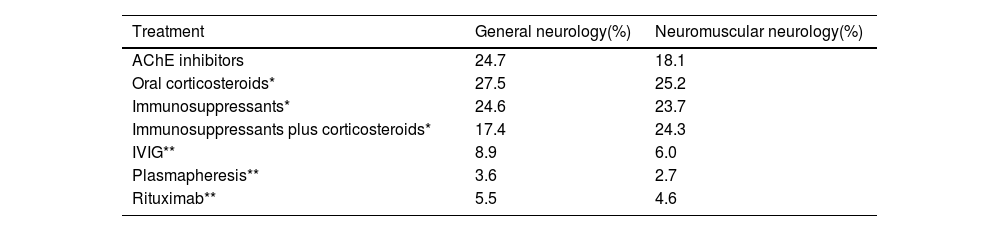
A higher percentage of patients received corticosteroids with or without AChE inhibitors: 27.5% and 25.2% in general neurology and neuromuscular neurology, respectively (Table 4). Among general neurologists, 24.7% of patients were receiving AChE inhibitors alone. This percentage decreased to 18.1% of patients for neuromuscular neurologists. A total of 24.6% and 23.7% of the patients from general neurology and neuromuscular neurology, respectively, were receiving immunosuppressants with or without AChE inhibitors. IVIG was administered in 8.9% and 6.0% of general neurology and neuromuscular neurology patients. Rituximab was used as an immunosuppressant in 5.5% and 4.6%, and plasmapheresis (plasma exchange) alone or combined with other systemic treatments was prescribed to 3.6% and 2.7% of general neurology and neuromuscular neurology patients, respectively.
Percentage of patients with myasthenia gravis under treatment.
| Treatment | General neurology(%) | Neuromuscular neurology(%) |
|---|---|---|
| AChE inhibitors | 24.7 | 18.1 |
| Oral corticosteroids* | 27.5 | 25.2 |
| Immunosuppressants* | 24.6 | 23.7 |
| Immunosuppressants plus corticosteroids* | 17.4 | 24.3 |
| IVIG** | 8.9 | 6.0 |
| Plasmapheresis** | 3.6 | 2.7 |
| Rituximab** | 5.5 | 4.6 |
AChE: acetylcholinesterase: IVIG: intravenous immunoglobulins.
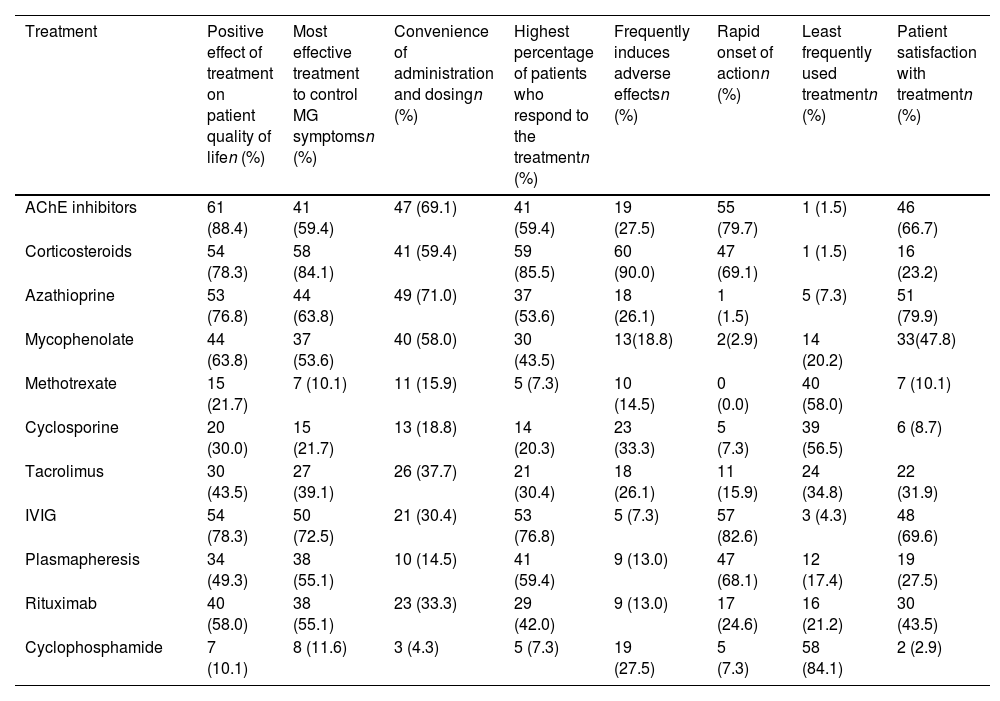
Neurologists were invited to provide an account of their experiences with a range of pharmacological treatments, specifically AChE inhibitors, corticosteroids, azathioprine, mycophenolate, methotrexate, cyclosporine, tacrolimus, IVIG, plasmapheresis, rituximab, and cyclophosphamide (Table 5).
Neurologists’ experience with myasthenia gravis treatments. Number of neurologists and percentage that agreed with each statement.
| Treatment | Positive effect of treatment on patient quality of lifen (%) | Most effective treatment to control MG symptomsn (%) | Convenience of administration and dosingn (%) | Highest percentage of patients who respond to the treatmentn (%) | Frequently induces adverse effectsn (%) | Rapid onset of actionn (%) | Least frequently used treatmentn (%) | Patient satisfaction with treatmentn (%) |
|---|---|---|---|---|---|---|---|---|
| AChE inhibitors | 61 (88.4) | 41 (59.4) | 47 (69.1) | 41 (59.4) | 19 (27.5) | 55 (79.7) | 1 (1.5) | 46 (66.7) |
| Corticosteroids | 54 (78.3) | 58 (84.1) | 41 (59.4) | 59 (85.5) | 60 (90.0) | 47 (69.1) | 1 (1.5) | 16 (23.2) |
| Azathioprine | 53 (76.8) | 44 (63.8) | 49 (71.0) | 37 (53.6) | 18 (26.1) | 1 (1.5) | 5 (7.3) | 51 (79.9) |
| Mycophenolate | 44 (63.8) | 37 (53.6) | 40 (58.0) | 30 (43.5) | 13(18.8) | 2(2.9) | 14 (20.2) | 33(47.8) |
| Methotrexate | 15 (21.7) | 7 (10.1) | 11 (15.9) | 5 (7.3) | 10 (14.5) | 0 (0.0) | 40 (58.0) | 7 (10.1) |
| Cyclosporine | 20 (30.0) | 15 (21.7) | 13 (18.8) | 14 (20.3) | 23 (33.3) | 5 (7.3) | 39 (56.5) | 6 (8.7) |
| Tacrolimus | 30 (43.5) | 27 (39.1) | 26 (37.7) | 21 (30.4) | 18 (26.1) | 11 (15.9) | 24 (34.8) | 22 (31.9) |
| IVIG | 54 (78.3) | 50 (72.5) | 21 (30.4) | 53 (76.8) | 5 (7.3) | 57 (82.6) | 3 (4.3) | 48 (69.6) |
| Plasmapheresis | 34 (49.3) | 38 (55.1) | 10 (14.5) | 41 (59.4) | 9 (13.0) | 47 (68.1) | 12 (17.4) | 19 (27.5) |
| Rituximab | 40 (58.0) | 38 (55.1) | 23 (33.3) | 29 (42.0) | 9 (13.0) | 17 (24.6) | 16 (21.2) | 30 (43.5) |
| Cyclophosphamide | 7 (10.1) | 8 (11.6) | 3 (4.3) | 5 (7.3) | 19 (27.5) | 5 (7.3) | 58 (84.1) | 2 (2.9) |
AChE: acetylcholinesterase; IVIG: intravenous immunoglobulins; MG: myasthenia gravis.
In relation to the positive impact of treatment on patients’ quality of life following treatment, AChE inhibitors received the highest score (61 responses, 88.4% of participants agreed). Corticosteroids were found to be the most effective treatment for managing symptoms of MG according to the experience of neurologists (58 responses, 84.1%). Azathioprine was considered the most convenient treatment option for administration and dosage, obtaining the highest score with 49 responses (71.0%). Based on the respondents’ experience, the highest percentage of patients who responded well to treatment were those managed with corticosteroids (59 responses, 85.5%). In terms of the frequency of adverse effects resulting from treatment, corticosteroids were found to induce the highest percentage of adverse effects, with 60 responses (90.0%). IVIG was considered the treatment with the fastest onset of action, receiving 57 responses (82.6%). The least commonly used treatment option included in this study was cyclophosphamide, with 58 responses (84.1%). According to neurologists’ experience, azathioprine exhibited the highest level of patient satisfaction, as evidenced by 51 responses (79.9%).
Management of double-seronegative patients (AChR and MuSK antibodies)Most neurologists (70.3% in general neurology and 80.0% in neuromuscular neurology) managed double-seronegative MG patients the same way as seropositive patients. This percentage increased with the years of experience of the respondents: 66.7%, 69.2%, 81.8%, and 81.8% for physicians with <5 years, 5–10 years, 11–15 years, and 16–20 years of experience, respectively.
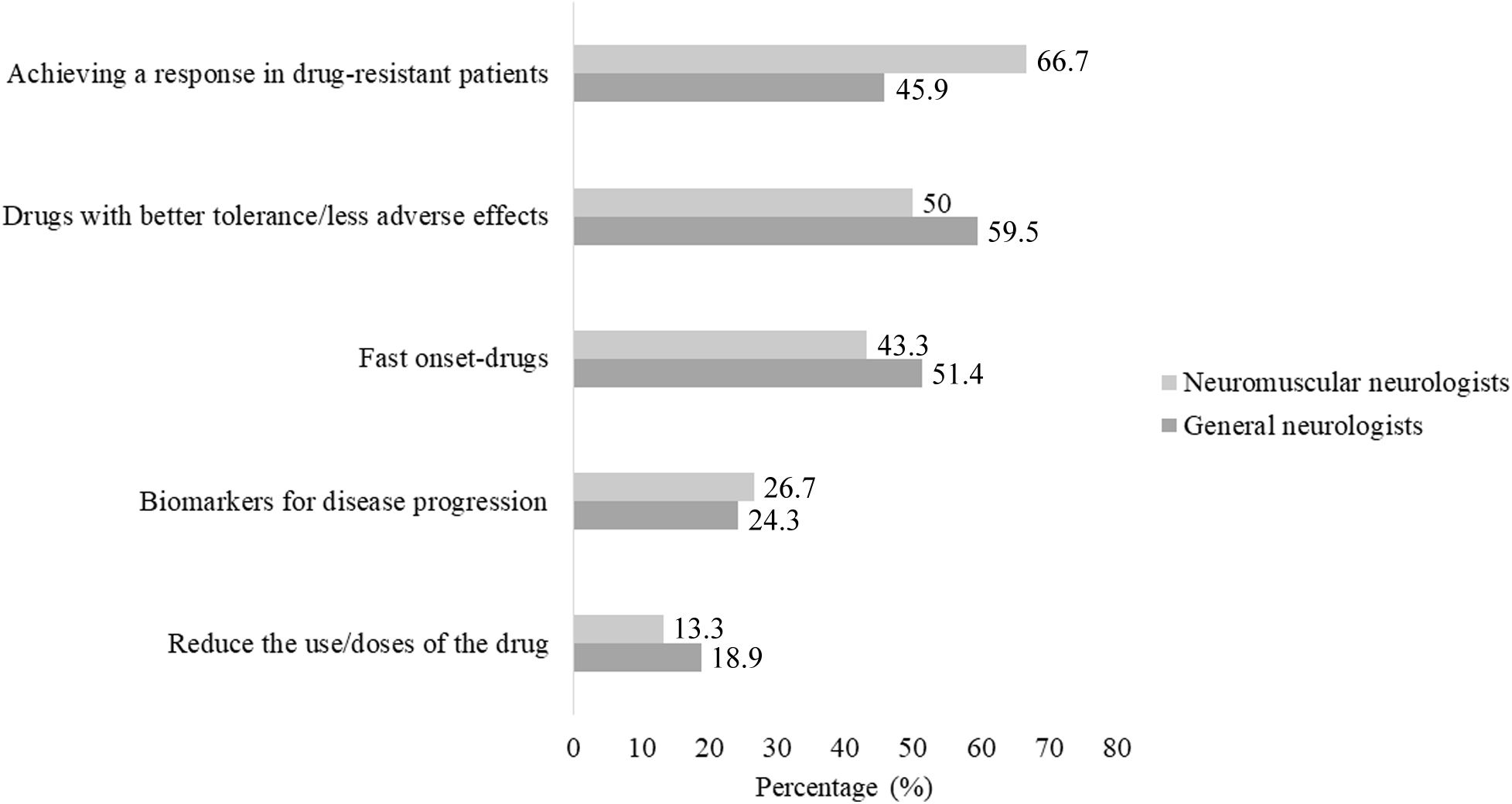
Unmet medical needsNeurologists were asked to select the two most relevant unmet medical needs in MG management, from the following options: (1) drugs with better tolerance/fewer adverse effects; (2) reducing the use/doses of the drug; (3) fast-onset drugs; (4) biomarkers for disease progression; and (5) achieving a response in refractory patients (Fig. 2). Overall, achieving a response in patients with drug-resistant disease and the need for drugs with better tolerance or fewer adverse effects appear to be the most relevant unmet medical needs, identified by 55.1% and 53.6% of respondents, respectively. Among general neurologists, 59.5% considered there to be a need for new drugs with better tolerance and fewer adverse effects, and 45.9% considered it necessary to offer a solution to patients with drug-resistant disease. Additionally, 18.9% of general neurologists considered it important to reduce the use/doses of treatments. Neuromuscular neurologists, on the other hand, prioritized the need to achieve a response in patients with drug-resistant disease (66.7%) and to have drugs with better tolerance or fewer adverse effects (50.0%).
Unmet needs identified by each participant group. The graph demonstrates that 66.7% of the neuromuscular neurologists prioritized the need to achieve a response in patients with drug-resistant disease. On the other hand, the majority of general neurologists identified the development of new drugs with better tolerance or fewer adverse effects as an unmet need.
MG management is a complex and challenging process that demands a multidisciplinary approach to cater to the unique needs of each patient. A comprehensive understanding of the current status is crucial in identifying areas for improvement and ensuring optimal patient care. This survey represents a preliminary study to better understand the status of MG management in Spain and identify the most relevant unmet medical needs.
The first description of MG in Spain dates from 1892,19 and the most recent epidemiological studies in the Iberian Peninsula reported a substantial increase in its prevalence in the elderly population.20 Early diagnosis by general practitioners and better access for elderly patients to specialized care are essential.21 However, patient referral from primary care was relatively low in our study, with only 1.3% of patients at small hospitals being referred from primary care.
Treatment-related adverse effects and tolerability problems contribute to the disease burden, especially in refractory phenotypes. Neurologists reported that 30.4% of patients presented active symptoms, and 18.2% presented self-limiting symptoms. A large cross-sectional study including 1660 patients with MG in Germany showed that the disease burden includes economic and social aspects, as well as patients’ emotional well-being. Moreover, 20% of patients with MG included in that study perceived no improvement in myasthenic symptoms after therapy. These results may be explained by lack of response to treatment or lack of access to experienced neurologists.12
Diagnosing MG in seronegative patients remains a challenge, particularly among those with double seronegativity for anti-AChR and anti-MuSK antibodies. Additionally, certain patient populations, such as those with anti-MuSK MG, thymomatous MG, refractory MG, and pregnant women, need particular attention.22 Both general and neuromuscular neurologists considered MG diagnosis to be somewhat challenging (2.9 and 2.5/5 respectively). A lack of antibody testing during MG diagnosis due to economic reasons was reported by 7 neurologists in our study. However, antibody detection is not only useful for diagnostic purposes, but also very important for immunophenotyping of patients to select the most appropriate therapy. For instance, MuSK-positive patients do not benefit from thymectomy,23 and rituximab is particularly effective in this subgroup of patients when they are refractory to other therapies.24 Therefore, accurate, timely diagnosis (using antibody testing) is crucial for organizing healthcare services and implementing preventive measures.25,26
In our study, thymectomy was performed in 34.3% of patients. Similar results were observed in an epidemiological study performed in the Spanish province of Ourense, where thymectomy was performed in 31.6% of cases.27 MG is induced by thymoma in 10% of patients, and thymectomy should always be performed, if technically possible, in this group. Moreover, thymectomy is a treatment option for patients with MG and thymic hyperplasia.28 Thymectomy for MG should only be performed when the patient is in a stable condition and it is deemed medically safe to perform this procedure, which may result in postoperative pain and limitations in respiratory function.29 In the study by Al-Bulushi et al.,30 the authors observed that, post-thymectomy, 21% of patients presented complete clinical remission, 76% presented significant clinical improvement, and 3% showed no apparent improvement in their clinical status.30
Wolfe et al.23 conducted a randomized trial to compare extended trans-sternal thymectomy plus alternate-day prednisone against the latter treatment alone, in 126 patients. The results showed that patients undergoing thymectomy had a lower time-weighted average quantitative MG score over a 3-year period compared to those who received prednisone alone (6.15 vs 8.99; P<.001). Patients in the thymectomy group also had a lower average requirement for alternate-day prednisone. Moreover, fewer patients in the thymectomy group required immunosuppression with azathioprine or were hospitalized due to exacerbations.23 In our study, at 3 years post-thymectomy, most patients are under some pharmacotherapy including immunosuppressants or corticosteroids. Pharmacotherapies included (1) AChE inhibitors, (2) immunosuppressants, and (3) immunomodulatory treatments. The sequences of different treatment options selected by the neurologists included in this study are in line with the international guidelines, and the clinical and immunological characteristics of each patient.31 AChE inhibitors such as pyridostigmine constitute the most widely used symptomatic therapy. According to the experience of our study participants, AChE inhibitors had the greatest positive effect on the quality of life of patients with MG. In line with international recommendations,29 respondents considered azathioprine as a first choice when immunosuppression was needed. Corticosteroids were considered the most effective treatment, but were also the therapy that caused the most adverse effects, according to respondents’ clinical experience. These results are consistent with those reported in the literature: although the effectiveness of corticosteroids in MG is documented, one of the main weaknesses of the treatment is the fact that patients may experience symptom worsening after corticosteroid treatment initiation (first 2 weeks), and time-dependent side effects.29,32,33 In this study, rituximab was considered as the last option in a sequence of treatments, with a lower percentage of patients receiving this treatment (5.5% and 4.6% of the patients reported by general neurologists and neuromuscular neurologists, respectively). According to the International Consensus Guidance for Management of MG, rituximab should be considered as an early therapeutic option in patients with anti-MuSK MG who have an unsatisfactory response to initial immunotherapy.29 The lower percentage of patients receiving treatment with plasmapheresis in this study can be explained by this treatment mainly being used for myasthenic crises, and the fact that plasma exchange is not available at all centers. Moreover, one of the disadvantages noted by some specialists is that plasmapheresis is excessively invasive to be used as a chronic treatment.34 Cyclophosphamide was the least frequently used treatment among the neurologists that participated in this survey. Although this drug has shown effective results in inducing remission, some data suggest a lack of effectiveness in the long term,35 and there is high risk of severe adverse effects. Some studies recommend long-term follow-up, alternative immunomodulation, and careful monitoring for adverse events when cyclophosphamide is used.36
Most of the participating neurologists followed the same therapeutic strategy for double-seronegative patients as for seronegative patients (70.3% in general neurology and 80.0% in neuromuscular neurology). Although seronegative patients represent around 10%–15% of all patients with MG, there are only very limited data on the clinical management and outcomes of these patients. Mergenthaler et al.37 observed that, although disease crises in seronegative MG affect younger patients after a longer duration of the disease, crisis treatment efficacy and outcomes do not differ compared to patients with anti-AChR MG.37
According to the general and neuromuscular neurologists that participated in this study, the two most urgent unmet needs in MG management are the development of new drugs with fewer adverse effects and better tolerance (59.5% and 50.5%, respectively), and achieving a response in refractory patients (45.9% and 66.7%, respectively). General neurologists and neuromuscular neurologists have different areas of expertise and training, which may have led to differences in their opinions about the most urgent unmet needs in MG management. While their opinions may differ on this subject, their ultimate goal is the same: to provide the best possible care for their patients with MG.
This study has some important limitations. Firstly, the questionnaire was unevenly distributed nationwide to members of the SEN, and the sample size is relatively small. Thus, our results may not be representative of all neurologists treating MG in Spain. Furthermore, the numbers and percentages cited in this study are estimated by the respondents, and do not correspond to real data. For instance, whereas the number of new cases diagnosed annually was estimated at 890, the incidence of MG is 8–10 new cases/million population/year; therefore, in Spain we would expect around 470 new cases/year. Nevertheless, we estimated that there are around 110 neurologists treating patients with MG, and obtained answers from 69. Moreover, this is the first study to specifically analyze the situation of MG in Spain.
ConclusionDue to the global prevalence of MG and its negative consequences for individuals and society, it seems necessary to take measures to achieve better therapies or to use supportive therapies to alleviate symptoms. The limitations associated with the diagnosis, such as the absence of a definitive diagnostic test and the overlap with other conditions, highlight the need for improvement in the field. Moreover, these results reinforce the importance of implementing new treatments that can effectively manage symptoms and improve outcomes for patients with MG.
CRediT authorship contribution statementAll authors participated in the planning and execution of the study. All participating authors have read and approved the paper as it is submitted here.
FundingThis research was funded by Argenx, although the design, data collection, and analysis were performed without participation from the company.
Conflict of interestElena Cortés-Vicente has participated in advisory boards/consultation activities or as speaker for Argenx, UCB, Alexion, and Janssen. The other authors declare that they have no conflicts of interest.
We thank all the Spanish neurologists who participated in the survey.