Amyotrophic lateral sclerosis (ALS) is a degenerative disease of unknown origin that affects the motor neurons. It has a rapid, fatal course.
MethodFor this study, an initial questionnaire of eleven items was developed by experts in the field, who evaluated the suitability and relevance of the items.
ResultsThe questionnaire was then applied to a pilot group of 22 patients diagnosed with ALS. Confirmatory factor analysis, based on estimating maximum likelihood, confirmed the three domains detected in the exploratory factor analysis. The reliability of the scale was tested using Cronbach's α (0.801) and the Kaiser–Meyer–Olkin test (0.770) confirmed the construct validity.
ConclusionsThe DEREDELA questionnaire is valid, in terms of its content, for monitoring the neurological dysphagia and respiratory deterioration suffered by patients diagnosed with ALS.
La esclerosis lateral amiotrófica (ELA) es una enfermedad degenerativa, de origen desconocido, que afecta a las neuronas motoras. Su evolución es rápida y mortal.
MétodosPara este estudio, desarrollamos un cuestionario inicial de 11 ítems, que fue evaluado por expertos en la materia, valorando la idoneidad y pertinencia de los ítems.
ResultadosA continuación se aplicó el cuestionario a un grupo piloto de 22 pacientes diagnosticados con ELA. El análisis factorial confirmatorio, basado en la estimación de máxima verosimilitud, confirmó los tres dominios detectados en el análisis factorial exploratorio. La confiabilidad de la escala se probó mediante el α de Cronbach (0,801) y la prueba de Kaiser-Meyer-Olkin (0,770) confirmó la validez de constructo.
ConclusionesEl cuestionario DEREDELA es válido, en cuanto a su contenido, para la monitorización del deterioro neurológico digestivo y el deterioro respiratorio que sufren los pacientes diagnosticados de ELA.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease of unknown origin that affects the motor neurons. The clinical course is rapid and fatal.1
In Spain, ALS currently affects more than 3000 people, with a prevalence ranging from 2–5 cases per 100,000 inhabitants. The frequency of appearance is slightly higher among male patients, with an incidence that increases after 40 years and peaks at 70–75 years, after which it decreases, approaching that of female patients. Death usually occurs 3–5 years after onset, usually as a consequence of respiratory failure.2,3
According to a meta-analysis published in 2020, the global prevalence of ALS has risen from 1.57 to 9.62 per 100,000 inhabitants and the incidence has risen from 0.42 to 2.76 per 100,000 inhabitants.4 The aetiology of ALS remains largely unknown. However, it has been suggested that genetic and/or environmental factors may be predisposing factors for its appearance and development.5
Despite unceasing efforts to improve the quality of life and dignity of patients and their families, no validated questionnaires have yet been made available for determining the existence and evaluating the impact of neurological dysphagia and respiratory deterioration. Such a test would be of great value, facilitating early intervention to address this condition and thus enhance the quality of life of those affected (which continues to be the main treatment focus).
In view of these considerations, our main study goal is to design and validate a questionnaire enabling physicians to monitor the progression of ALS and its possible complications. This instrument would help them make decisions in areas such as the use of mechanical ventilation and percutaneous gastrostomy to improve the quality of life of patients and their families.
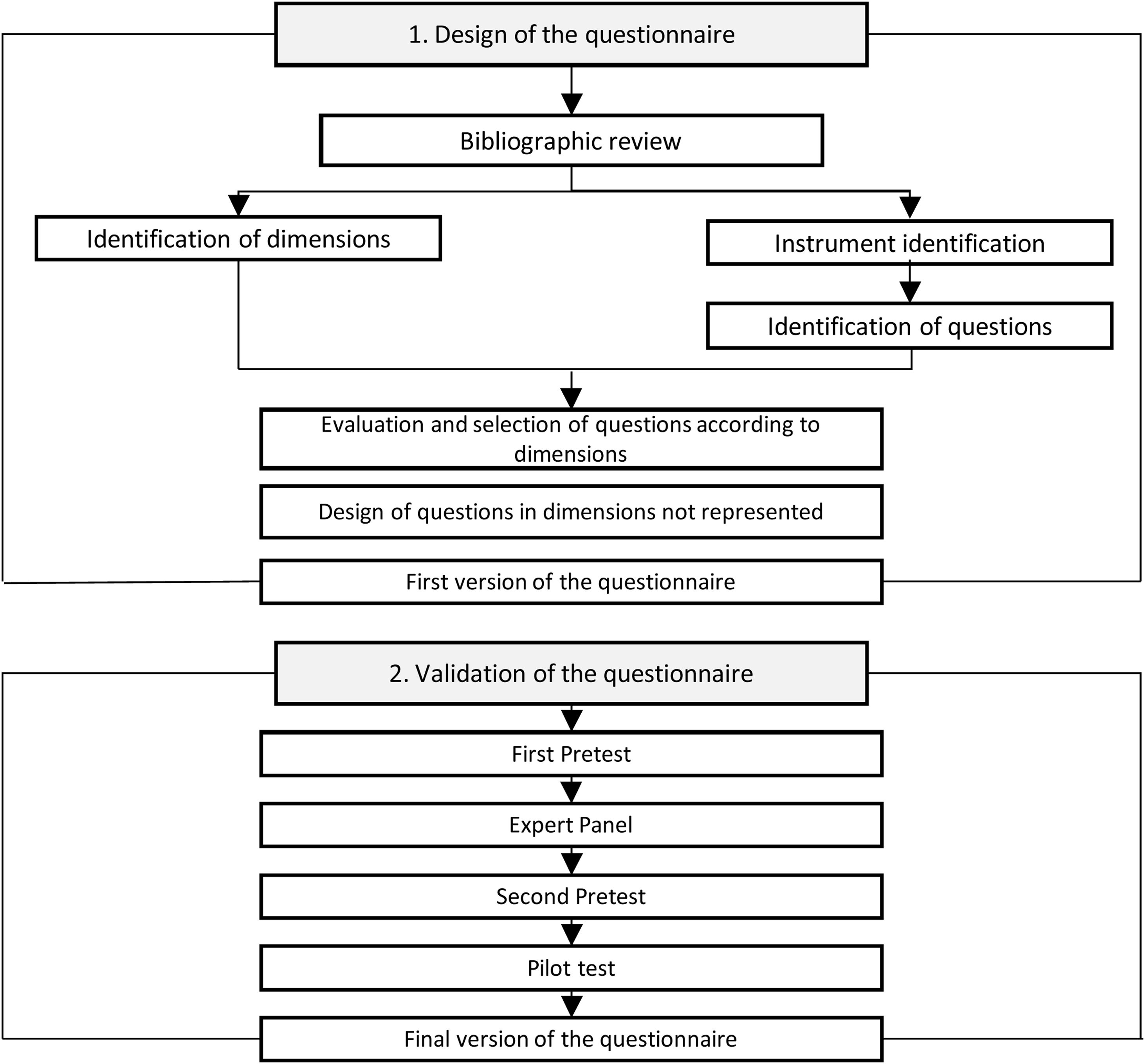
Study methodThis instrumental study was conducted to design, develop and validate DEREDELA, a questionnaire to monitor neurological dysphagia and respiratory deterioration in patients diagnosed with ALS, in a process involving the following stages:
- 1)
Literature review and analysis;
- 2)
Questionnaire design;
- 3)
Validation (see Fig. 1).
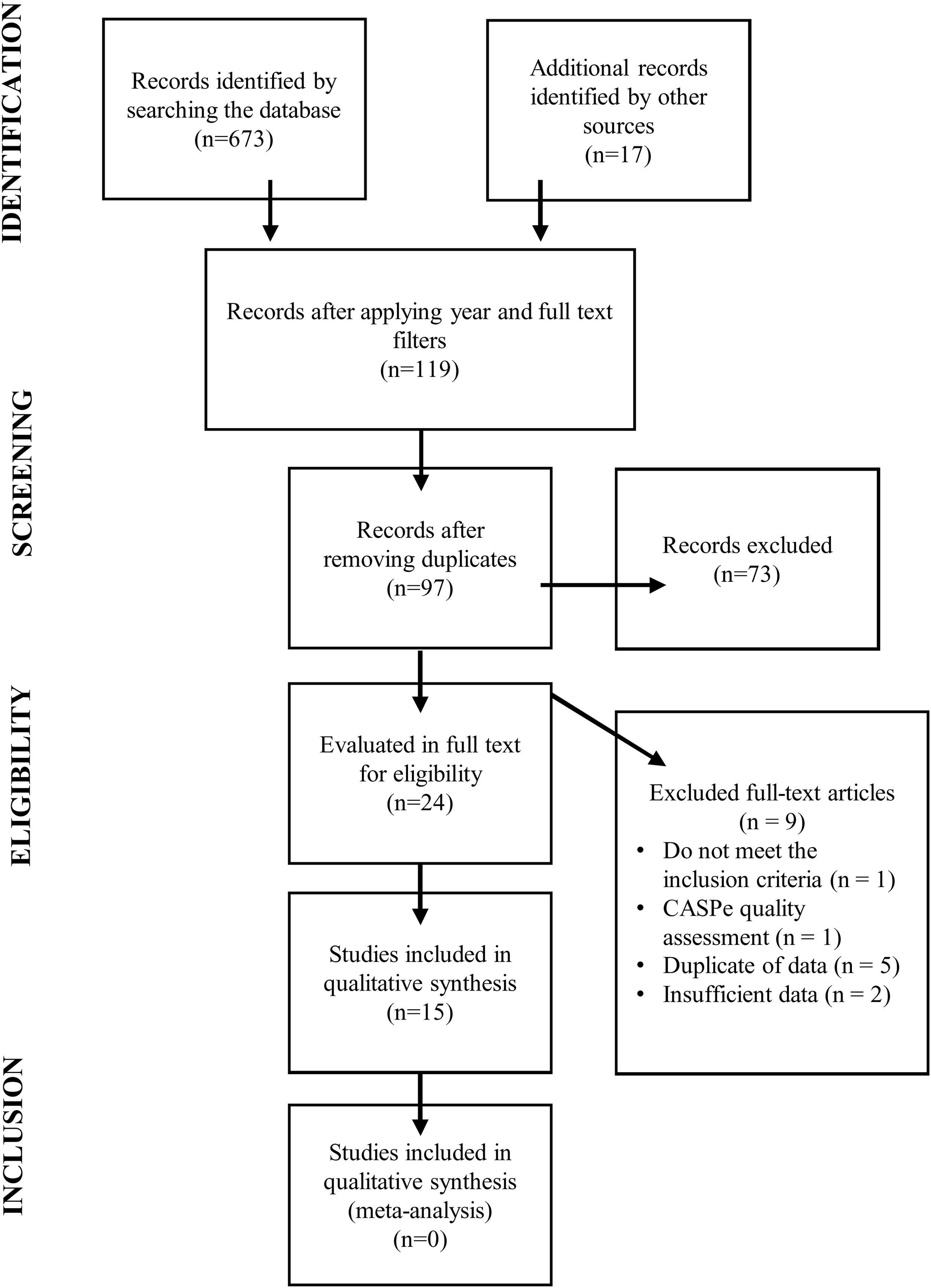
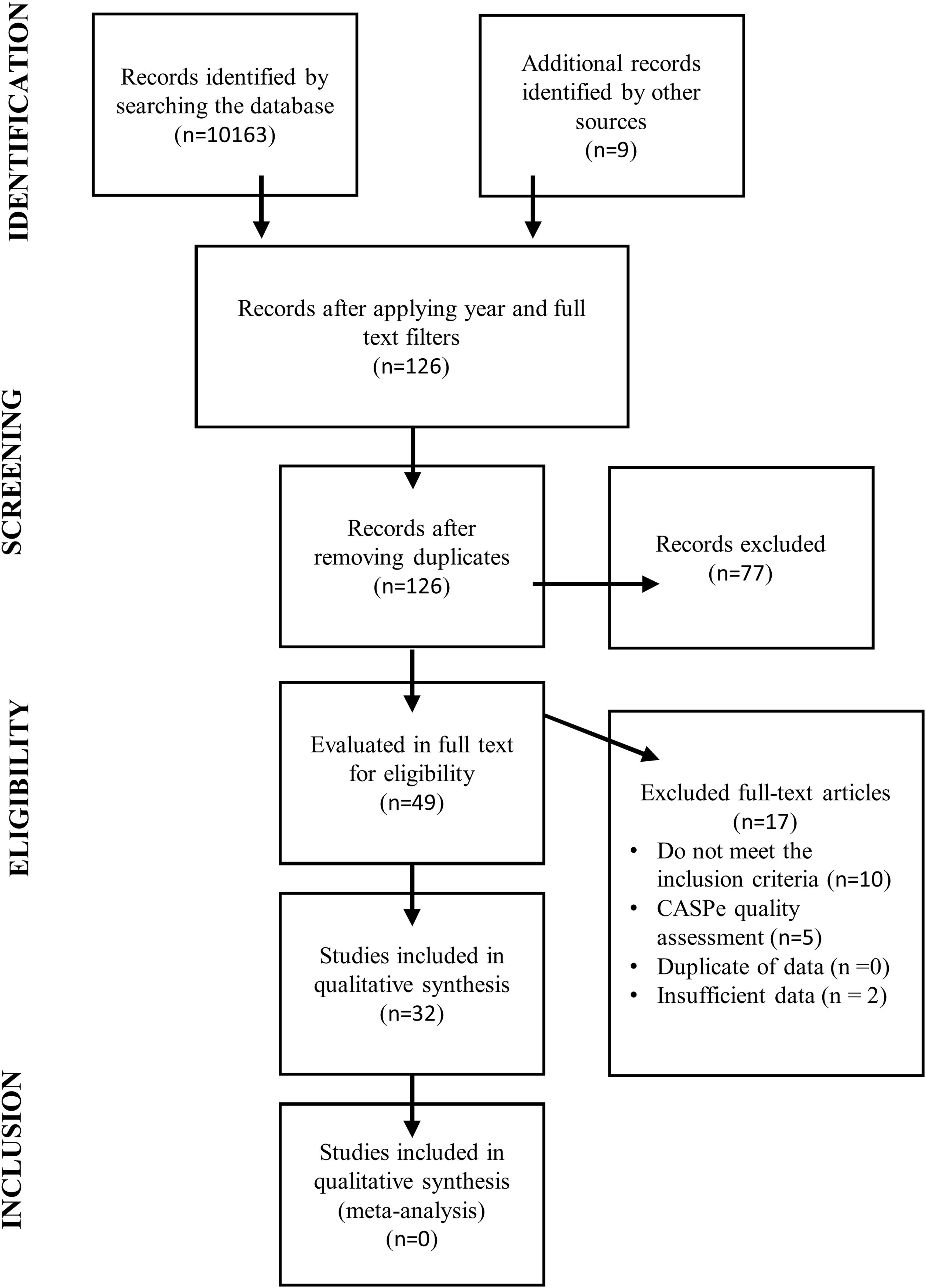
An initial literature review was carried out to determine the existence or otherwise of validated questionnaires to monitor neurological dysphagia and respiratory deterioration in patients diagnosed with ALS (see Fig. 2).6 A second literature review was then conducted to identify the main signs and symptoms of the early onset of this deterioration (see Fig. 3).
This review process identified 15 suitable studies in which a questionnaire was used as a diagnostic tool for patients with suspected ALS. The second step in developing our questionnaire was to stipulate the items required to address the dimensions of the disease, ensuring that each one was addressed by a single item.
A committee of experts was then established, each of whom had a doctorate or master's degree in nursing or medicine and at least ten years’ experience of working with patients diagnosed with ALS. As recommended by Polit et al., this committee was composed of six persons: three neurologists, each with over 30 years’ experience in the field of neurology; two nurse case managers, in direct contact with patients diagnosed with ALS and each with over 15 years’ experience; and a psychologist, expert in case management and difficult diagnoses, with over 20 years’ experience.7
These experts evaluated the questionnaire items, individually and jointly, in terms of their relevance, probable effectiveness and appropriateness for the patients concerned. The committee was asked to complete its assessment within 15 days and to provide an opinion on the overall suitability of the questionnaire.
The initial questionnaire consisted of three dimensions and eleven items. The data analysis was carried out as recommended by Polit and Beck,7 calculating the content validity index (CVI) and the modified Kappa coefficient (K) for each item. The overall coefficient of content validity (CCV) was also calculated for each dimension and for the global assessment.
Selection of patients for the study sampleOnce the questionnaire had been drafted, patients were selected to form the study sample, taking into account previous literature in this respect. According to Cattel, a sample size of 3–6 individuals is sufficient; Gorsuch recommended a sample size of five, while Everitt argued that it should include at least ten patients.8–10
The pilot questionnaire, entitled “DEREDELA – Questionnaire for monitoring neurological dysphagia and respiratory deterioration in patients with ALS” was delivered to a statistically significant sample of 22 patients, who were asked to complete it unaided and anonymously. The main purpose of this questionnaire was to assess the patients’ understanding of the items and to invite suggestions for improvement.
The questionnaire was accompanied by a brief introduction to the study, explaining its purpose and content, and stipulating its compliance with the provisions of Spanish legislation (the Organic Law on Personal Data Protection and Guarantees for Digital Rights, No. 3/2018, of 5 December).
The final version of the questionnaire contained 18 questions characterising the study sample and 11 items, each of which presented 6 response alternatives, ranging from the lowest to the highest degree of neurological impact, on a scale where 1=little or no impact and 6=very severe impact.
After this pilot study, the study sample was then expanded to 105 individuals diagnosed with ALS, a sample size that was considered sufficient for our purposes.11 Study data were obtained via the following channels:
- -
Self-administered survey, via Google Forms.
- -
Self-administered survey in pdf format, via email.
- -
Collaborating researchers, in in-person or telephone interviews, conducted in 2020.
All participants were informed of the purpose of the study and of its anonymous nature, and of its mandatory compliance with the Organic Law on Personal Data Protection and Guarantees for Digital Rights, No. 3/2018, of 5 December. Completion of the questionnaire, therefore, implied the existence of informed voluntary consent to participate. In addition, the study was approved by the Ethics Committee of Torrecárdenas University Hospital (Almería, Spain).
ResultsInitial validity of the DEREDELA questionnaireThe results obtained by the Kaiser–Meyer–Olkin test (0.77) and Bartlett's test of sphericity (chi-square: 271.17; p<0.001) mean that, for the data matrix, the correlation obtained by DEREDELA in the factor analysis was appropriate for our purposes. The inter-item correlations were also determined.
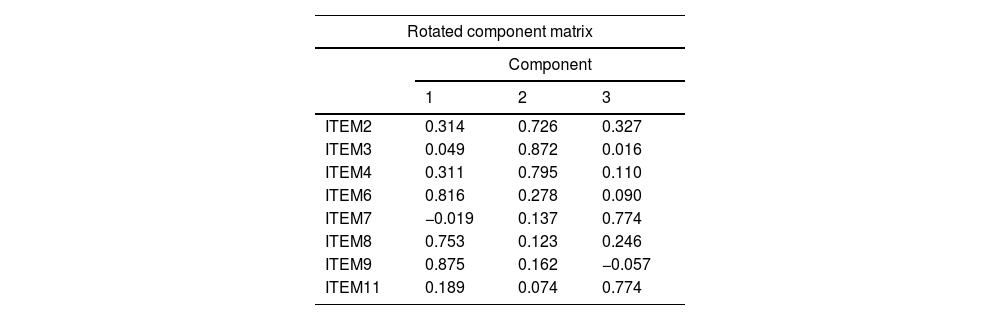
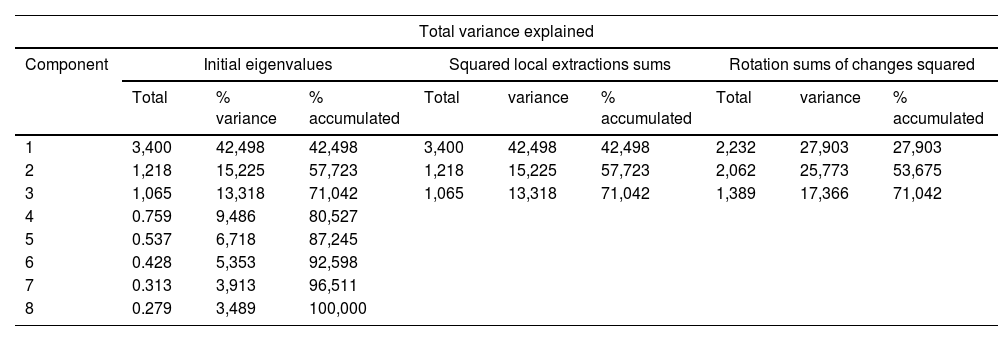
Factor extraction by principal component analysis and Varimax rotation initially yielded three primary factors that jointly explained 62.05% of the total variance of DEREDELA. After eliminating items 1, 5 and 10, the same factor extraction process (Table 1) yielded three primary factors that jointly explained 71.04% of the total variance of DEREDELA (Table 2).
Rotation method: Varimax with Kaiser normalization. The rotation has converged in 4 iterations.
| Rotated component matrix | |||
|---|---|---|---|
| Component | |||
| 1 | 2 | 3 | |
| ITEM2 | 0.314 | 0.726 | 0.327 |
| ITEM3 | 0.049 | 0.872 | 0.016 |
| ITEM4 | 0.311 | 0.795 | 0.110 |
| ITEM6 | 0.816 | 0.278 | 0.090 |
| ITEM7 | −0.019 | 0.137 | 0.774 |
| ITEM8 | 0.753 | 0.123 | 0.246 |
| ITEM9 | 0.875 | 0.162 | −0.057 |
| ITEM11 | 0.189 | 0.074 | 0.774 |
Extraction method: principal component analysis.
| Total variance explained | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Component | Initial eigenvalues | Squared local extractions sums | Rotation sums of changes squared | ||||||
| Total | % variance | % accumulated | Total | variance | % accumulated | Total | variance | % accumulated | |
| 1 | 3,400 | 42,498 | 42,498 | 3,400 | 42,498 | 42,498 | 2,232 | 27,903 | 27,903 |
| 2 | 1,218 | 15,225 | 57,723 | 1,218 | 15,225 | 57,723 | 2,062 | 25,773 | 53,675 |
| 3 | 1,065 | 13,318 | 71,042 | 1,065 | 13,318 | 71,042 | 1,389 | 17,366 | 71,042 |
| 4 | 0.759 | 9,486 | 80,527 | ||||||
| 5 | 0.537 | 6,718 | 87,245 | ||||||
| 6 | 0.428 | 5,353 | 92,598 | ||||||
| 7 | 0.313 | 3,913 | 96,511 | ||||||
| 8 | 0.279 | 3,489 | 100,000 | ||||||
The domains identified in this analysis were ‘Respiratory difficulty’ (defined by items 6, 8, 9), ‘Nutritional status’ (defined by items 2, 3, 4) and ‘Impact on quality of life’ (defined by items 7 and 11). Our initial theoretical model is explained by the factorial solution thus obtained (Table 3). All analyses were performed using SPSS version 24.0 statistical software.
Domains of the DEREDELA questionnaire.
| Dimension | Items | Explained variability |
|---|---|---|
| Respiratory difficulty | 6- Breathing tubes, tracheostomy, laryngectomy, Respiratory medication: Oxygen, Inhalers, others | 42,498% |
| 8- Presence of respiratory distress when lying down | ||
| 9- Respiratory devices | ||
| Nutritional condition | 2- Have you made any changes regarding the amount of food and liquids consumed each day? | 57,723% |
| 3- Take vitamins or nutritional supplements (protein shakes, sachets, pharmaceutical preparations, creams... | ||
| 4- Do you follow any type of diet? | ||
| Impact on quality of life | 7- Level of consciousness | 71,042% |
| 11- Changes in the habitual sleep pattern: difficulty falling asleep, sleep interruptions, non-restorative sleep, sleeping during the day, prolonged wakefulness, having nightmares | ||
The reliability of the DEREDELA questionnaire was measured according to the internal consistency between the items, applying Cronbach's alpha coefficient, which obtains values in the range from 0 to 1, where 0.7 is the minimum acceptable value, below which the internal consistency of the scale is considered low.12
In the present case, after carrying out the exploratory factor analysis and excluding the three items mentioned, the global coefficient obtained for the questionnaire was 0.80, a value that has been considered good to excellent.13
In our study, the test-retest technique could not be applied since the rapid progression of the disease made it difficult or impossible to repeat the questionnaire with the same users. The reliability of the questionnaire was also evaluated using the split-half method, which revealed no differences in any of the domains found, or in the global evaluation.
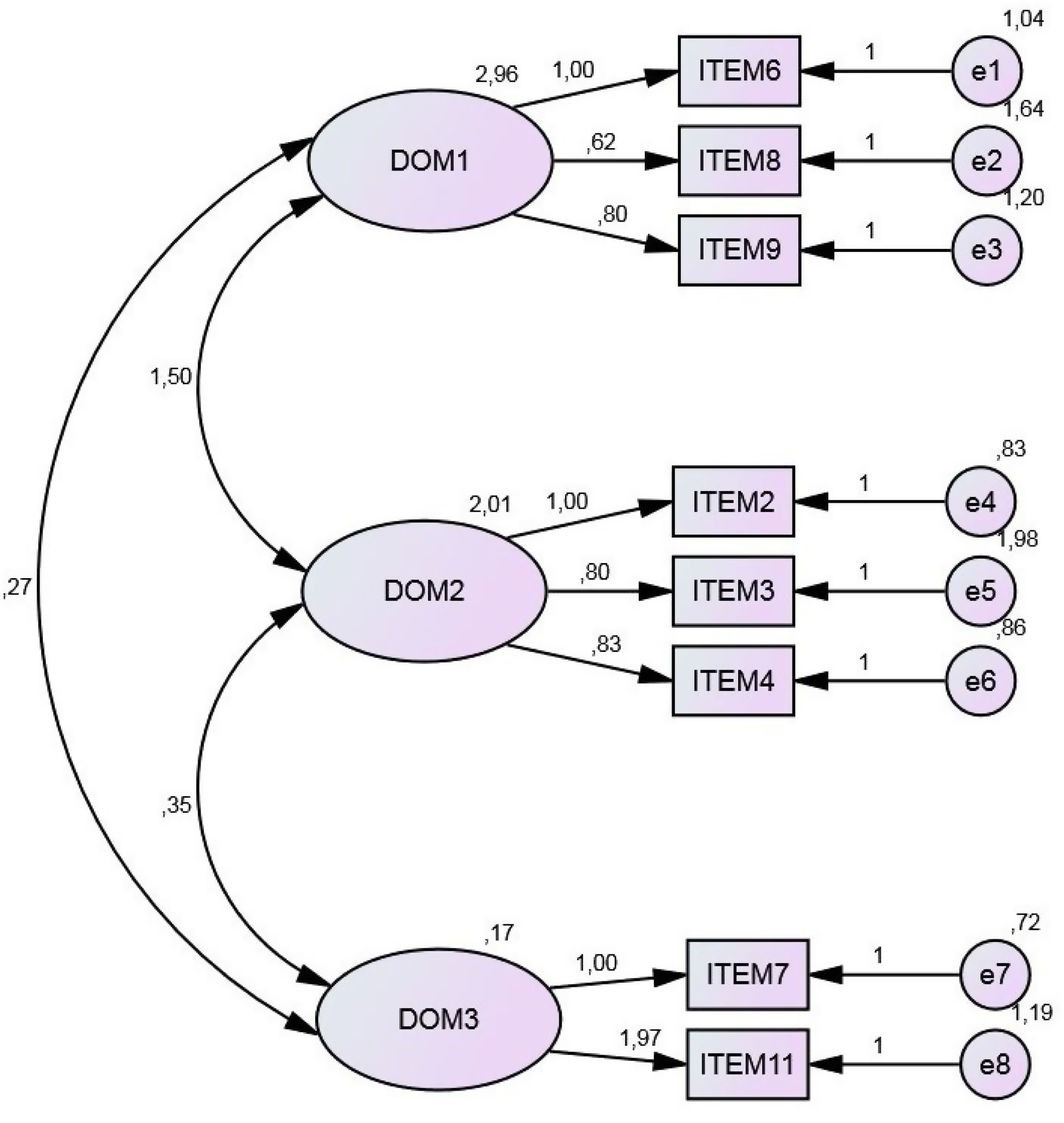
Confirmatory factor analysisConfirmatory factor analysis, which is considered an appropriate means of determining the underlying conceptual structure of a questionnaire11,14,15 was performed, using the AMOS program. The results obtained are shown in Fig. 4.
Maximum likelihood estimation confirmed the three domains detected in the exploratory factor analysis. The chi-square test result (p=0.13) was not statistically significant. Moreover, the chi-square/degrees of freedom ratio obtained (1.50) was less than 3.
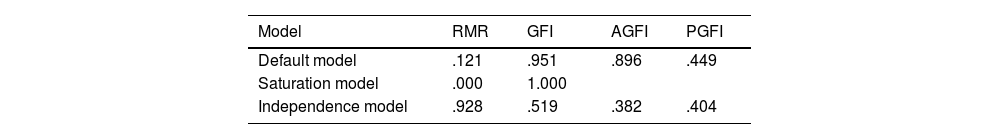
The significance of the chi-square test is strongly dependent on the sample size. In our case, the sample was composed of 105 patients, which exceeds the minimum figure recommended in previous studies (6–8 patients). The absolute goodness of fit indices obtained were reasonably close to 1 (Table 4).
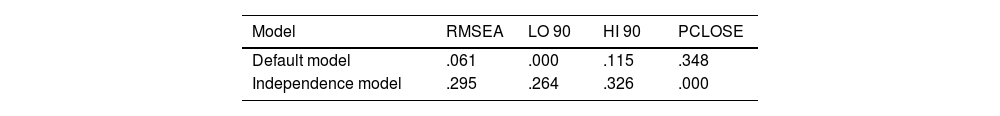
Interpretation of the RMSEA index may be subjective, but values below 0.08 are usually considered indicative of a good fit of the model. In our case, the value obtained (0.061) was below this threshold (Table 5).
As concerns the incremental indices of goodness of fit, the value obtained for the normed fit index (NFI) (0.91) was close to 1, and hence acceptable. The non-normed fit index (NNFI) (or Tucker-Lewis index, TLI) was applied to determine the degrees of freedom of both the null model and the proposed model, thus overcoming the sample-size limitations of the NFI. The NNFI values obtained also exceeded 0.9. The comparative fit index (CFI) measures the improvement observed in the non-centrality of a model. With this test, we obtained a value of 0.97, close to the recommended 1.8, which corroborates the suitability of our model. In summary, the CFI and NNFI results for our model were within the recommended ranges.
The NFI and GFI values were above the recommended minima, and the RMSEA value was well above the minimum level (Table 6). Accordingly, we conclude that the final eight-item questionnaire, with the domains described above, is fit for purpose and that evaluation by maximum likelihood estimation provides acceptable results.
DiscussionIdentifying validated questionnaires for monitoring the progression of ALSPredicting the course of neuromuscular deterioration in ALS is a difficult task, as the rate and extent of neurological deterioration may change from month to month, or even week to week. Despite multiple organ involvement and the existence of a battery of tests for identifying signs of deterioration, early diagnosis continues to be a challenge. Moreover, choosing which test to use may also pose problems. Indeed, two people with the same disease may have a different presentation and course: a patient with bulbar involvement will have a different course from someone mainly suffering lower limb weakness. In addition, the clinician must take into consideration that some patients will survive less than a year from diagnosis.16
ALS is a neurodegenerative disease which, due to its poor prognosis and the associated psychosocial impact, greatly affects the quality of life and dignity of patients and their families. Studies have observed a close relationship between clinical deterioration and worsening quality of life.17 Therefore, one of the crucial objectives for the multidisciplinary team managing ALS is to monitor the progression of the disease.
Nevertheless, and despite ceaseless efforts to improve the quality of life and the dignity of patients and their families, no validated questionnaire has yet been offered to predict the existence of neurological dysphagia and respiratory deterioration. Such an instrument would be very useful, facilitating early intervention to address this deterioration.6
From a nursing perspective, learning more about the main prognostic factors for neurological dysphagia and respiratory deterioration in patients diagnosed with ALSWhen ALS is diagnosed, the fear generated by uncertainty about the short and long-term prognosis leads many patients and their families to reject the use of complementary therapies such as percutaneous gastrostomy or mechanical ventilation. Therefore, it is very important that these complementary approaches be presented and discussed at an early stage.18
Among other functions, home nurses help patients and their families in areas such as optimising the operation and comfort of mechanical ventilation in the home, maintaining the gastrostomy tube, ensuring the prescribed treatments are received correctly and providing health education and other support, from diagnosis until the end of life.19
ALS is a devastating degenerative disease, with a severe negative impact on the quality of life of patients and their families. As the disease progresses and patients become more dependent, they and their families are affected emotionally, psychologically, socially and economically. Although there is still no curative treatment for this disease, there exist therapeutic measures that can enhance the patient's quality of life and safety, as part of the care strategies adopted by a multidisciplinary team. Studies have shown that a multidisciplinary approach can prolong survival, improve the quality of life and facilitate access to therapies, as well as promoting acceptance of invasive non-ventilatory systems or enteral feeding.20–22
Designing and validating a questionnaire to monitor neurological dysphagia and respiratory deterioration in patients diagnosed with ALSThe study we present is based on an initial questionnaire consisting of three dimensions and 11 items, the “DEREDELA – Questionnaire for monitoring neurological dysphagia and respiratory deterioration in patients with ALS”. This questionnaire was first completed as a pretest by a statistically significant sample of 22 patients, via a self-administered form. After this pretest, the study sample was expanded to 105 patients diagnosed with ALS.
The results obtained show that DEREDELA is a reliable instrument for monitoring neurological dysphagia and respiratory deterioration in patients with ALS. It obtained a good Cronbach's alpha coefficient (0.801) and the results of the exploratory and confirmatory factor analyses were satisfactory.23,24
Therefore, the study aim, to design and validate an appropriate questionnaire for monitoring neurological dysphagia and respiratory deterioration in patients diagnosed with ALS, was fulfilled. This questionnaire is a useful instrument, offering psychometric properties that reduce the need for invasive and unpleasant clinical tests and improve decision-making regarding the use of resources such as gastrostomy or non-invasive mechanical ventilation, thus improving the quality of life of patients and their families.23–25
In view of these considerations, we believe the DEREDELA questionnaire is a valuable complementary tool in decision-making for and by patients diagnosed with ALS.
ConclusionsOur initial review of the literature in this field showed that, despite the constant work undertaken to improve the quality of life and dignity of patients and their families, as yet there is no validated tool for healthcare professionals to predict the existence of neurological dysphagia and respiratory deterioration in patients diagnosed with ALS. A questionnaire performing this function would be very useful, facilitating early intervention and thus improving the quality of life and dignity of patients and their families, in line with the main focus of approach to this disease, at present.
A second literature review enabled us to lay the foundations for DEREDELA, the questionnaire we propose. Due to the special characteristics of ALS, a multidisciplinary approach is essential. Patients must be assisted by diverse medical specialists, including neurologists, rehabilitators, pulmonologists, nutritionists, nurses, pharmacists, physiotherapists, occupational therapists and social workers, working in close coordination from the moment of diagnosis, agreeing on tests and treatments to be carried out, on the type of information that the patient should properly receive, on the psychological and social support to be offered and on decision-making regarding end-of-life instructions, both in outpatient care and on admission to hospital. Furthermore, these aspects should be monitored by primary care physicians and in nursing care. Accordingly, a high degree of coordination and resource management is necessary to ensure the best possible health care is provided. Success in this area would reduce the variability in care and increase the satisfaction of patients, caregivers and family members.
In view of the content and construct validity tests performed on the DEREDELA questionnaire, the reliability observed and the positive results obtained, we believe it can be accepted as an effective, useful instrument for monitoring neurological dysphagia and respiratory deterioration in patients diagnosed with ALS.
Conflicts of interestThe authors declare no conflicts of interest.