Tirbanibulin, a novel antineoplastic agent, received FDA approval in 2020 for the topical management of actinic keratosis (AK). This regulatory endorsement was substantiated by pivotal clinical investigations, encompassing phase III trials and studies in real clinical practice that underscored its efficacy and safety profile.1,2 Formulated from a synthetic analog of hemiasterlin, tirbanibulin exerts its effects through the disruption of microtubule dynamics, culminating in cell cycle arrest and apoptosis.3 With its approval, a new therapeutic weapon appeared for AK and its field of cancerization, a very common pathology in primary care. However, the singularity of its mechanism of action, coupled with its relatively favorable tolerability, has prompted exploration for its potential extension into novel therapeutic applications.
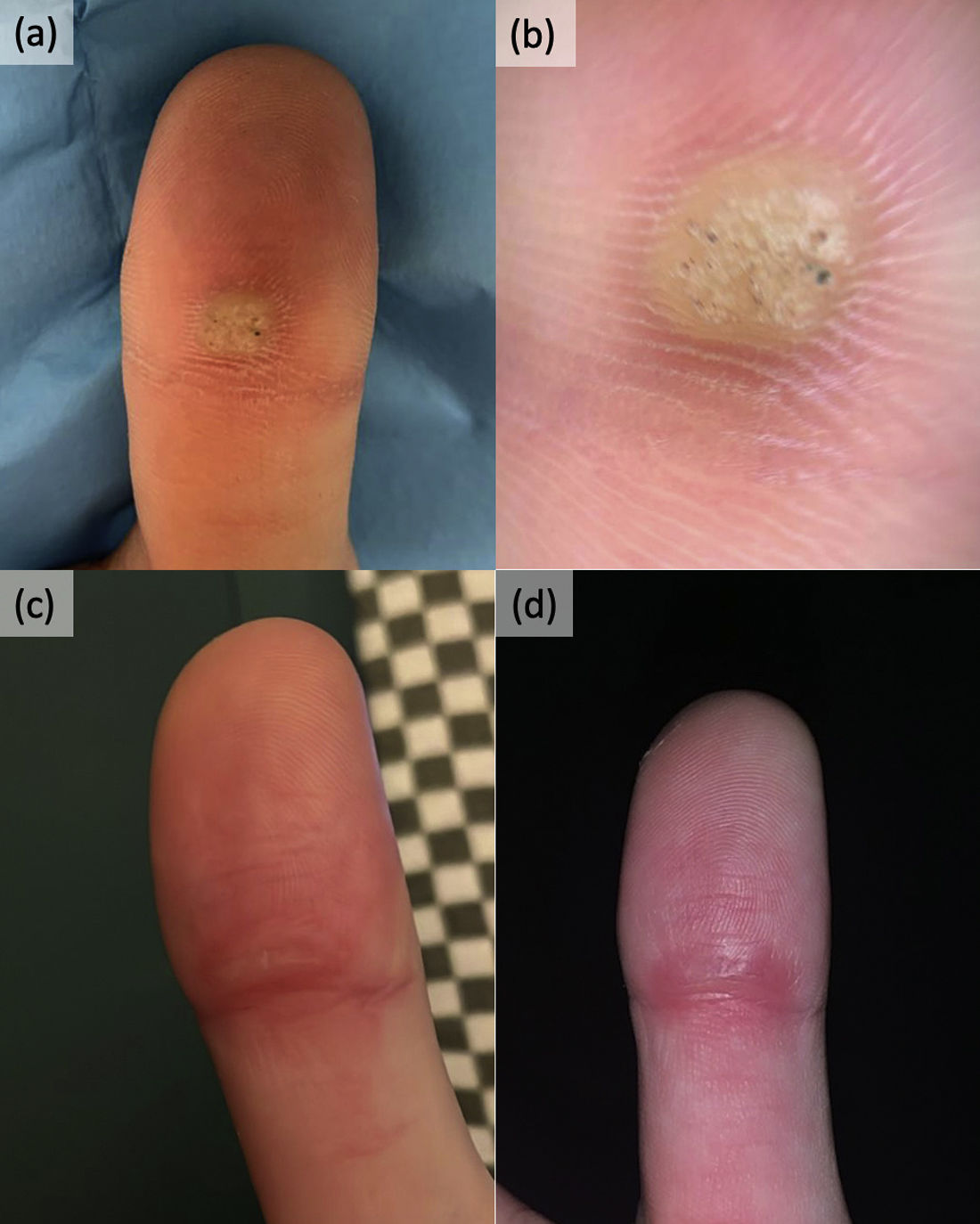
A 28-year-old woman, with no notable medical history sought evaluation for a viral wart (VW) located on the fingertip of her right index finger. She reported the lesion for approximately 4 years. She had undergone three cryotherapy sessions, one electrocoagulation treatment, and a topical combination of salicylic acid and 5-fluorouracil, all yielding only partial responses with subsequent relapses. Upon examination, a 0.8cm×0.7cm papule was observed proximally on the fingertip (Fig. 1a and b). Dermoscopy revealed a slightly velvety surface and fine superficial puncta consistent with a VW (Fig. 1b). Faced as refractory VW, superficial curettage followed by application of aluminum chloride to remove the surface of the wart. Ten days post-curettage, topical application of 1% tirbanibulin ointment for 5 consecutive nights was initiated. At the 3-month follow-up, the patient showed no recurrence, albeit experiencing mild dysesthesias at the application site (Fig. 1c). At the 6-month mark, no lesions were present, and the patient remained entirely asymptomatic (Fig. 1d). No adverse effects were reported during tirbanibulin treatment.
Recently, a new drug, tirbanibulin 1% ointment, has become available for the treatment of actinic keratosis (AK) and its field of cancerization. Initially, its approval was based on studies involving a 25cm2 application on immunocompetent patients with lesions located on the head and scalp.1 However, recent studies have demonstrated its potential effectiveness and safety in broader applications, such as areas exceeding 25cm2, different anatomical locations beyond the head and scalp, and among immunocompromised patients.2 Furthermore, other authors have attempted to push the boundaries, expanding its use to other indications. Thus, recently, case series have been reported with a favorable response and an appropriate safety profile in the treatment with tirbanibulin 1% ointment for in situ squamous cell carcinoma, invasive squamous cell carcinoma, superficial basal cell carcinoma or condyloma acuminata.4,5
In conclusion, we present tirbanibulin 1% ointment as a potential new therapeutic option that is effective and safe for the treatment of refractory VW. This utility opens the possibility of its use as a treatment for recalcitrant viral warts in primary care.
EthicsProcedures followed here were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983. We have not use patients’ names, initials, or hospital numbers.
FundingNo specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
AuthorshipAll authors had access to the data and played a role in writing this manuscript.
Authors’ contributions- -
Miguel Mansilla-Polo and Daniel Martín-Torregrosa managed clinical treatment and procedures, contributing to the development of this paper.
- -
Carlos Abril-Pérez supervised the work.
Oral and written consent was obtained to publish this image.
Conflicts of interestThe authors have declared no conflicts of interest.