Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosHirschsprung-associated enterocolitis (HAEC) is a complication with a multifactorial etiology that can cause morbidity and mortality in patients with Hirschsprung disease (HSCR). The histopathological degree of HAEC according to Teitelbaum's criteria can be used to predict the clinical development of HAEC. S-100 immunohistochemistry identifies enteric ganglion cells and nerve cell proliferation, the absence of ganglion cells, and hypertrophy of nerve fibers – all features of HSCR.
MethodsPatients were children with HSCR who underwent leveling colostomy or a pull-through procedure; this sample came from the ganglionic segment of dilatation in the distal zone. The histopathological grade of HAEC was examined, and the size of the nerve diameter was measured by immunohistochemical S-100. Data were analyzed with the Spearman rank test and the Fisher's exact test.
ResultsThis study had 26 samples, dominated by boys (73.1%) and the age group 2–3 years (38.5%). The histopathological class distribution was reasonably fair except for class V, where no sample was found in this group. Measurement of the dominant nerve diameter was a size of ≥40μm (84.6%). Statistically, the p-value was not significant to confirm the relationship between histopathological class size and size of nerve diameter (r=0.067).
ConclusionIf the diameter of the nerve experiencing hypertrophy is higher, the risk of HAEC is greater, but this relationship is not statistically significant.
Hirschsprung disease (HSCR), or congenital aganglionic megacolon, is a condition that involves functional intestinal obstruction in part or all of the colon at the aganglion level. HSCR occurs due to the failure of cephalocaudal migration of ganglion cells in the 12th week of pregnancy. The development of intrinsic components in the enteric nervous system is abnormal and characterized by the absence of ganglion cells in the myenteric plexus and the submucosa in the distal intestine. These cells are responsible for normal peristalsis.1 A fatal complication of HSCR is Hirschsprung-associated enterocolitis (HAEC), the most significant and potentially life-threatening cause of morbidity and mortality due to HSCR. Due to the difficulty in diagnosis, a high level of wariness is required when dealing with HSCR, as it is currently still a significant challenge for pediatric surgeons and paediatricians.2
HAEC is a complication of HSCR, with clinical manifestations ranging from mild symptoms – such as mild abdominal distension, watery stools with perianal excision, and fever – to severe symptoms – which include explosive diarrhea, foul-smelling diarrhea, and loose stools accompanied by blood, vomiting, lethargy, and shock due to the life-threatening toxic megacolon characterizing HSCR.3 HAEC incidence was found to vary, from 6 to 26% before and 5 to 42% after diversion or definitive resection of the aganglionic bowel; this variance might be due to an overlap of symptoms with other pathological conditions, as well as differences in standards for diagnosing HAEC.4
Although several hypotheses have been posited as to its etiopathogenesis, HAEC's biological mechanism is still unknown. Various biomechanisms have been described, ranging from the level of gene expression to a biochemical imbalance in the digestive tract that causes complex disorders. Colon obstruction appears as histopathologic changes in the structure of the intestinal wall. Another supported theory is that changes in the mucin ratio in the colonic mucosa can predispose it to microorganism infection, increasing the incidence of infection.5,6 Teitelbaum et al. propose that patients with HAEC will experience significant changes in their intestinal tissue, which is characterized by mucin retention and dilatation of crypts that become the basis of the classification of a histopathologic HAEC.5
Speedy, accurate diagnosis is the primary key in handling this disease. With the right diagnosis obtained quickly, young patients can get the therapy they need to prevent further complications.1 However, until now, an examination of the primary essential pathology is used to diagnose this disease. Unfortunately, the examination of enteric ganglion cells is a separate problem faced by pathologists7; therefore, a much simpler method of identification is needed. Immunohistochemical examination is an alternative approach with strong potential for diagnosing this disease. In the S-100 immunohistochemical method, the ganglion cells are negatively colored, and the size of the nerve diameter is μ 40μm.8 Nerve cells undergoing proliferation are colored using this method. An absence of ganglion cells and a discovery of hypertrophy from nerve fibers indicates HSCR. It has been previously reported that 90% of patients with HSCR showed a nerve diameter ≥40μm.9 We hypothesize that there is a relationship between the severity of the histopathological grade and the size of nerve diameters. To evaluate this hypothesis, we conducted a study to identify a correlation between the histopathological level of enterocolitis and the size of nerve fiber diameters.
MethodsWe conducted a cross-sectional study with consecutive sampling using children with HSCR who were confirmed by histopathological examination to have HAEC. The study took place in the Dr. Wahidin Sudirohusodo Hospital in Makassar, Indonesia from December 2019 to April 2020. The HSCR diagnosis was confirmed by the results of a rectal biopsy before a leveling colostomy or pull-through procedure was performed. Intestinal samples were collected intraoperatively and were histopathologically assessed based on Teitelbaum's HAEC classification (hematoxylin and eosin staining under a light microscope). Our sample was taken in the distal zone of the dilatation. The inclusion criteria for the study consisted of all pediatric HSCR patients age ≤18 years whose diagnoses were proven based on the results of biopsy pathology of full-thickness rectal anatomy and whose HAEC were confirmed with colonic histopathology based on the Teitelbaum criteria (grade IV) and with size of nerve diameter (determined by immunohistochemistry S-100) when a leveling colostomy or pull-through procedure was performed. The exclusion criteria consisted of an HSCR patient only undergoing barium enema examination, and an examination of damaged histopathological tissue. For this study, the HAEC degree was established through histopathological diagnosis and then related to the results of the nerve diameter measurements. The Institutional Ethics Committee approved this study. The collected data were compared using the Spearman rank test and the Fisher's exact test. Statistical analysis was performed using SPSS version 24.0 for Windows, and a p-value of ≤0.05 was considered statistically significant.
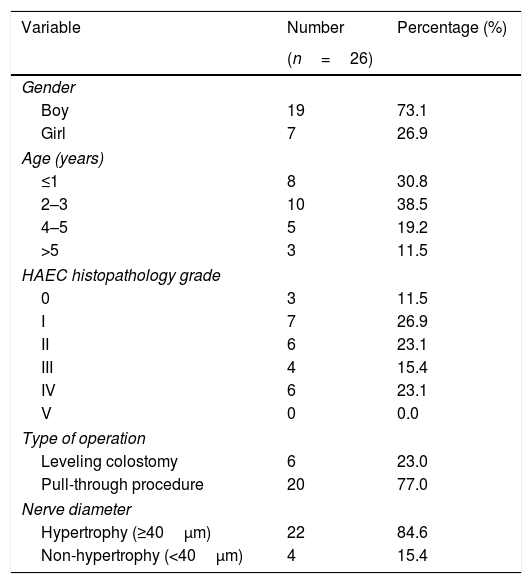
ResultsTwenty-eight colon tissue samples were taken from patients undergoing leveling colostomy diversion or definitive pull-through surgery at our institution and were used for histopathological examination. The diameter of nerve hypertrophy was measured using S-100 immunohistochemistry in order to analyze the relationship with the severity of HAEC. During the measurement of nerve diameter with S-100 immunohistochemistry, two obtained samples were damaged during the measurement process; these were excluded from this study, leading to a total sample population of 26 tissue samples. The majority of the sample population were boys (n=19) (73.1%); the ages of the population ranged from 5 months to 14 years, although the dominant age at the time of the surgical procedure was 2–3 years. The sample population is categorized into four age groups. Demographic details and characteristics of the study population are provided in Table 1.
Patient characteristics.
| Variable | Number | Percentage (%) |
|---|---|---|
| (n=26) | ||
| Gender | ||
| Boy | 19 | 73.1 |
| Girl | 7 | 26.9 |
| Age (years) | ||
| ≤1 | 8 | 30.8 |
| 2–3 | 10 | 38.5 |
| 4–5 | 5 | 19.2 |
| >5 | 3 | 11.5 |
| HAEC histopathology grade | ||
| 0 | 3 | 11.5 |
| I | 7 | 26.9 |
| II | 6 | 23.1 |
| III | 4 | 15.4 |
| IV | 6 | 23.1 |
| V | 0 | 0.0 |
| Type of operation | ||
| Leveling colostomy | 6 | 23.0 |
| Pull-through procedure | 20 | 77.0 |
| Nerve diameter | ||
| Hypertrophy (≥40μm) | 22 | 84.6 |
| Non-hypertrophy (<40μm) | 4 | 15.4 |
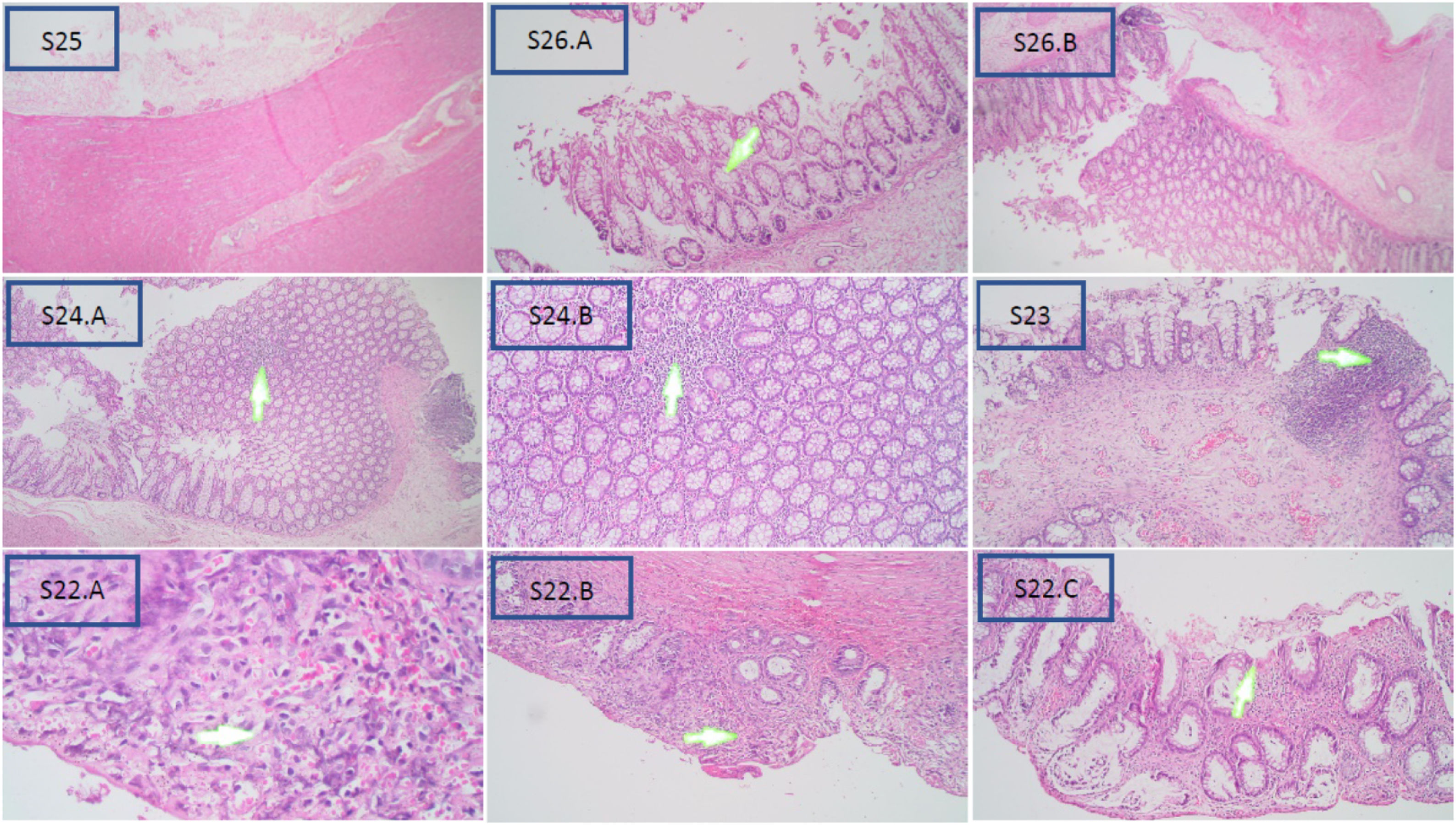
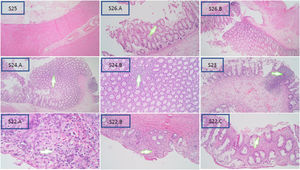
Based on the Teitelbaum classification (Fig. 1), S25 is grade 0 and shows no abnormalities; S26 is class I and represents a widening crypt (A) and retention of mucin (B); S24 is class II and is characterized by cryptitis, or two crypt abscesses at enlargement 4× (A) and 10× (B); S23 is grade III and is considered as womb multiple crypt abscesses; and S22 is grade IV, with fibrinopurulent debris (A) and 4× (B) and 10× (C) enlarged mucosal ulcerations. Grade V was not obtained in this study, which would process transluminal necrosis or perforation in this sample.
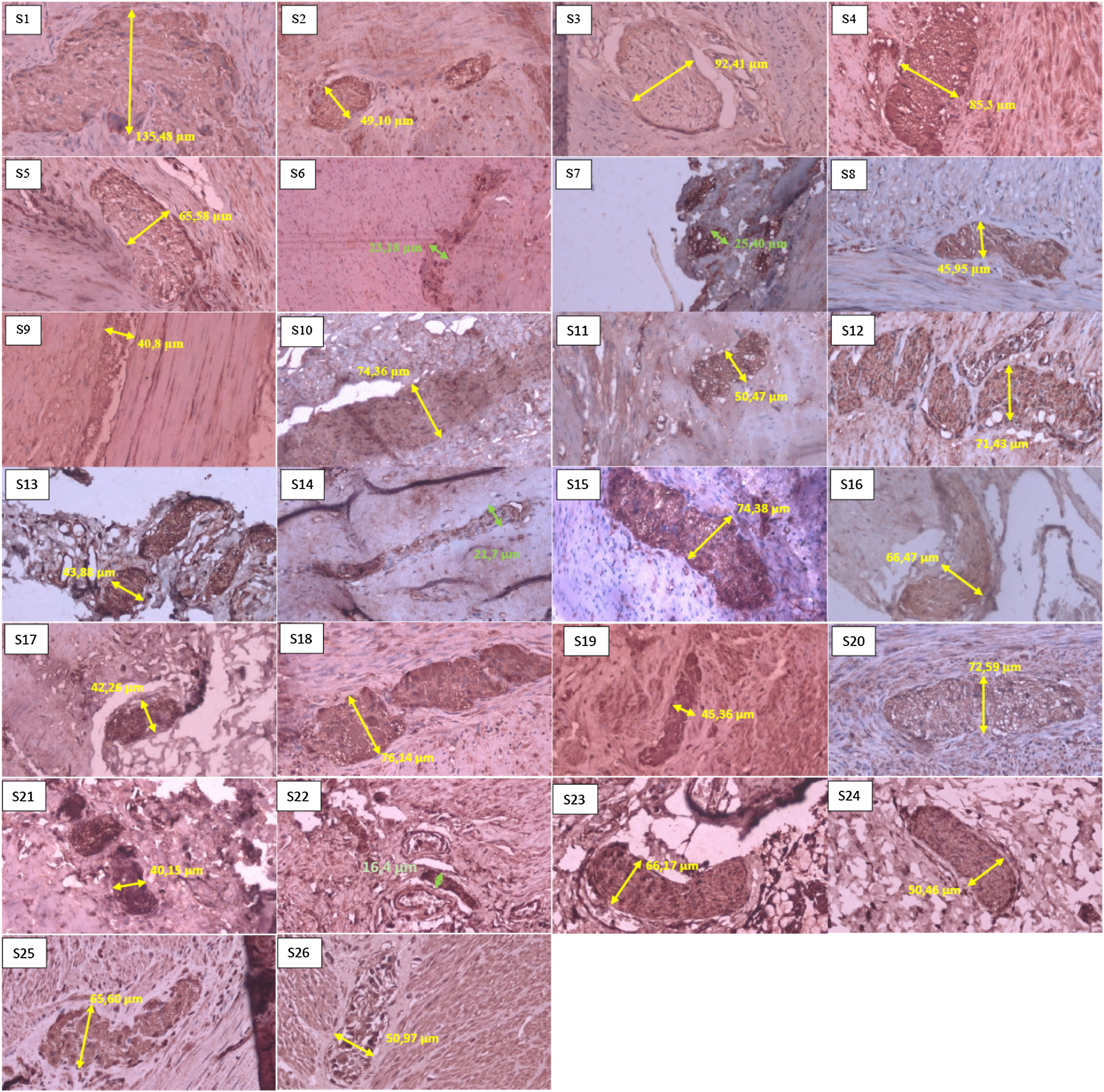
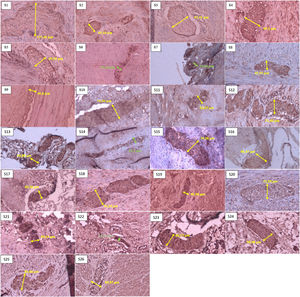
In the histopathological examination results, the most samples in one grader were in grade I with seven samples (26.9%), then in grades II and IV with six samples (23.1%); we had no samples in grade V, based on the Teitelbaum classification. In the results of measuring nerve diameter with immunohistochemical S-100, using a cut-off value of ≥40μm as a reference value for nerve enlargement. We found that 22 patients (84.6%) experienced neurological hypertrophy, while four patients (15.4%) did not have an enlarged nerve diameter (Fig. 2).
Sample of neural hypertrophy diameter measurement using S-100 immunohistochemistry, with 40× magnification. The green line shows the diameter of a nerve that does not experience hypertrophy (nerve diameter<40μm). In comparison, the yellow line shows the diameter of a nerve that experiences hypertrophy (nerve diameter≥40μm).
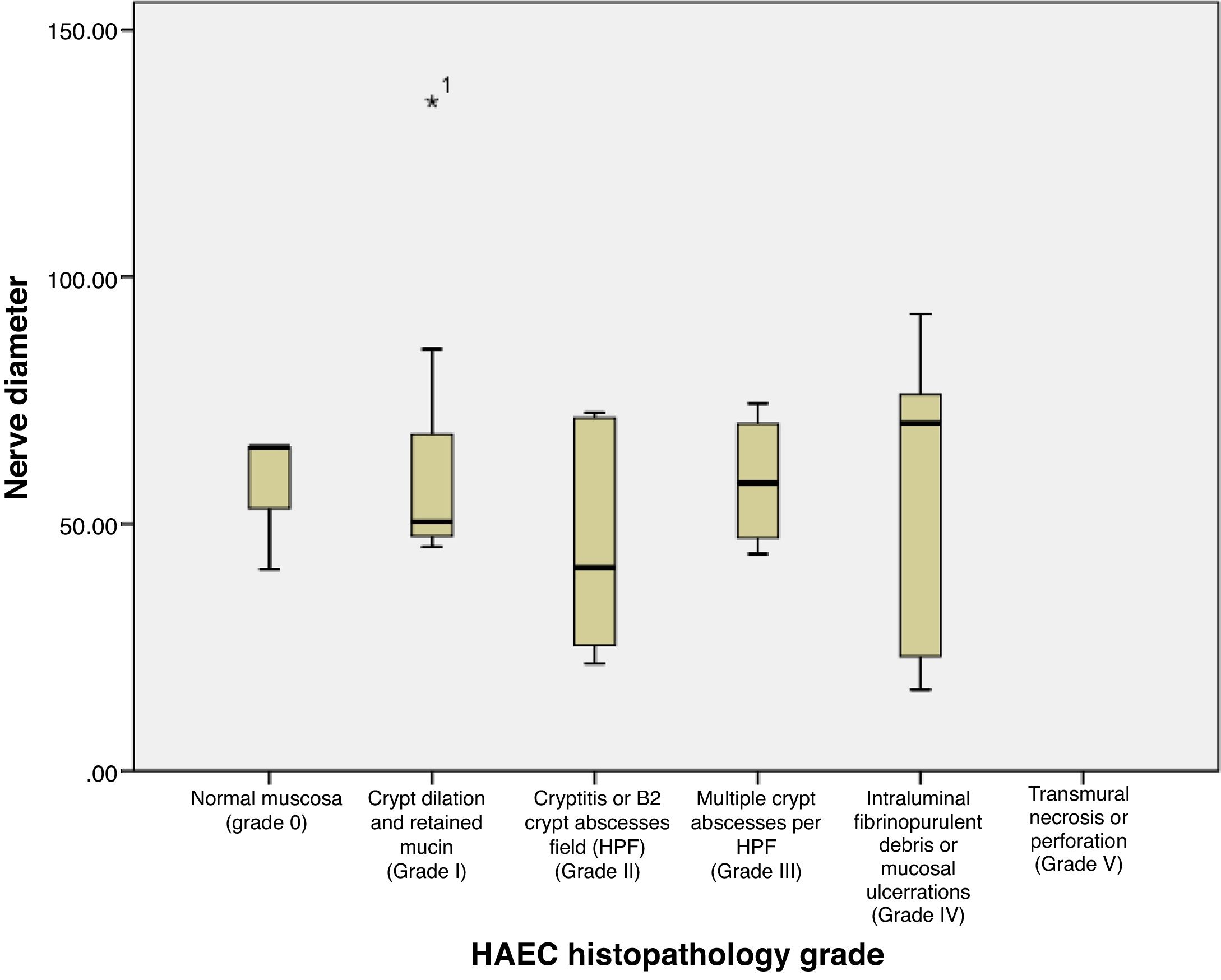
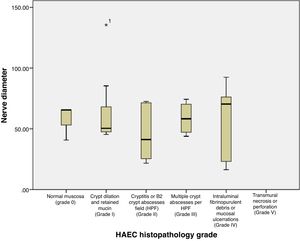
Based on Table 2 and Fig. 3, the mean±SD of nerve diameter measurements was 57.38±25.58, with the smallest nerve diameter at 16.40μm and the largest at 135.48μm. The above results indicate that there is no significant relationship between the size of the nerve diameter and the histopathological grade, with a correlation coefficient of r=0.067. Furthermore, when nerve diameters and HAEC grade were divided into two groups and a Fisher's exact test was conducted, no significant relationship was seen (p=0.136) (Table 3).
The relationship of nerve diameter to the degree of histopathology of HAEC.
| Measurement | Histopathology grade | Total | p* | r | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| Mean | 57.32 | 68.69 | 45.58 | 57.07 | 58.16 | 57.38 | 0.745 | 0.067 |
| Standard deviation | 14.31 | 36.04 | 21.98 | 12.68 | 30.96 | 25.58 | ||
| Median | 65.58 | 50.03 | 41.20 | 50.47 | 70.41 | 50.72 | ||
| Minimum | 40.80 | 45.36 | 21.70 | 43.88 | 16.40 | 16.40 | ||
| Maximum | 65.60 | 135.48 | 72.59 | 74.38 | 92.41 | 135.48 | ||
The relationship of nerve diameter to the degree of histopathology of HAEC.
| Histopathology HAEC score | p* | ||
|---|---|---|---|
| Grade<III | Grade≥III | ||
| Nerve fiber diameter | |||
| Hypertrophy | 0 (0%) | 4 (100%) | |
| Non-hypertrophy | 10 (45.5%) | 12 (54.5%) | 0.136 |
| Total | 10 (38.5%) | 16 (61.5%) | |
Hypertrophic nerve fibers have long been known as a additional way to confirm HSCR. Several previous studies have proven that a confirmed hypertrophic submucosal nerve diameter is highly correlated with HSCR. Measurement of nerve diameter can be used in conjunction with clinical symptoms when diagnosing a transition zone pull-through.10,11 In our study, using ≥40μm as the cutt-off value for enlargement of the nerve (Fig. 3), we found that 84.6% of samples had neuronal hypertrophy, either in the distal zone of the dilatation or more proximal to the transition zone during pull-through or leveling colostomy, which we considered ganglionic.
According to Kapur, standard ≥40μm nerve fibers are normal in the distal rectum after one year of age12; therefore, diagnosing transition zone pull-throughs based on nerve fibers of > 40μm is unreliable among children older than one year,13,14 the submucosal nerve becomes larger and moves more into the distal rectum as children age, as well as move higher in the rectum than in the colon (which is more proximal at any age).13,15
We had two patients with high degrees of HAEC but with a nerve diameter of <40μm. These results may be due to how nerve size cut-off values are determined (based on an age of three years) along with the biopsy's location in the colon, or possibly due to histopathological changes in HAEC. It has been reported that the cut-off value is only 40μm in the submucosal plexus,16 which has a smaller ganglion than the myenteric plexus.17 One explanation could be that myenteric nerve bonds are more related to intestinal motility, while submucosal nerves mainly have a secretory function. Therefore, it is expected that if this (40μm) is limit value for the submucosal plexus, a higher limit value is expected for myenteric plexus (68μm).16 However, some previous studies have used a cut-off value of >40μm to explain neural hypertrophy in both the submucosal and myenteric plexuses, as these ganglion cells are often obtained together and overlap in both plexuses.18
Although pathologists around the world agree that the basis for establishing an HSCR diagnosis depends on the presence or absence of ganglion cells and on hypertrophy of the nerve, the results of our study indicate that the presence or absence of hypertrophy from the nerve stem is not significant to determine on its own a correlation to varying degrees of HAEC scores. This is likely because the diameter of nerve fibers seems to change with age.14 Another study has found that histopathological changes in HAEC can not only occur in the aganglionic and transitional segments, but also affect the intestinal ganglionic segments in HSCR. This abnormality can last for a long time or can be permanent and susceptible to the incidence of postoperative enterocolitis, so that ongoing management of long-term prevention and observational action must still be conducted to combat the risk of enterocolitis.19,20
ConclusionIn our study, we found that a higher diameter of a nerve experiencing hypertrophy is correlated with a greater risk of having HAEC, but this correlation is not sufficiently significant, showing a feeble level of strength.
Ethical approvalAll procedure for this study has been approved by Ethics Commission Faculty of Medicine, Hasanuddin University Number: 265/UN4.6.4.5.31/PP36/2020.
ConsentThe research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The patients have given their written informed consent on admission to use their data base and files for research work.
Authors’ contributionsYTP, NM, FNM, UAM, AAZ, UM, TH and MF wrote the manuscript and participated in the study design. YTP, NM, AAZ, UM, TH, and MF drafted and revised the manuscript. YTP, FNM, AW, NM, UM and TH performed the treatment and surgery. YTP, AAZ, MIK, Pri, and MF performed bioinformatics analyses and revised the manuscript. All authors read and approved the final manuscript.
FundingThe authors declared that this study has received no financial support.
Conflicts of interestThe authors declare that they have no conflict of interests.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.