Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosLower Urinary Tract Symptoms (LUTS) can be a complication of ureteral stent insertion. Clinically, PDE5 inhibitors (Tadalafil) and alpha blockers (Tamsulosin) are the preferred drugs for treating lower urinary tract symptoms after ureteral stent insertion, also called ureteral stents-related symptoms (uSRS). This study aims to compare the effectiveness of 0.4mg/day Tamsulosin and 10mg/day Tadalafil for treating uSRS.
MethodsThis study used a double-blinded experimental design. Fifty patients with uSRS were randomly assigned to one of 2 groups; group I (n=25) received 10mg/day Tadalafil, while group II (n=25) received 0.4mg/day Tamsulosin. The Ureteral Symptoms Score Questionnaire (USSQ) was administered on the 7th, 14th, 21st, and 28th day after insertion of the ureteral stent. p values of <0.05 were considered to be statistically significant.
ResultsThe administration of 10mg/day Tadalafil had a good effect in reducing the LUTS score: mean scores were VS=11.03, p=4.21, GC=3.8, WA=1.29, SA=1.94, and AS=3.21. The administration of 0.4mg/day Tamsulosin also had a good effect in reducing the LUTS score: mean scores were VS=13.73, p=9.89, GC=3.85, WA=4.31, SA=0.59, and AS=4.69.
ConclusionAdministration of 10mg/day Tadalafil had significantly better results compared to 0.4mg/day Tamsulosin in improving uSRS, except in VS week 2 and in GC weeks 2 and 4.
Ureteral stent insertion can cause complications for patients, one of which is urinary complaints or Lower Urinary Tract Symptoms (LUTS), also called ureteral stents-related symptoms (uSRS). Common uSRS include frequency (50–60%), urgency (57–60%), dysuria (40%), incomplete emptying (76%), and incontinence (25%). Discomfort associated with a ureteral stent may be caused by mucosal bladder irritation (especially the trigonum), coils in the bladder, smooth muscle spasms, and urine reflux.1 One study has revealed that uSRS can begin the second week after stent insertion.1 Other studies have shown that the international prostate symptom score (IPSS) in patients with stent insertion increases in the first week after insertion and decreases after the ureteral stent is removed.2
In a study conducted by Shalaby et al. (2013) compared 4 groups: no therapy (Group I), 0.4mg Tamsulosin (Group II), 10mg Solifenacin (Group III), and a combination of Tamsulosin-Solifenacin (Group IV). There was a significant difference between scores of all experimental groups compared to the control group (p value<0.005). Additionally, Group IV showed statistically significant differences in total IPSS, quality of life scores, and OAB-q scores compared to groups II and III (p-values<0.001).3
In other research, a group with Solifenacin had total symptom, urgency, and incontinence scores that were significantly lower than the control group.4 In a study with patients receiving either 10mg of Tadalafil or a placebo once a day for 3 weeks, the Tadalafil group had significantly lower urgency, frequency, dysuria, hematuria, and incomplete emptying of the bladder.5
This study aims to compare the effectiveness of 0.4mg of Tamsulosin 0.4 per day and 10mg of Tadalafil per day in treating uSRS in Makassar, Indonesia.
MethodsThis study was conducted in a referral hospital in eastern Indonesia. The study used a double-blind experiment design. The sample consisted of 50 participants, selected based on the entry queue in the hospital (consecutive random sampling). Participants who were included were treated at a central referral hospital in eastern Indonesia and were recruited based on admission order to the hospital in June–July 2019. The Ureteral Symptoms Score Questionnaire (USSQ) was administered on the 7th, 14th, 21st, and 28th day (weeks 1, 2, 3, and 4, respectively) after the insertion of the ureteral stent.
Inclusion criteria: willing to undergo voluntary research; age 18–79 years old; has indications of endoscopic ureteral stent insertion; is diagnosed with ureteral stones <10mm (with or without dilatation of pelvic, calix, ureter), ureteral stenosis, and/or kidney stones and will undergo shockwave lithotripsy (ESWL); has had the first insertion of ureteral stent; and has indications for the unilateral ureteral stent insertion. Exclusion criteria: history of malignancy in the urinary tract; history of hypertrophic prostate (prostate enlargement more than 20cc in the ultrasonogram); history of previous sexual dysfunction; having had a urinary tract infections (UTI) in the previous 6 months; pregnancy; history of diabetes mellitus, cardiovascular disease, or hypertension; having or undergoing radiation therapy, hormonal therapy, and/or minor pelvic surgical procedures; previous surgery of ureteral reconstruction; history of alcoholism; history of stroke; having Alzheimer's disease; having central nervous system trauma; having accompanying stones in the bladder; history of urethra diverticula in women; and hypersensitivity to Tadalafil or Tamsulosin.
The participating uSRS patients were randomly assigned to one of 2 groups: The participating uSRS patients were randomly assigned to one of 2 groups: Group I (n=25) received 10mg of Tadalafil per day, and group II (n=25) received Tamsulosin 0.4mg/day (both started from day-7 to day-28 after insertion of ureteral stent). Observation and data collection were conducted for four weeks, with evaluation of six complaints of the lower urinary tract (LUTS) that cause discomfort in patients: voiding symptoms (VS) score, pain (P) score, general condition (GC) score, work activity (WA) score, sexual activity (SA) score, and additional symptoms (AS) score.
Data were analyzed using the SPSS 21.0 program (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.), with the significance level set at p<0.05. Ethical approval was obtained from the local Institutional Review Board, No. 686/UN4.6.4.5.31/PP36/2019.
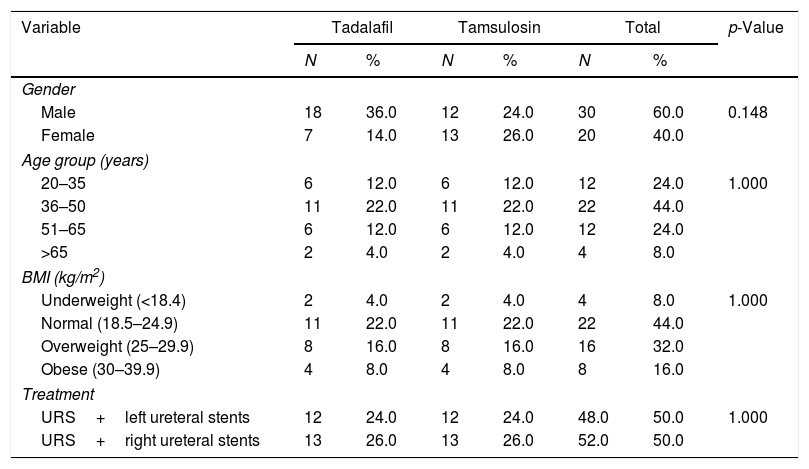
ResultsTable 1 shows that in the total sample, 30 participants (60.0%) were male and 20 (40.0%) were female. The most represented age group of the samples was ages 36–50 years (44%), while the smallest age group was >65 years (4%). In total, 52% of patients underwent ureteroscopy (URS) and right ureteral stent insertion, while 48% of patients underwent a URS and left ureteral stent insertion. Based on the Body Mass Index (BMI) measures in the two groups studied, many patients had a healthy body weight, with a BMI of 18.5–24.9 (44.0%).
Patient characteristics.
| Variable | Tadalafil | Tamsulosin | Total | p-Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Gender | |||||||
| Male | 18 | 36.0 | 12 | 24.0 | 30 | 60.0 | 0.148 |
| Female | 7 | 14.0 | 13 | 26.0 | 20 | 40.0 | |
| Age group (years) | |||||||
| 20–35 | 6 | 12.0 | 6 | 12.0 | 12 | 24.0 | 1.000 |
| 36–50 | 11 | 22.0 | 11 | 22.0 | 22 | 44.0 | |
| 51–65 | 6 | 12.0 | 6 | 12.0 | 12 | 24.0 | |
| >65 | 2 | 4.0 | 2 | 4.0 | 4 | 8.0 | |
| BMI (kg/m2) | |||||||
| Underweight (<18.4) | 2 | 4.0 | 2 | 4.0 | 4 | 8.0 | 1.000 |
| Normal (18.5–24.9) | 11 | 22.0 | 11 | 22.0 | 22 | 44.0 | |
| Overweight (25–29.9) | 8 | 16.0 | 8 | 16.0 | 16 | 32.0 | |
| Obese (30–39.9) | 4 | 8.0 | 4 | 8.0 | 8 | 16.0 | |
| Treatment | |||||||
| URS+left ureteral stents | 12 | 24.0 | 12 | 24.0 | 48.0 | 50.0 | 1.000 |
| URS+right ureteral stents | 13 | 26.0 | 13 | 26.0 | 52.0 | 50.0 | |
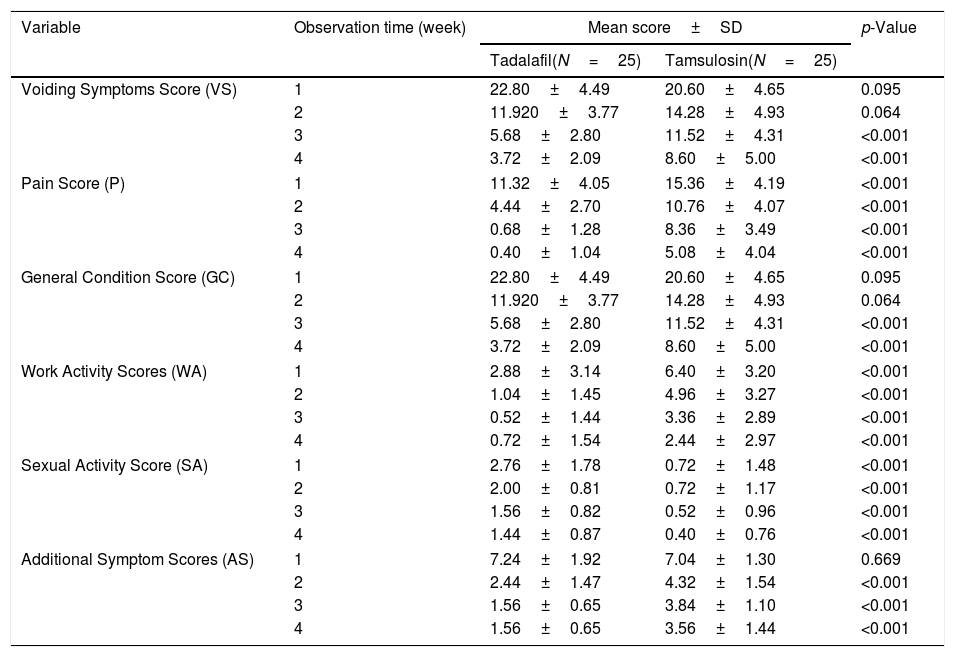
Table 2 shows the results of the USSQ scores of the participants, according to group and to week of assessment.
Score value in uSRS.
| Variable | Observation time (week) | Mean score±SD | p-Value | |
|---|---|---|---|---|
| Tadalafil(N=25) | Tamsulosin(N=25) | |||
| Voiding Symptoms Score (VS) | 1 | 22.80±4.49 | 20.60±4.65 | 0.095 |
| 2 | 11.920±3.77 | 14.28±4.93 | 0.064 | |
| 3 | 5.68±2.80 | 11.52±4.31 | <0.001 | |
| 4 | 3.72±2.09 | 8.60±5.00 | <0.001 | |
| Pain Score (P) | 1 | 11.32±4.05 | 15.36±4.19 | <0.001 |
| 2 | 4.44±2.70 | 10.76±4.07 | <0.001 | |
| 3 | 0.68±1.28 | 8.36±3.49 | <0.001 | |
| 4 | 0.40±1.04 | 5.08±4.04 | <0.001 | |
| General Condition Score (GC) | 1 | 22.80±4.49 | 20.60±4.65 | 0.095 |
| 2 | 11.920±3.77 | 14.28±4.93 | 0.064 | |
| 3 | 5.68±2.80 | 11.52±4.31 | <0.001 | |
| 4 | 3.72±2.09 | 8.60±5.00 | <0.001 | |
| Work Activity Scores (WA) | 1 | 2.88±3.14 | 6.40±3.20 | <0.001 |
| 2 | 1.04±1.45 | 4.96±3.27 | <0.001 | |
| 3 | 0.52±1.44 | 3.36±2.89 | <0.001 | |
| 4 | 0.72±1.54 | 2.44±2.97 | <0.001 | |
| Sexual Activity Score (SA) | 1 | 2.76±1.78 | 0.72±1.48 | <0.001 |
| 2 | 2.00±0.81 | 0.72±1.17 | <0.001 | |
| 3 | 1.56±0.82 | 0.52±0.96 | <0.001 | |
| 4 | 1.44±0.87 | 0.40±0.76 | <0.001 | |
| Additional Symptom Scores (AS) | 1 | 7.24±1.92 | 7.04±1.30 | 0.669 |
| 2 | 2.44±1.47 | 4.32±1.54 | <0.001 | |
| 3 | 1.56±0.65 | 3.84±1.10 | <0.001 | |
| 4 | 1.56±0.65 | 3.56±1.44 | <0.001 | |
For complaints of voiding symptoms, administration of treatment in the first 2 weeks did not show any significant difference. However, at weeks 3 and 4, a significant difference was seen, wherein the patients given Tadalafil (Group I) showed a decrease in VS score compared to the patients given Tamsulosin (Group II) (p<0.001).
For pain complaints, significant differences were seen throughout the entire study, wherein Group I showed a greater decrease in the average P score greater than Group II (p<0.001).
For complaints of general conditions, the score differences fluctuated every week: in the first week, Group II more significantly reduced complaint scores, but in the second week, there did not appear to be any difference between the two groups. In the third and fourth weeks of observation, Group I showed a greater decrease in the average GC score than Group II (p<0.001).
For complaints of work activities, a significant difference was seen for the entirety of the study, wherein Group I showed a greater decrease in the mean WA score than Group II (p<0.001).
For complaints of sexual activity, significant differences were seen throughout the entire study, wherein Group I showed a greater decrease in the mean SA score than Group II (p<0.001).
For complaints about additional symptoms, administration of treatment in the first week did not show a significant difference. However, in all subsequent weeks, a significant difference was seen, wherein Group I showed a greater decrease in AS scores than Group II (p<0.001).
DiscussionIn terms of demographic variables, male participants were 60.0% of our sample. The age groups with the greatest numbers of participants were ages 36–50 years (44.0%) and ages 51–65 years (24.0%), with an average age of 41 years. Meanwhile, URS ureteral stents installation is slightly more dominant on the right side, occurring in 52.0% of the cases.
This result is similar to the research by Pansota et al., who examined the indications and complications of eighty ureteral stent installations in Pakistan.6 Their study had a plurality of patients (44.0%) ages 36–50 years, with a male/female ratio of 1.5:1. Of eighty patients who had a ureteral stent installed because of the obstructive uropathy of the upper region, most received a ureteral stent after surgery. Additionally, Pansota et al. reported that the most common cause of obstructive uropathy was kidney stone disease, whether ureteric or combined (87.5%); the remaining 12.5% of patients experienced Ureteropelvic Junction (UPJ) obstruction, carcinoma, and pregnancy.6
In this study, several side effects were experienced after the ureteral stents were inserted at our institution, including complaints related to voiding, pain, general conditions, work activities, sexual activity, and additional symptoms. Pain is considered to be a result of urinary trigonal irritation by the coil in the bladder when it passes through the midline or forms an incomplete loop. A previous randomized clinical trial showed that urgency symptoms and urinary pain are more common with longer stents but have a negative impact on patients’ quality of life. Additionally, kidney colic is associated with ureteral stent movement in the ureter and with ureteral spasms. Pain is also thought to occur due to distension of the kidney capsule due to urine reflux through the ureteral stent, symptoms that are also caused by urinary tract infections and encrustation.7
The results of the current study indicate that therapy using 0.4mg of Tamsulosin per day for 3 weeks in patients with ureteral stents insertion can reduce ongoing complaints related to voiding symptoms (58.25%), pain (64.62%), general conditions (69.87%), work activity (61.87%), sexual activity (44.44%), and additional symptoms (49.43%). The greatest effectiveness in Tamsulosin therapy at 0.4mg/day for 3 weeks in patients with ureteral stents is in reducing complaints of general conditions (69.87%).
The results of this study are in line with prospective studies conducted at Stanley Government Hospital from February 2013 to January 2014. In this study, a total of 180 patients were randomly divided into two groups. The group of patients prescribed with non-selective α adrenergic receptors (such as Tamsulosin) after ureteral stent insertion experienced significant benefits, as the symptoms and complaint that they experienced were far lower than those experienced by controls. Patients who received 0.4mg of Tamsulosin after the insertion of a ureteral stent showed lower overall symptoms and also showed a significant reduction in the symptoms of their initial complaints. Specifically, the patients who received Tamsulosin experienced a significant reduction in irritation scores, pelvic pain, and complaints of voiding symptoms.8
At the same time, the results of the current study indicate that therapy using 10mg of Tadalafil per day for 3 weeks in patients with a ureteral stent can reduce ongoing complaints related to voiding symptoms (83.68%), pain (96.47%), general conditions (95.83%), work activity (81.94%), sexual activity (47.82%) and additional symptoms (78.45%). The greatest effectiveness of therapy using 10mg of Tadalafil per day for 3 weeks in patients who received ureteral stents is in reducing pain complaints (96.47%).
The results of this study are in line with a study in which 72 patients underwent 5mg Tadalafil therapy and experienced lower/fewer urinary tract symptoms related to stents, pain, sexual function, and overall scores. Tadalafil therapy can be used as a treatment modality to reduce lower urinary tract symptoms and improve quality of life in patients. In that study, patients taking Tadalafil were more able to engage in strenuous activity and had less pain during sexual intercourse, which increased their overall satisfaction compared to the placebo group. Tadalafil not only reduced pain during urination, but also reduced genital pain that affected PDE receptors on the bladder neck and trigonum. Tadalafil also improved patients’ status of sexual performance and sexual satisfaction.9
The current study compared the effectiveness of 0.4mg of Tamsulosin versus 10mg of Tadalafil in patients who had received ureteral stents. The results showed significant differences in scores of voiding symptoms, pain, complaints of general conditions, complaints of work activities, complaints of sexual activity, and additional symptoms after the administration of therapy for 3 weeks. The results show that Tadalafil was more effective than Tamsulosin in reducing LUTS in all observed complaints; thus, Tadalafil is considered a more effective drug than Tamsulosin in reducing LUTS after the insertion of a ureteral stent in our hospital in Makassar.
These results are in line with the results of a study of 144 patient with ureteral stent symptoms, randomized into two groups with 72 patients each. For 3 weeks, Group I patients were given 5mg Tadalafil, and Group II patients were given 0.4mg Tamsulosin. Tadalafil was found to be more effective than Tamsulosin in reducing body aches and in increasing general health index scores, performance, and sexual function scores. Tadalafil was found to be more effective than Tamsulosin in relieving body aches and sexual symptoms and in improving general condition and work activity, but t was less effective in improving urinary symptoms.10
Other studies have suggested that Tadalafil and Tamsulosin are comparable in eliminating complaints of urinary symptoms, general condition complaints, and work activity complaints, but Tadalafil is more effective than Tamsulosin in relieving body aches, sexual problems, and additional problems.
Other studies have indicated that 0.4mg Tamsulosin and 5mg Tadalafil specifically can reduce urinary symptoms. Studies comparing the safety and effectiveness of antimuscarinic (Solifenacin), alpha-blockers (Tamsulosin), and PDE inhibitors (Tadalafil) in eliminating uSRS have found that all drugs are useful in reducing scores in all domains of the USSQ, compared to placebo.11 However, a prospective randomized study conducted from January 2015 to March 2017 found different results. Patients in Group I received 10mg Tadalafil, while patients in Group II received 0.4mg Tamsulosin. While Tamsulosin and Tadalafil are equally effective in reducing stent-related symptoms, Tadalafil has fewer side effects than Tamsulosin.12
Tamsulosin is an α1-blocker that causes a significant decrease in peak contraction pressure, which causes ureteral dilatation.13–15 Alpha blockers relieve pelvic pain by reducing muscle spasms and intrarenal urine reflux. Irritative symptoms (frequency, dysuria, and urgency) can be improved due to blocked alpha receptors in the bladder trigone. Tamsulosin selectively antagonizes α1A- and α1D-adrenergic receptors, inhibiting contraction of smooth muscle in the distal ureter, bladder trigonum, and neck, thus eliminating LUTS and pelvic pain.3,16,17 Additionally, as an α1-adrenergic receptor antagonist, Tamsulosin reduces ureteral spasm, bladder outlet resistance, and pressure canceling,3,18 thereby reducing urinary symptoms and pain related to urine reflux. Reduction in symptoms and pain in the urinary process leads to an increase in work activity, general conditions, and sexual function scores.10
Tadalafil, associated with Phosphodiesterase-5 (PDE5) inhibitors in sexual dysfunction, eliminates urinary tract obstruction related to lower urinary tract obstruction (LUTS).19 The PDE5 receptor is present in the lower ureter, trigone, and bladder neck. Tadalafil relaxes the ureter by blocking the PDE5 receptor present in the lower ureter, thereby reducing spasms and reflux. By blocking the PDE5 receptors in the bladder trigone and neck, Tadalafil can overcome irritation symptoms.20,21 Tadalafil is effective in relieving urinary symptoms and body aches and increasing sexual health, general condition, and work activity. Increased improvement in sexual symptoms can be caused by resolution of erectile dysfunction and decreased body aches.16 PDE5 receptors are present in the bladder, prostate, proximal urethra, and ureter; PDE5 inhibitors cause smooth muscle relaxation in these organs.20 Tadalafil (PDE5 inhibitor) has been approved by the FDA for the treatment of erectile dysfunction and LUTS because of this muscle relaxation activity.20 Relaxation of the smooth muscle in the lower ureter and bladder trigone decreases symptoms and urinary pain. Improved sexual health with Tadalafil can be associated with decreased pain, improved urinary symptoms, and positive effects on underlying erectile dysfunction.19,22
Tadalafil was found to be more effective in relieving body aches, promoting better sexual health, improving work activity and general condition scores than Tamsulosin.22 Tadalafil is a safe, effective drug that can be used as the first choice in relieving symptoms related to ureteral stents and related problems, especially in sexually active patients.10
ConclusionIn this study, the highest LUTS score that appeared in patients with uSRS was voiding symptoms complaints, followed by complaints of pain, general condition, work activities, sexual activity, and additional symptoms. The administration of 10mg Tadalafil per day is significantly effective in reducing the average LUTS score, but administering 0.4mg Tamsulosin per day is also effective in reducing the average LUTS score. Statistical test results show that the Tadalafil treatment was more effective than the Tamsulosin treatment day in improving uSRS.
Conflict of interestThe authors declare no conflict of interest.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.