Anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) is a rare and life-threatening autoimmune disease. Immunoadsorption (IA) is a potential approach in treating AAV.
Patients and methodsA 76-year-old male patient was admitted with hemoptysis and oliguria, progressed rapidly into pulmonary hemorrhaging, acute kidney damage, and multi-organ failure. He was diagnosed as MPO-ANCA-positive vasculitis by immunological detection and kidney biopsy in the case report. IA combined with methylprednisolone to induce and alleviate the disease effectively, and cyclophosphamide (0.2g every other day, a total of 1g for the first time, after the patients tolerated, 10mg/kg every 3 weeks for 6 months in total) combined with prednisone for maintenance therapy.
Results and discussionAlthough both kidneys suffered severe deterioration requiring long-term hemodialysis replacement therapy, their pulmonary function was restored. Furthermore, clinical and serological symptoms of the disease were successfully controlled. Consequently, IA treatment may quickly remove IgG and ANCA to efficiently control clinical symptoms, especially in patients presenting with alveolar hemorrhaging and acute renal failure.
La vasculitis asociada a anticuerpos frente al citoplasma de los neutrófilos (ANCA) es una enfermedad autoinmunitaria poco frecuente y potencialmente mortal. La inmunoadsorción (IA) constituye un posible tratamiento para dicha enfermedad.
Pacientes y métodosVarón de 76 años que ingresó por hemoptisis y oliguria, que progresó rápidamente a hemorragia pulmonar, insuficiencia renal aguda y fallo multiorgánico. Fue diagnosticado de vasculitis asociada a ANCA y mieloperoxidasa positivos mediante análisis inmunológico y biopsia renal. Se trató inicialmente con IA combinada con metilprednisolona y ciclofosfamida, junto a prednisona de mantenimiento.
Resultados y discusiónSi bien el grado de deterioro de la función renal requirió terapia de sustitución con hemodiálisis a largo plazo, la función pulmonar se recuperó completamente. Los síntomas clínicos y serológicos se controlaron con éxito. En conclusión, el tratamiento con IA puede constituir una terapia capaz de eliminar rápidamente IgG y ANCA para el control eficaz de los síntomas clínicos, especialmente en pacientes que presentan hemorragia alveolar e insuficiencia renal aguda.
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare systemic autoimmune disease characterized by necrotizing inflammation of blood vessels infiltrated by neutrophils. It includes three distinct entities: granulomatosis with polyangiitis (GPA, formerly Wegener's granulomatosis), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA).1 Clinical symptoms of AAV are both diverse and varied. It can affect the respiratory tract, eyes, kidneys, skin, and nervous system. In some patients, renal insufficiency may progressively worsen to end-stage renal disease (ESRD). Patients experiencing respiratory issues may experience massive hemorrhage and asphyxiation. Diseases that progress rapidly are commonly associated with high clinical misdiagnosis rates and mortality, creating an urgent need for accurate diagnosis and timely therapy. Since, ANCA has direct pathogenicity; its levels are closely related to disease activity and recurrence.2 Thus, reducing ANCA levels is an extremely important measure for controlling the disease.
Plasma exchange (PE) may be a good method to eliminating ANCA, which reduces organ damage caused by AAV.3 However, the scarcity of blood resources restricts the viability of its application, and the effect of immunosuppressive therapy alone is relatively slow. Immunoadsorption (IA) is another possible approach that can be used to efficiently remove pathogenic antibodies or circulating immune complexes from contaminated plasma. It is characterized by strong selectivity, high efficiency, avoidance of disease transmission, and retention of useful plasma components, and it may be more effective than PE. Unfortunately, there are not enough reports on the efficacy of IA in AAV cases. This article evaluates the clinical therapeutic effect of IA when combined with glucocorticoids and immunosuppressive therapy in an AAV patient.
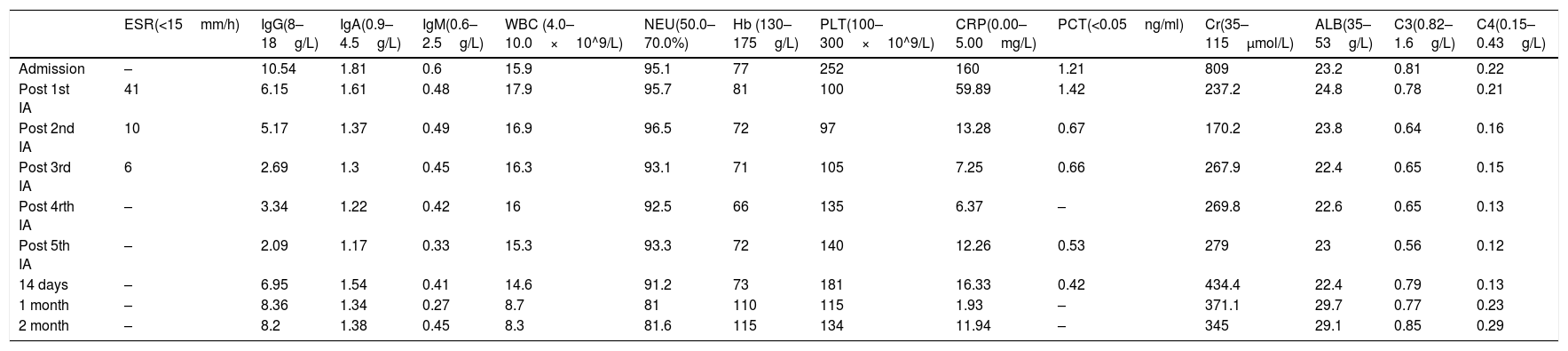
A 76-year-old man (height, 158cm; weight, 64.2kg) was admitted with fever, chest tightness, and oliguria. Three months prior to admission, he developed a serious cough and hemoptysis. He was diagnosed with a pulmonary infection, and his symptoms improved after receiving medical treatment at his local hospital. At the time of arrival, his creatinine level was 102μmol/L. However, this time the patient was found to be suffering from severe lung damage with pulmonary hemorrhage, which progressed rapidly to acute respiratory failure. At the same time, acute renal failure caused creatinine levels to spike up to 809μmol/L (Table 1). As a result, the patient was intubated and mechanically ventilated, and hemodialysis was initiated. Further workups revealed that both perinuclear-ANCA and anti-myeloperoxidase (MPO) antibody IgG were positive. At this point, the patient was finally diagnosed with AAV causing kidney damage due to microvasculitis, acute renal failure, alveolar hemorrhage, acute respiratory failure, and pulmonary infection.
Changes in various indicators of the patient during the whole treatment.
| ESR(<15mm/h) | IgG(8–18g/L) | IgA(0.9–4.5g/L) | IgM(0.6–2.5g/L) | WBC (4.0–10.0×10^9/L) | NEU(50.0–70.0%) | Hb (130–175g/L) | PLT(100–300×10^9/L) | CRP(0.00–5.00mg/L) | PCT(<0.05ng/ml) | Cr(35–115μmol/L) | ALB(35–53g/L) | C3(0.82–1.6g/L) | C4(0.15–0.43g/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission | – | 10.54 | 1.81 | 0.6 | 15.9 | 95.1 | 77 | 252 | 160 | 1.21 | 809 | 23.2 | 0.81 | 0.22 |
| Post 1st IA | 41 | 6.15 | 1.61 | 0.48 | 17.9 | 95.7 | 81 | 100 | 59.89 | 1.42 | 237.2 | 24.8 | 0.78 | 0.21 |
| Post 2nd IA | 10 | 5.17 | 1.37 | 0.49 | 16.9 | 96.5 | 72 | 97 | 13.28 | 0.67 | 170.2 | 23.8 | 0.64 | 0.16 |
| Post 3rd IA | 6 | 2.69 | 1.3 | 0.45 | 16.3 | 93.1 | 71 | 105 | 7.25 | 0.66 | 267.9 | 22.4 | 0.65 | 0.15 |
| Post 4rth IA | – | 3.34 | 1.22 | 0.42 | 16 | 92.5 | 66 | 135 | 6.37 | – | 269.8 | 22.6 | 0.65 | 0.13 |
| Post 5th IA | – | 2.09 | 1.17 | 0.33 | 15.3 | 93.3 | 72 | 140 | 12.26 | 0.53 | 279 | 23 | 0.56 | 0.12 |
| 14 days | – | 6.95 | 1.54 | 0.41 | 14.6 | 91.2 | 73 | 181 | 16.33 | 0.42 | 434.4 | 22.4 | 0.79 | 0.13 |
| 1 month | – | 8.36 | 1.34 | 0.27 | 8.7 | 81 | 110 | 115 | 1.93 | – | 371.1 | 29.7 | 0.77 | 0.23 |
| 2 month | – | 8.2 | 1.38 | 0.45 | 8.3 | 81.6 | 115 | 134 | 11.94 | – | 345 | 29.1 | 0.85 | 0.29 |
ESR: erythrocyte sedimentation rate; IgG: immunoglobulin G; IgA: immunoglobulin A; IgE: immunoglobulin E; WBC: white blood cell; NEU: neutrophilic granulocyte percentage; Hb: hemoglobin; PLT: platelet; CRP: C-reactive protein; PCT: procalcitonin; Cr: creatinine; ALB: albumin; C3: Complement C3; C4: Complement C4.
IA was chosen to selectively remove immunoglobulins and immune complexes from the plasma. The patient began IA treatment eight days after admission, and received five sessions over eight days. 3.6L of plasma was processed during the initial session and 4.8L at each of the following four sessions. Simultaneously, the therapeutic regimen was combined with 500mg of methylprednisolone intravenously for three days and then reduced to a daily intravenous dose of 40mg. Imipenem cilastatin sodium to fight infection as well as other symptomatic treatments were administered. As the patient's condition became stable, intravenous cyclophosphamide (0.2g every other day, a total of 1g for the first time, after the patients tolerated, 10mg/kg every 3 weeks for 6 months in total) combined with low-dose prednisone therapy was administered to control the disease, and compound sulfamethoxazole was used to prevent infection.
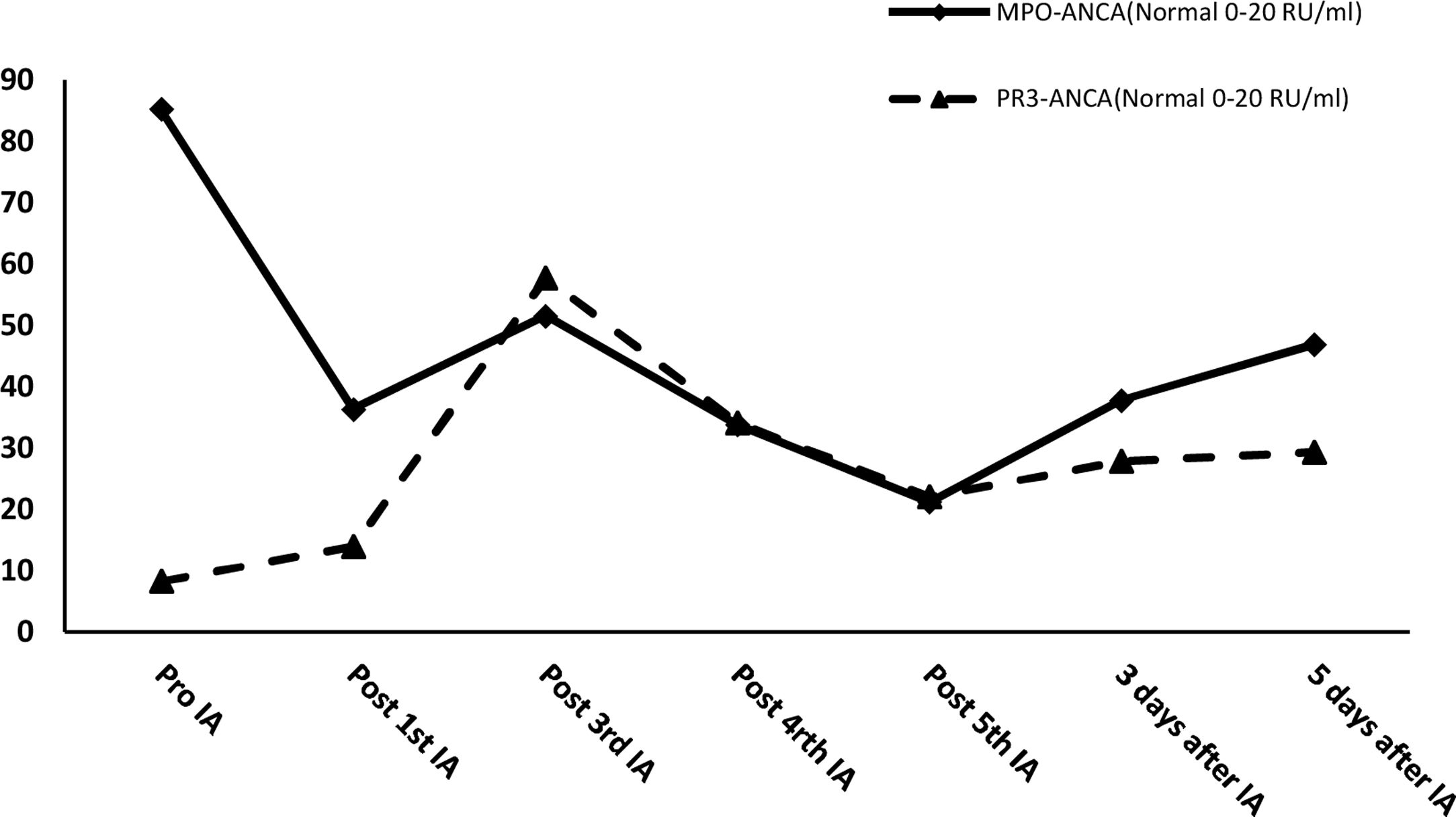
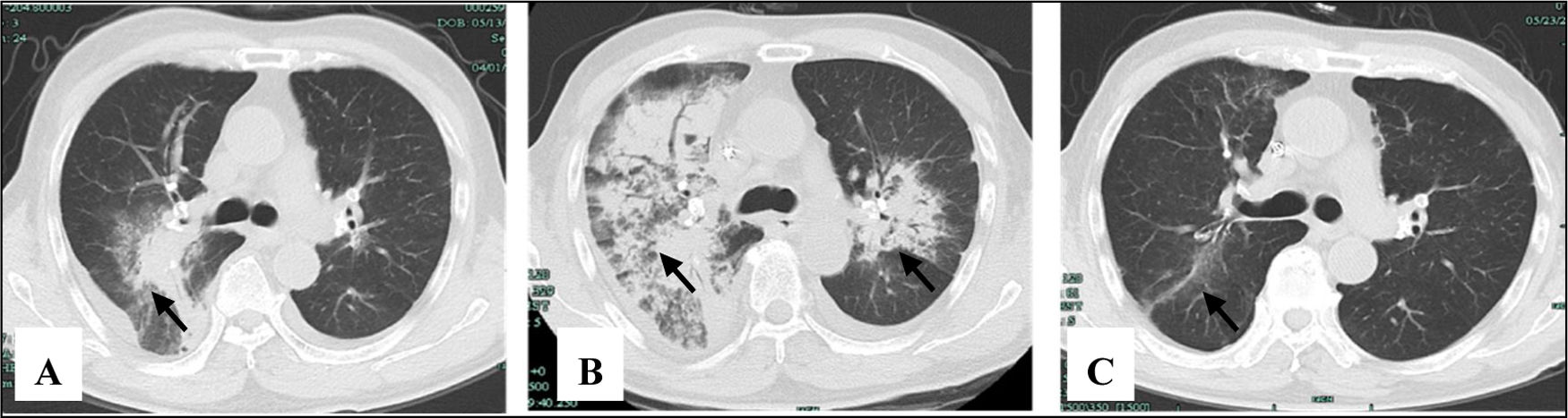
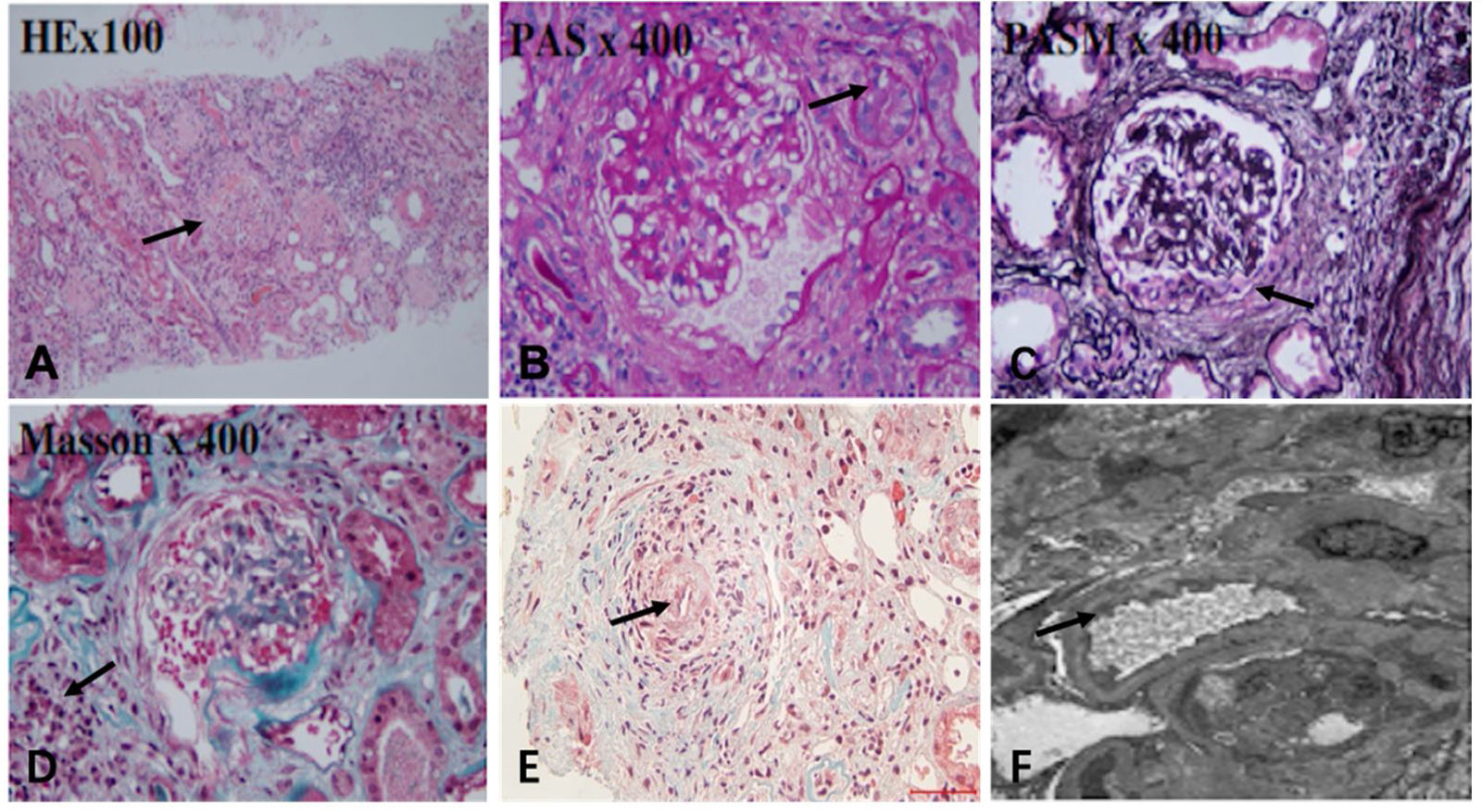
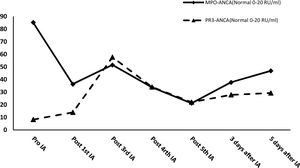
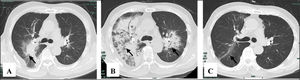
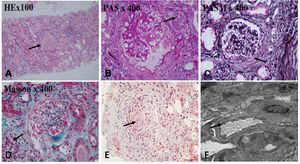
After effective treatment, patient's IgG levels dropped significantly (Table 1), and treatment was further supplemented with immunoglobulins to enhance immune function in preventing infection. MPO-ANCA titer decreased as well (Fig. 1). Although the levels did not reach the standard values, the patient's clinical symptoms significantly improved. After the initial treatment with IA, a remarkable improvement in alveolar hemorrhage was seen, and there was no need for mechanical ventilation, therefore, the patient was extubated and given high-flow oxygen. Pulmonary imaging revealed that alveolar hemorrhage had noticeably resolved, but a few areas of interstitial fibrosis remained (Fig. 2). The patient required long-term hemodialysis because his renal failure was irreversible and kidney biopsy showed necrotizing glomerulonephritis with more than 50% crescents (Fig. 3).
The pulmonary imaging before and after IA. (A) Admission: there were some exudative lesions (arrow) in the right lungs. (B) Before IA: multiple infiltration of the lower lobes of both lungs (arrow). (C) The 35th day after IA: the exudative lesions were absorbed, but a few areas of interstitial fibrosis remained (arrow).
The renal biopsy findings of the patient. Renal biopsy was routinely stained with HE, PAS, PASM, Masson. (A) 50% of glomerulus sclerosis (arrow), (B) vacuolar and granular degeneration of renal tubular epithelium(arrow), (C) crescent formation(arrow), (D) multifocal inflammatory cell infiltration in the renal interstitium with fibrosis (arrow), (E) thickening of the arterial wall, segmental hyaline degeneration, and suspicious fibrinoid necrosis of one of the arterioles(arrow). (F) There was no obvious thickening of the glomerular basement membrane, extensive fusion of the foot processes (arrow), and no exact electron dense deposits were found.
In this case report, the patient was MPO-ANCA-positive AAV, which had mainly affected the lungs and kidneys. The clinical symptoms were successfully controlled by IA combined with the appropriate immunosuppressant treatment. Pulmonary function had almost recovered completely despite interstitial fibrosis persisting in some places, which could progress to delayed interstitial pneumonia. However, the kidneys were severely damaged and the patient required long-term hemodialysis replacement therapy. If the patient had been accurately diagnosed with AAV when clinical symptoms were first reported, the degree of damage to the organs would have been smaller qualitatively. The patient is currently being treated with cyclophosphamide, corticosteroids, and hemodialysis. Although the ANCA titer did not turn negative, the clinical symptoms and serological disease parameters were successfully controlled.
A successful AAV therapeutic regimen includes remission induction and maintenance therapy to decrease the recurrence rate and morbidity, and improve renal prognosis, quality of life, and survival.4,5 In this case, IA treatment was combined with methylprednisolone to alleviate the disease symptoms, and cyclophosphamide was combined with prednisone for maintenance therapy. PE may play an active role in induction therapy. Although some clinical trials have indicated that PE cannot reduce the risk of mortality or development of ESRD in patients with severe AAV,6 most studies support the effectiveness of PE. European League against Rheumatism/The European Renal Association–European Dialysis and Transplant Association recommends the use of PE in patients with severe renal insufficiency or diffuse alveolar hemorrhage.5 PE may also result in loss of coagulation factors, albumin, and immunoglobulins. Moreover, the total treated volume of plasma is limited, and many complications, including bleeding, allergies, and infections, must be considered.7 This implies that more effective and safer treatments for AAV are required. Therefore, treatment with IA was selected instead of PE.
IA may improve clinical symptoms by effectively reducing or eliminating ANCA and all other IgG subclasses. Theoretically, the largest advantage of IA is the unlimited volume of plasma that can be processed at each treatment, whereas PE is usually restricted to a finite plasma volume. Simultaneously, the antibody reduction in IA is more than 85%, which is significantly higher than that in PE (70%).8 IA reduced the level of IgG in this patient by 41.65% after the first session. After the fourth session, IgG dropped to the lowest recorded level, with its level decreasing by 74.45%, before supplementation with immunoglobulins. If immunoglobulin had not been administered, this level would have been even lower. At the same time, IA successfully controlled MPO-ANCA titers and kept them at a relatively low level. After five courses of IA, the MPO-ANCA level decreased by 75.07%. In this case, IA treatment quickly cleared the pathogenic IgG and ANCA, which resulted in rapid improvement in alveolar hemorrhage and the patient's life could be saved. This novel treatment also created an opportunity for early extubation, reducing the risk of secondary infections. However, little information is available regarding the potential use of IA in AAV. A few case reports have shown that administering IA in the early treatment of AAV is beneficial and sound clinical effects can be achieved.9 However, there is insufficient evidence regarding the superiority of IA over PE and a randomized controlled trial has not been performed to compare these therapies. A single study compared the renal response of AAV patients to IA and PE, with 78% and 65% effectiveness, respectively.10 Renal and overall survival rates were similar, and no significant difference in adverse events was found.
IA treatment may quickly remove IgG and ANCA to efficiently control clinical symptoms, especially in patients presenting with alveolar hemorrhage. Furthermore, the large volume of plasma that can be processed by IA provides an innovative approach to AAV treatment. However, further studies are needed that can confirm the therapeutic potential of IA treatment in preventing further progression of the disease with statistical confidence.
Funding statementThis work was supported by the National Natural Science Foundation of China (grant number 81971857).
Conflict of interestThe authors declare they have no conflict of interest.