COVID-19 represents a worldwide pandemic and vaccination remains the most effective preventive strategy. Among hematological patients, COVID-19 has been associated with a high mortality rate. Vaccination against SARS-CoV-2 has shown high efficacy in reducing community transmission, hospitalization and deaths related to severe COVID-19 disease. However, patients with impaired immunity may have lower sero-responsiveness to vaccination.
MethodsThis study focuses on hematopoietic stem cell transplantation (HSCT) recipients. We performed a unicenter, prospective, observational study of a cohort of 31 allogeneic and 56 autologous-HSCT recipients monitored between March 2021 and May 2021 for serological response after COVID-19 vaccination with two doses of mRNA1273 vaccine (Moderna). In order to determine seroconversion, serological status before vaccination was studied.
ResultsAt a median range of 75 days after the second vaccine dose, seroconversion rates were 84% and 85% for the autologous and allogeneic-HSCT groups, respectively. We confirmed some potential risk factors for a negative serological response, such as receiving anti-CD20 therapy in the previous year before vaccination, a low B-lymphocyte count and hypogammaglobulinemia. Neutralizing antibodies were quantified in 44 patients, with a good correlation with serological tests. Adverse events were minimal.
ConclusionmRNA1273 vaccination is safe and effective in HSCT recipients, especially in those presenting recovered immunity.
Entre los pacientes hematológicos, la COVID-19 se ha asociado a una mayor mortalidad. La vacunación frente a SARS-CoV-2 es la principal estrategia de prevención y ha demostrado eficacia en la reducción de la transmisión, de la hospitalización y de la tasa de mortalidad. Aun así, los pacientes oncohematológicos con un sistema inmunológico disfuncional podrían presentar una respuesta menor a la vacunación.
MétodosEstudio unicéntrico, prospectivo y observacional, con una cohorte de 31 receptores de un trasplante alogénico de progenitores hematopoyéticos y de 56 receptores de un trasplante autólogo que recibieron la vacunación frente a SARS-CoV-2 entre marzo de 2021 y mayo de 2021, con 2 dosis de la vacuna mRNA1273 (Moderna). Para poder determinar la tasa de seroconversión, se determinó el estado serológico previamente a la vacunación y posteriormente se monitorizó la respuesta serológica.
ResultadosCon un tiempo medio de seguimiento de 75 días después de la segunda vacuna, la tasa de seroconversión fue del 84%, y del 85% en el grupo receptor de trasplante autólogo y alogénico, respectivamente. Se confirmaron algunos potenciales factores de riesgo para la ausencia de respuesta serológica, como haber recibido terapias anti-CD20, un recuento bajo de linfocitos B y la hipogammaglobulinemia. En 44 pacientes se cuantificaron títulos de anticuerpos neutralizantes, con buena correlación con los test serológicos. Los efectos adversos de la vacuna fueron mínimos.
ConclusiónLa vacunación con mRNA1273 es segura y efectiva en los pacientes receptores de un trasplante de progenitores hematopoyéticos, especialmente en los que presentan reconstitución inmune previa.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), was declared a worldwide pandemic by the World Health Organization (WHO) on 11 March 2020.1 After two years of learning how to manage this infection, vaccination remains the most effective preventive strategy. Results from the Moderna and Pfizer-BioNTech vaccine clinical trials showed 94–95% efficacy for the prevention of symptomatic severe acute SARS-CoV-2 infection at 14 days after the second dose,2,3 and both vaccines were approved by the Food and Drug Administration (FDA) for emergency use in December 2020. However, the Pfizer and Moderna clinical trials excluded immunocompromised patients.2,3
Given the immunosuppression associated with hematopoietic stem cell transplantation (HSCT), the recipients who develop COVID-19 have a poor overall prognosis,4 with a survival at 30 days of 68% in allogeneic HSCT recipients and 67% in autologous HSCT recipients.4 Therefore, HSCT recipients have been considered a priority population for vaccination. Despite this, these patients usually remain immunosuppressed for months after transplant, due to the conditioning regimens, maintenance therapies, immunosuppressive drugs, hypogammaglobulinemia or development of graft-versus-host disease (GvHD) after allogeneic HSCT. All these factors may lead to an impaired immune response and may compromise vaccine efficacy.5 Thus, it is often recommended that patients receive vaccination about 6 months after the procedure to ensure an adequate immune reconstitution.5
Prior experience with influenza vaccines showed lower serological response in HSCT patients.6 Although patients receiving influenza vaccine at 6 months or later after HSCT have a lower risk for influenza infection, patients vaccinated earlier than 6 months after HSCT may present T-cell responses after vaccination.6 Undoubtedly, influenza vaccination has shown clinical benefit in HSCT recipients and it is specially recommended when the risk of influenza is high.
Reports on antibody response after SARS-CoV-2 vaccination in hematological patients confirm the lower antibody response rates compared to the general population in retrospective studies.7,8 The characterization of humoral and cellular response in HSCT recipients and the identification of patients with poor response could help to design more efficacious vaccination programs. The main objective of this study was to prospectively assess SARS-CoV-2 seroconversion by the detection of SARS-CoV-2-reactive IgG antibodies at 2–4 months after full vaccination of HSCT recipients with the mRNA1273 vaccine (Moderna). Safety and tolerability were also determined in this group of patients.
Materials and methodsStudy designFollowing authorization of mRNA vaccination, since March 2021 vaccination has been offered to patients undergoing consecutive autologous and allogeneic transplantation for hematological malignancies in our center from February 2015 to November 2020. At 4–72 months after transplantation, all adult patients (>18 years) gave written informed consent before study enrollment and COVID vaccination. The study was performed in accordance with the Declaration of Helsinki.
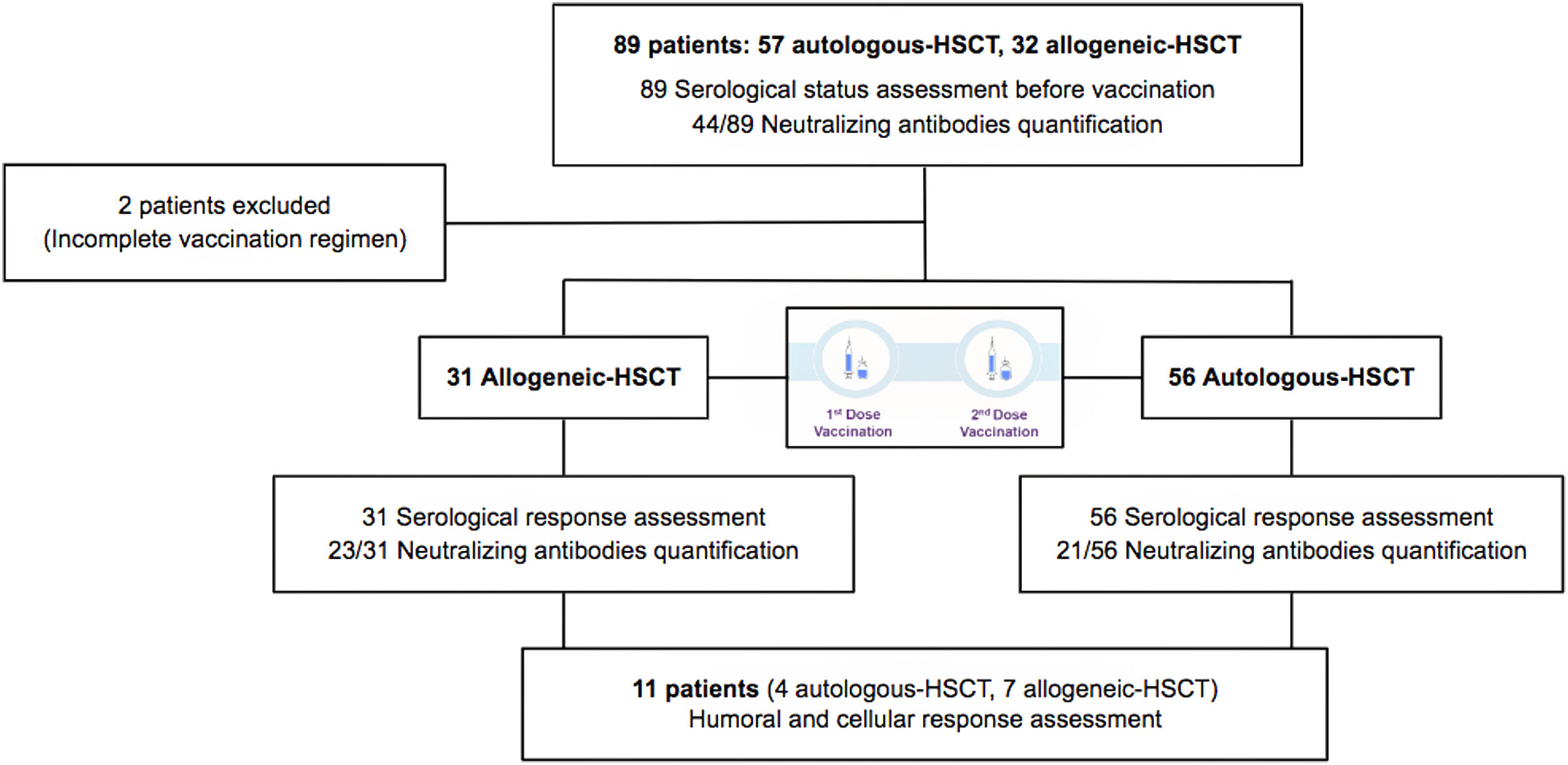
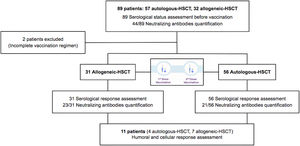
Eighty-nine HSCT recipients (57 autologous-HSCT, 32 allogeneic-HSCT) were initially included and data on the hematological disease characteristics, conditioning regimen, type of donor, GvHD prophylaxis, immunosuppressive treatment, prior history of COVID-19, date of vaccination and self-reported adverse effects after vaccination were registered (supplementary figure). Any patient didn’t receive treatment with intravenous immunoglobulins during this study.
SARS-CoV-2 serological status before vaccination and immunological status (blood count, lymphocytes subpopulations and serum immunoglobulins) were determined. Moderna vaccines were administered as two doses 4 weeks apart and serological response was assessed at 2–4 months after complete vaccination. Quantification of neutralizing antibodies against SARS-CoV-2 was performed in 44 out of 87 patients. In cases without serological response after two vaccines, we reassessed humoral and cellular response 2–6 weeks after having received a third vaccine dose.
SafetyPatients were instructed to report any suspected adverse event and were actively screened for any local reactions: pain at vaccination site, swelling or erythema, and for systemic reactions: fever, chills, fatigue, myalgia, nausea/vomiting or headache, within 30 days after vaccination.
Serology assaysEvaluation of anti-Spike IgG antibodies was performed using Covid-19 (SARS-CoV-2) IgG Elisa Quantitative® (Demeditec Diagnostics GmbH, Germany) as indicated by the manufacturer.
Quantification of neutralizing antibodies against SARS-CoV-2 was performed by HIV reporter pseudoviruses expressing SARS-CoV-2 S protein and Luciferase which were generated as previously described.9 pNL4-3.Luc.R-.E-was obtained from the NIH AIDS Reagent Program.10 SARS-CoV-2.SctΔ19 was generated (GeneArt) from the full protein sequence of the original WH1 SARS-CoV-2 spike (Genbank MN908947.3) with a deletion of the last 19 amino acids in C-terminal,11 human-codon optimized and inserted into pcDNA3.1(+). Expi293F cells were transfected using ExpiFectamine293 Reagent (Thermo Fisher Scientific, USA) with pNL4-3.Luc.R-.E- and SARS-CoV-2.SctΔ19 (WH1), at an 8:1 ratio, respectively. Control pseudoviruses were obtained by replacing the S protein expression plasmid with a VSV-G protein expression plasmid as reported previously.12 Supernatants were harvested 48h after transfection, filtered at 0.45μm, frozen, and titrated on HEK293T cells overexpressing WT human ACE-2 (Integral Molecular, USA).
Neutralization assays were performed in duplicate. Briefly, in Nunc 96-well cell culture plates (Thermo Fisher Scientific), 200 TCID50 of pseudovirus were preincubated with three-fold serial dilutions (1/60–1/14,580) of heat-inactivated plasma samples for 1h at 37°C. Then, 2×104 HEK293T/hACE2 cells treated with DEAE-Dextran (Sigma–Aldrich, USA) were added. Results were read after 48hours using the EnSight Multimode Plate Reader and BriteLite Plus Luciferase reagent (PerkinElmer, USA). The values were normalized, and the ID50 (reciprocal dilution inhibiting 50% of the infection) was calculated by plotting and fitting all duplicate neutralization values and the log of plasma dilution to a 4-parameters equation in Prism 9.0.2 (GraphPad Software, USA). This neutralization assay has been previously validated in a large subset of samples with a replicative viral inhibition assay.13
For the analysis of specific cellular immune response, we performed an in house whole-blood stimulation assay. Briefly, we incubated fresh sodium heparin whole blood with a mix of 15-mer peptides covering the sequence domain aa 689–895 from the spike (S) (PepTivator® SARS-CoV-2, Miltenyi Biotec). A positive Phytohaemagglutinin P control and a negative control with no stimulus were cultivated in parallel. After an overnight incubation, the supernatants were collected and the expression of interferon gamma (IFNγ) was measured with the LEGEND MAX Human IFN-γ ELISA kit® (BioLegend, California). Positive results were considered when concentration of IFN-γ was 10pg/mL or higher in the stimulated condition over negative control.
Statistical analysisAll the patient characteristics and study variables were described as median and range for continuous variables and frequency and percentage for categorical variables. Comparisons between groups and associations between variables were analyzed by Pearson's Chi-squared, Fisher's exact test or median test, as appropriate. The median test was used to compare antibody response and titer of neutralizing antibodies between groups. The statistical analysis was performed using SPSS (v.24). Two-sided values of p<0.05 were considered statistically significant. Graphics were obtained with GraphPad Prism software (La Jolla, CA).
ResultsInitially, 89 patients were included but two did not receive the complete vaccination regimen (one patient died due to non-COVID-19 pneumonia and one patient developed COVID-19 infection before the second dose), and were excluded from the analysis. Eighty-seven HSCT patients (56 autologous-HSCT and 31 allogeneic-HSCT) received the complete vaccination regimen including two doses between March 23rd and May 5th, 2021.
The vaccines were well tolerated with no severe adverse effects (AEs). Patients reported mild/moderate AEs after the first dose in 27% of cases (only one case with moderate AEs). After the second dose, 34% of the patients reported mild/moderate AEs, with only one case of moderate AEs. The AEs most commonly reported were local pain at the puncture site, followed by fatigue and myalgia.
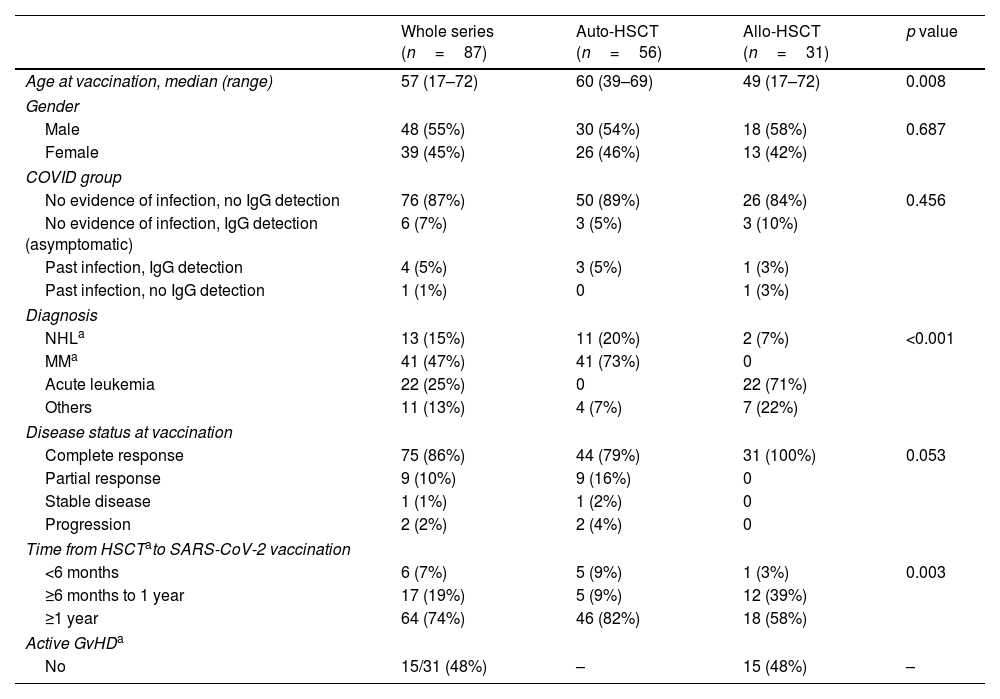
Clinical and laboratory characteristics by patient category are summarized in Table 1. The median age was 57 years (18–72). Autologous-HSCT recipients were significantly older and multiple myeloma was the most frequent indication for HSCT (n=41, 73%), followed by non-Hodgkin lymphoma (n=11, 20%). Acute lymphoblastic and myeloblastic leukemia were the most frequent diagnosis (n=22, 71%) in the allogeneic-HSCT recipients. In this group, progenitors were from a human leukocyte antigen (HLA)-identical sibling donor in 10 patients (32%), 9–10/10 matched unrelated donor in 16 cases (52%) and haploidentical related donor in 5 patients (16%).
Patient characteristics.
| Whole series (n=87) | Auto-HSCT (n=56) | Allo-HSCT (n=31) | p value | |
|---|---|---|---|---|
| Age at vaccination, median (range) | 57 (17–72) | 60 (39–69) | 49 (17–72) | 0.008 |
| Gender | ||||
| Male | 48 (55%) | 30 (54%) | 18 (58%) | 0.687 |
| Female | 39 (45%) | 26 (46%) | 13 (42%) | |
| COVID group | ||||
| No evidence of infection, no IgG detection | 76 (87%) | 50 (89%) | 26 (84%) | 0.456 |
| No evidence of infection, IgG detection (asymptomatic) | 6 (7%) | 3 (5%) | 3 (10%) | |
| Past infection, IgG detection | 4 (5%) | 3 (5%) | 1 (3%) | |
| Past infection, no IgG detection | 1 (1%) | 0 | 1 (3%) | |
| Diagnosis | ||||
| NHLa | 13 (15%) | 11 (20%) | 2 (7%) | <0.001 |
| MMa | 41 (47%) | 41 (73%) | 0 | |
| Acute leukemia | 22 (25%) | 0 | 22 (71%) | |
| Others | 11 (13%) | 4 (7%) | 7 (22%) | |
| Disease status at vaccination | ||||
| Complete response | 75 (86%) | 44 (79%) | 31 (100%) | 0.053 |
| Partial response | 9 (10%) | 9 (16%) | 0 | |
| Stable disease | 1 (1%) | 1 (2%) | 0 | |
| Progression | 2 (2%) | 2 (4%) | 0 | |
| Time from HSCTato SARS-CoV-2 vaccination | ||||
| <6 months | 6 (7%) | 5 (9%) | 1 (3%) | 0.003 |
| ≥6 months to 1 year | 17 (19%) | 5 (9%) | 12 (39%) | |
| ≥1 year | 64 (74%) | 46 (82%) | 18 (58%) | |
| Active GvHDa | ||||
| No | 15/31 (48%) | – | 15 (48%) | – |
Serological status was determined in all patients at day 0 before vaccination. Serological response was assessed in all the patients, while neutralizing antibody quantification was performed in 44 patients (23 allogeneic-HSCT and 21 autologous-HSCT). Most of the patients (87%) had no prior history of COVID-19 infection and presented a negative serological status. Six patients without a prior history of COVID-19 infection presented a positive serological status (had had asymptomatic infection). Five patients presented a previously documented COVID-19 infection (positive polymerase chain reaction – PCR-test) but only 4 showed seropositivity.
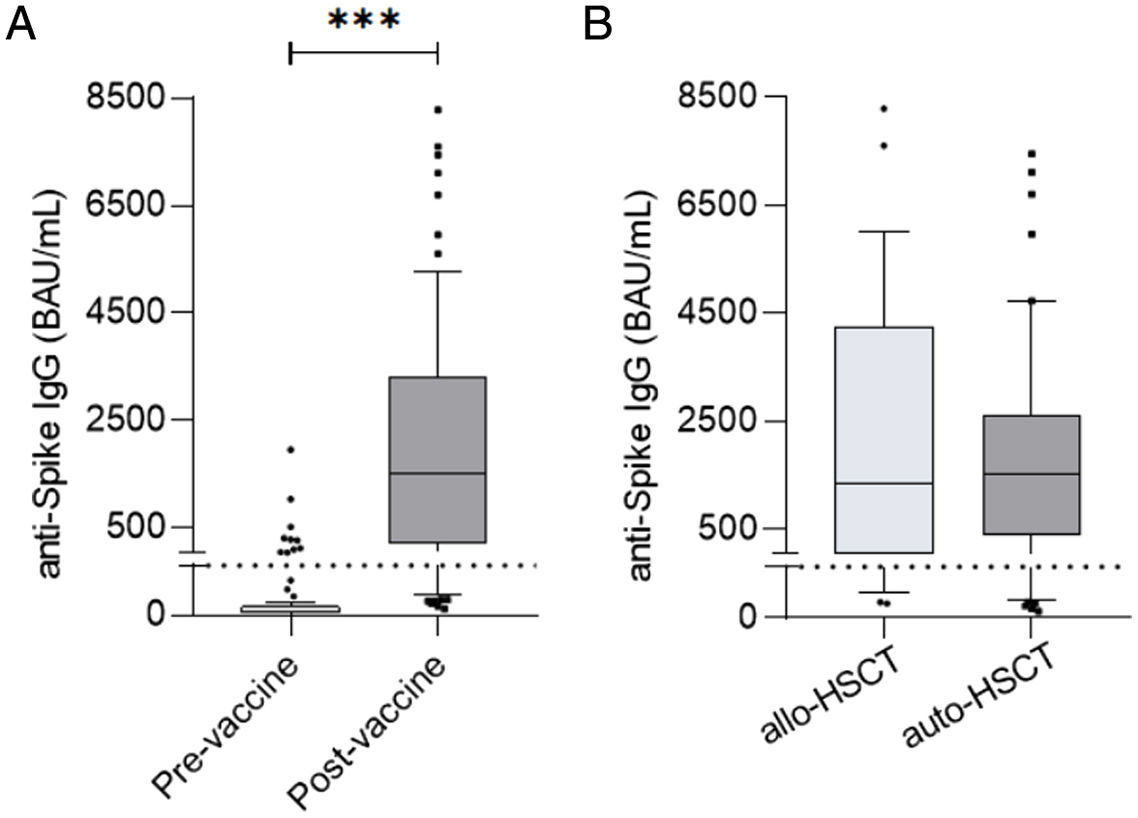
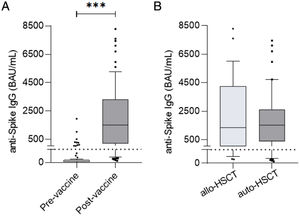
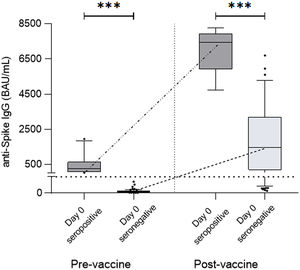
At a median time of 75 days (33–176) after complete regimen vaccination, 75/87 (86%) patients presented a positive serological response (seropositivity). Levels of specific IgG after vaccination were significantly higher compared to pre-vaccine levels (57.43±245.3 vs 5759±16,902, p<0.0001) (Fig. 1a). No statistical differences were detected in positivity percentages and specific IgG levels between the allogeneic-HSCT and autologous-HSCT groups (86% vs 87%, p=1.000; 2578±13,060 vs 1674±18,628, p=0.4909) (Fig. 1b).
(a) Antibody titers at day 0 (pre-vaccine) and post-second dose (post-vaccine) of all participants in the study (p<0.05, p<0.0001). (b) No significant post-vaccine differences were detected between allogeneic-HSCT and autologous-HSCT patients (p=0.4909). The dotted line indicates the cutoff value of positivity, 39BAU/mL.
Regarding seroconversion, 65/77 (84%) patients with a previous negative serological status presented positive serological status with no differences between the autologous-HSCT (84%) and allogeneic-HSCT (85%) groups. Concerning the 65 patients with seroconversion, the antibody response rate was higher in the allogeneic-HSCT group, with a median of 2578BAU/mL (range: 40.8–42,793.41BAU/mL), compared to 1675.5 (range: 67.2–10,686.09BAU/mL) for autologous-HSCT patients, but without significant differences (p=0.384).
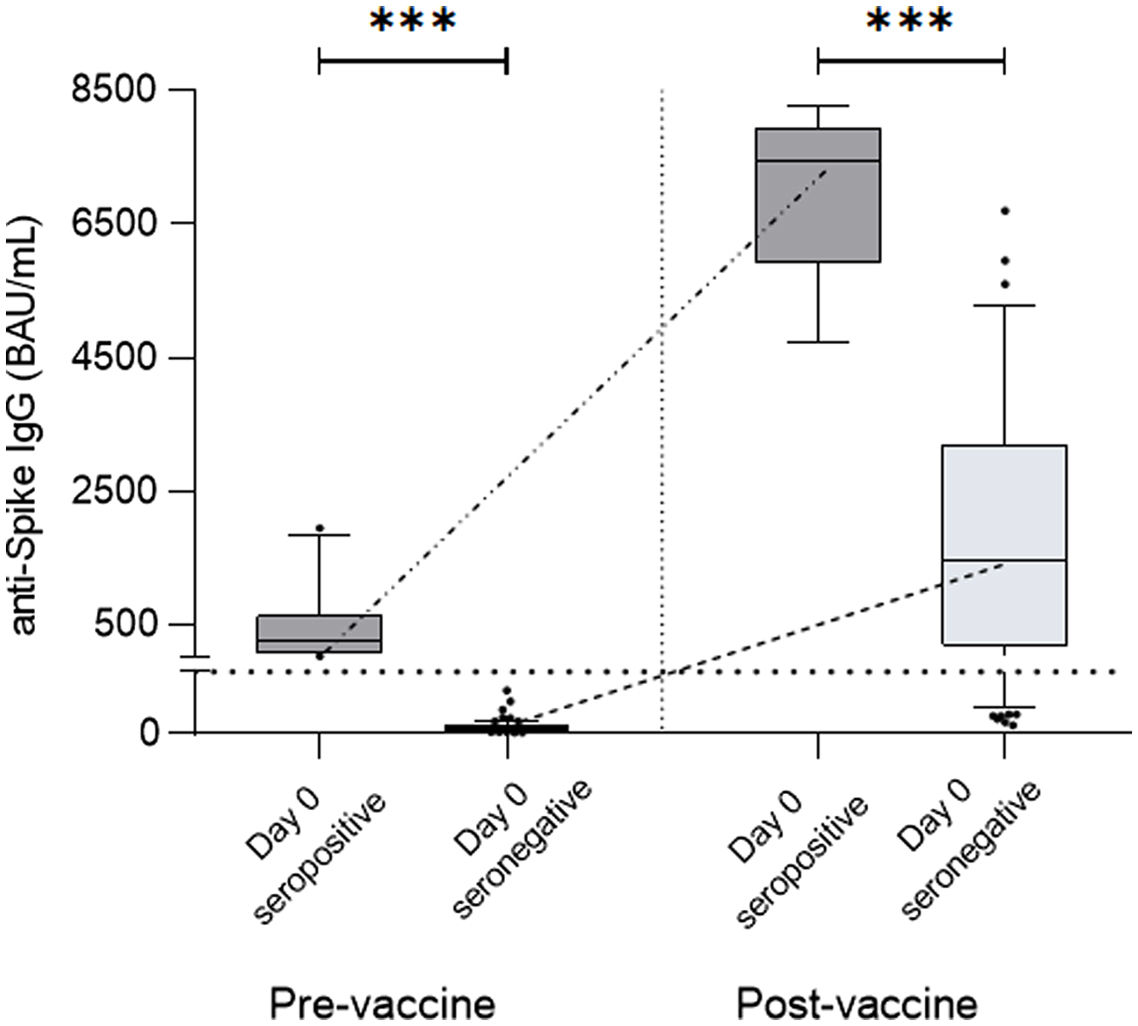
When comparing seropositive and seronegative patients before vaccination, seropositive patients at the basal time-point achieved higher levels of specific antibodies after complete vaccination compared to non-infected seronegative patients (12,488±42,410 vs 1482±5394BAU/mL, p<0.0001) (Fig. 2).
Antibody titers from pre-vaccine seropositive patients (Day 0 seropositive) compared to non-seropositive patients (Day 0 seronegative). These two groups were compared before vaccination, with Day 0 seropositive patients presenting significantly higher titers of specific IgG compared to Day 0 seronegative patients (278.00±601.60 vs. 2.68±4.25BAU/mL, p<0.0001) and after vaccination, at which time these differences were maintained or even increased (12,488±42,410 vs. 1482±5394BAU/mL, p<0.0001).
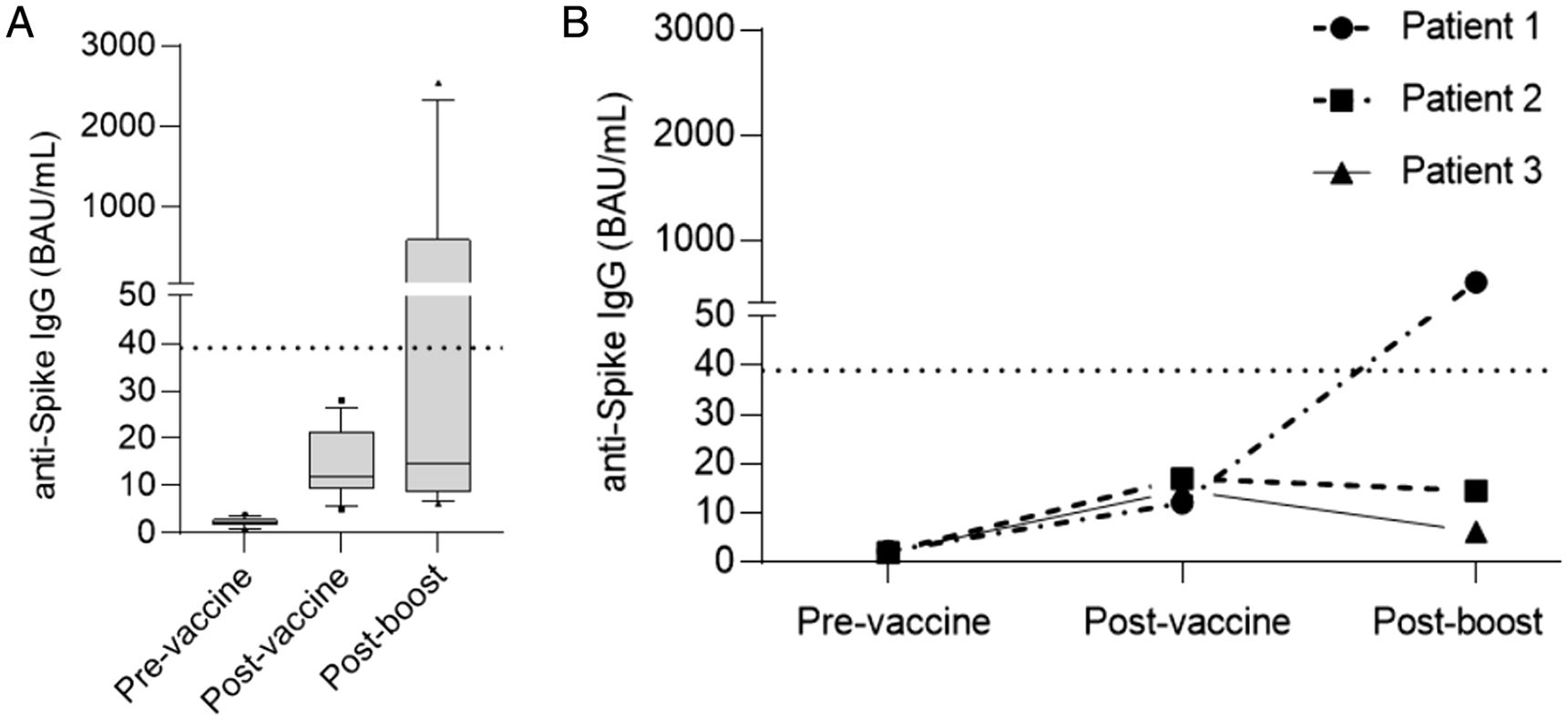
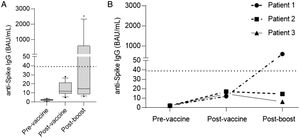
Twelve patients showed a persistent negative serological status after vaccination: 8 patients had received an autologous-HSCT (6 with non-Hodgkin lymphoma and 2 with multiple myeloma). Eleven of the 12 patients without seroconversion received a third dose of the vaccine. At 2–6 weeks after the third dose, humoral and cellular response were reassessed. Five of the 11 patients achieved seroconversion and 6/11 patients presented cellular response, two without seroconversion (Fig. 3a).
(a) Serology levels in non-seroconverters at the basal time-point, after two doses of vaccine and after the booster. Note that IgG levels rose after two doses of vaccine compared to pre-vaccine levels but still remained below the cutoff level of positivity (dotted line 39BAU/mL). Five patients achieved seroconversion after the booster dose. (b) Specific IgG levels in patients treated with anti-CD20 therapy. Only one achieved seropositivity after the booster.
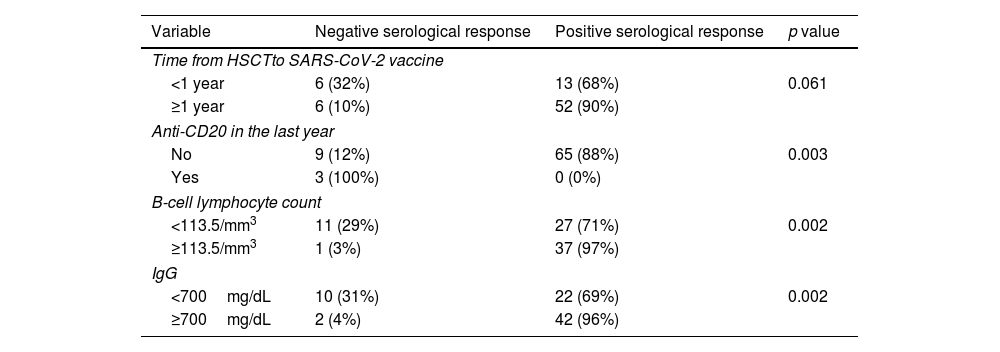
We found an association between negative serological response and lower B-cell lymphocyte count, hypogammaglobulinemia and receiving anti-CD20 therapy in the last year before vaccination (Table 2). Only 3% of the patients with a B-cell lymphocyte count≥113.5/mm3 presented a negative serological response versus 29% of the patients with a B-cell lymphocyte count<113.5/mm3 (p=0.002); 4% of the patients with IgG≥700mg/dL presented a negative serological response versus 31% of the patients with IgG<700mg/dL (p=0.002); 100% of the patients who had received anti-CD20 therapy in the last year before vaccination presented a negative serological response versus 12% of the patients who did not receive anti-CD20 (p=0.003). One of these patients was able to induce specific antibodies after the booster (Fig. 3b).
Association of patient characteristics with negative serological response.
| Variable | Negative serological response | Positive serological response | p value |
|---|---|---|---|
| Time from HSCTto SARS-CoV-2 vaccine | |||
| <1 year | 6 (32%) | 13 (68%) | 0.061 |
| ≥1 year | 6 (10%) | 52 (90%) | |
| Anti-CD20 in the last year | |||
| No | 9 (12%) | 65 (88%) | 0.003 |
| Yes | 3 (100%) | 0 (0%) | |
| B-cell lymphocyte count | |||
| <113.5/mm3 | 11 (29%) | 27 (71%) | 0.002 |
| ≥113.5/mm3 | 1 (3%) | 37 (97%) | |
| IgG | |||
| <700mg/dL | 10 (31%) | 22 (69%) | 0.002 |
| ≥700mg/dL | 2 (4%) | 42 (96%) | |
HSCT: hematopoetic stem cell transplantation.
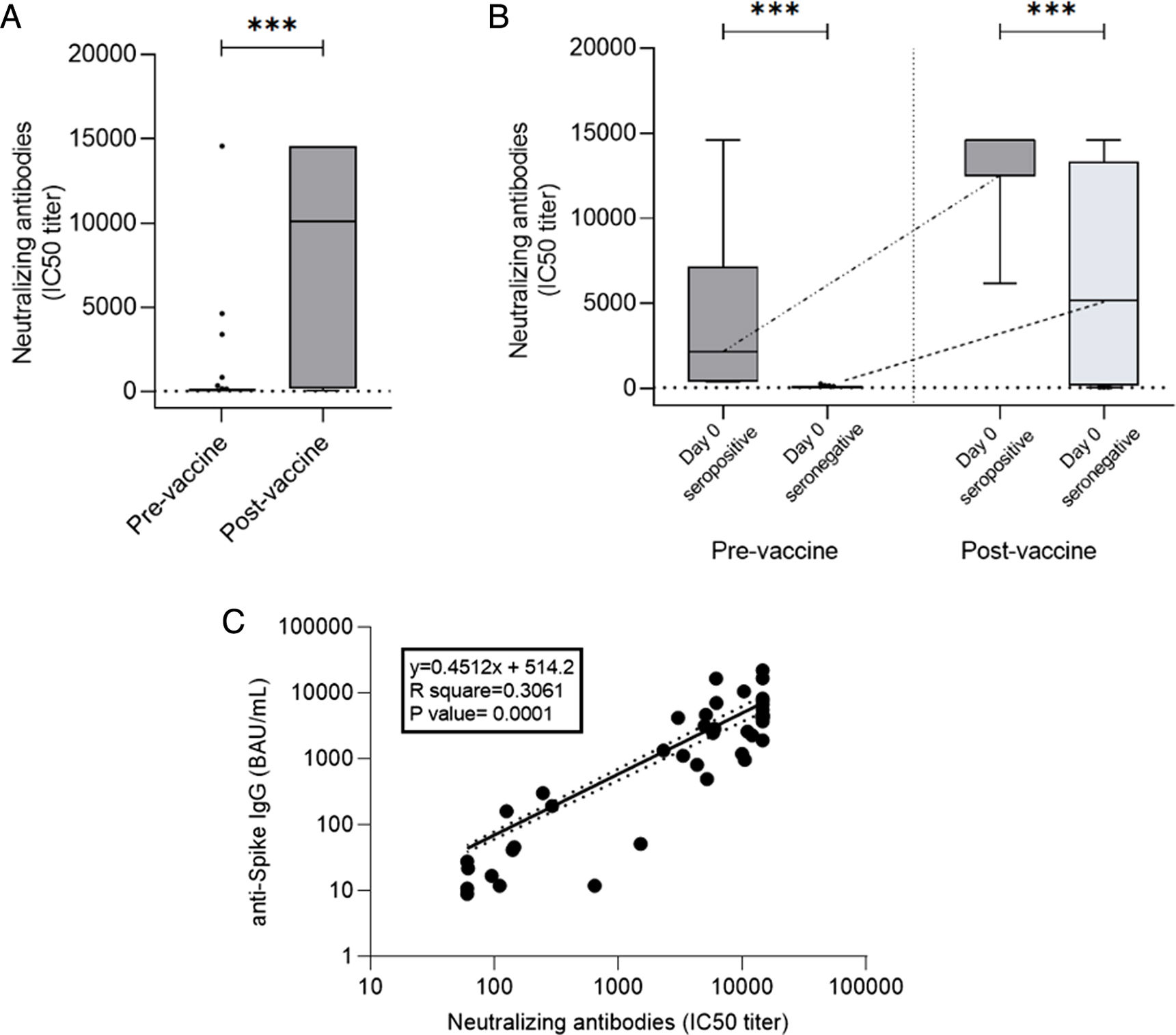
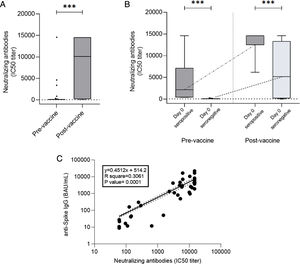
Quantification of neutralizing antibodies against SARS-CoV-2 was performed in 44/87 patients. In one case the analysis could not be performed because the patient presented a previous HIV infection. Neutralizing antibody quantification was determined before vaccination in 43 patients, with seven presenting a positive result. Among the 36 patients with an initial negative result, 27 (75%) reached seroconversion with two vaccine doses. The mean titer of neutralizing antibodies after two doses was 8224±6375BAU/mL measured as 50% inhibitory concentration (IC50) neutralizing capacity titer without significant differences between the autologous and allogeneic HSCT groups. There was also a good correlation between serological response and neutralizing antibody determination (p=0.0001), with only one case of negative serological response with a positive value for neutralizing antibodies and 4 cases with a positive serological response without detection of neutralizing antibodies (Fig. 4).
(a) Neutralizing antibodies pre-vaccine and post-vaccine (907±2877 vs 8224±6375, p<0.0001). (b) Neutralizing antibody titers from pre-vaccine seropositive patients (Day 0 seropositive) compared to non-seropositive patients. (Day 0 seronegative) before vaccination (4042±5452 vs 72.54±38.37, p<0.0001) and after vaccination (13,179±3433 vs 6551±5825, p=0.0064). Note that the kinetics of neutralizing antibodies were similar to those observed with total anti-Spike IgG. (c) Correlation of total specific and neutralizing antibodies (p=0.0001).
After a median follow-up of 385 (34–412) days after the second dose, COVID-19 infection was reported in one of the 87 patients. This patient was diagnosed with COVID-19 five months after the third vaccine dose and presented a pauci-symptomatic infection. The patient didn’t receive treatment with convalescent plasma. Two deaths were reported by non-COVID-19 pneumonia in both cases. Two of the 31 allogeneic-HSCT recipients presented a GvHD exacerbation at 6 months after complete vaccination.
DiscussionThis study prospectively evaluated the safety profile and response to the mRNA1273 vaccine (Moderna) in a consecutive cohort of HSCT patients. We found that in this population the vaccine was safe and, similarly to previously reported data,14 AEs were mild and resolved within a short time. Contrarily to our study, most previous reports have been retrospective and some patient selection effect cannot be ruled out.
Most HSCT recipients responded to the vaccine, although serological response after mRNA SARS-CoV-2 vaccination was lower than that reported in the general population (>98%).15 Regarding efficacy, our findings are consistent with recent experiences in oncohematological patients receiving mRNA vaccines,7,16,17 which detected >75% of seroconversion. The response rate in our cohort of autologous and allogeneic HSCT patients was 84% and 85%, respectively, which is higher than what has been observed in solid organ transplant recipients (antibody response of 15% after 1 dose and 54% after 2 doses).18 Specifically, previous studies reported the development of antibodies following 2 doses of the vaccine in only 18% of heart transplant recipients19 and 38% of kidney transplant recipients.20 The response rate in patients with chronic lymphocytic leukemia was 55% among patients without previous treatment and 16% in patients under treatment at the time of vaccination.21
Following HSCT patients are often immunosuppressed due to the conditioning regimens, maintenance therapy, persistent hypogammaglobulinemia, GvHD or persistent requirements of immunosuppressive drugs (in case of allogeneic-HSCT). These have been identified as strong risk factors for poor responses to vaccination.22 However, we found no significant differences between autologous-HSCT and allogeneic-HSCT recipients, as has previously been described.16 Despite the absence of differences in serological response, antibody response rate was higher in the allogeneic-HSCT group. Some sensible differences were detected between auto and allogeneic recipients that could explain the difference in the antibody response rate: autologous-HSCT recipients were significantly older and with higher humoral immunosuppression due to more frequent use of anti-lymphocyte therapies.
Patients with low lymphocyte counts have also poorer responses, as reported by Sharma et al.,4 with a worse survival for the patients with an absolute lymphocyte count of 0.3×109/L or less. Likewise, our study confirmed receiving anti-CD20 therapy in the last year before vaccination, a low B-lymphocyte count (<113.5/mm3) and hypogammaglobulinemia (IgG<700mg/dL) as potential risk factors for a negative serological response. Interestingly, intensive immunosuppressive therapy and active GvHD did not predict response to the vaccine. Nonetheless, we cannot exclude that this could be related to the limited number of patients that received allo-HSCT.
GvHD exacerbation was noted in 2/87 patients (about 6%) of the patients after allogeneic-HSCT, similar to previous study results,23 and was easily controlled. Studies performed in healthy individuals showed that, concurrent with the production of neutralizing antibodies and the stimulation of virus-specific T cells, there was a marked release of immunomodulatory cytokines, which may alter T cell function and induce or exacerbate GvHD.23,24 Similarly, in patients with immunological disbalance such as autoimmune diseases, COVID vaccine administration has been related to inflammatory flares of the disease.25 In our study, the two patients with GvHD exacerbation required systemic steroids to control the flare and the exacerbations were resolved within several days-weeks. In a recent report, three patients presented with worsening of GvHD and two experienced a novo onset GvHD in a cohort of 87 patients receiving allogeneic-HSCT exposed to COVID-19 vaccination. Moreover, in this study, allogeneic-HSCT recipients who experienced an adverse reaction to mRNA COVID-19 vaccination are more likely to present durable SARS-CoV-2 specific immune responses. Concomitantly, it has been noted that systemic adverse reactions seem to be more predictive of immunoreactivity compared with local adverse reactions.26
Pre-vaccine seropositive patients demonstrated higher levels of anti-Spike IgG titers after two vaccine doses compared to patients with negative serology at the basal time-point. These results are consistent with studies that show greater and broader humoral response in infected patients plus two doses of the vaccine compared to those without infection receiving the vaccines.27 This suggests that after infection, HSCT patients can achieve immunological memory similar to that observed in healthy infected people, and can show stronger immune response to vaccines.
As reported in previous studies, there seems to be a good correlation between serological response and neutralizing antibody determination. Jian et al.28 demonstrated that neutralizing titers were significantly correlated with IgG levels in convalescent patients and, moreover, neutralizing antibodies remained positive in the long term, depending on the age, sex, and/or disease severity.
We recognize several limitations in our study, especially in relation to the small number of patients and the lack of an immunocompetent control group. In addition, we could only determine neutralizing antibody response in only 46 patients and T-cell response was analyzed after a third vaccination dose in only 11 patients.
The absence of SARS-CoV-2 infections among our patients is due to the protective effect of the vaccine, but also HSCT recipients are usually more respectful with social distancing, mask wearing and handwashing than the general population. The protective effect of the SARS-CoV-2 vaccine against the new variants has not been fully established in the HSCT population. However, a lower protective efficacy can likely be expected compared to the general population.29 More studies in HSCT patients are needed to evaluate the final benefit of recurrent vaccination, especially in those patients that do not achieve positive serological status. This may be particularly warranted taking into account that patients with positive SARS-CoV-2 serology have recently shown infection susceptibility albeit with generally less severe disease.
Although serological response to vaccination is lower than in the general population, a robust polyfunctional memory T-cell and serological immune responses induced after a second dose of vaccine have been reported in HSCT recipients, suggesting that repeated vaccination in these patients improves immune responsiveness.30 Considering the incidence of COVID-19 infection in vaccinated patients, SARS-CoV-2 vaccination seems to have clinical benefit in preventing COVID-19 infection in HSCT recipients. Vaccination remains the most important effective strategy in the fight against COVID-19 and onco-hematologic patients are at higher risk of presenting severe COVID-19 and they should be considered a priority in all vaccination programs.31 Despite effectiveness degree and the duration of response of SARS-CoV-2 vaccines in onco-hematological patients are still under evaluation, transplant recipient's vaccination programs must include a complete immunization against SARS-CoV-232 and the disease status and therapeutic strategy should be considered in each case in order to maximize the chances of achieving an immunological response.
Contribution statementConceived the idea: Christelle Ferrà, Eudald Felip, Teresa Morán, Bibiana Quirant.
Reviewed medical chart and collected data: Maria Huguet, Anna Torrent, Laura Abril, Christelle Ferrà.
Performed laboratory analysis: Marc Boigues, Bibiana Quirant, Edwards Pradenas, Silvia Marfil, Benjamin Trinite, Julià Blanco.
Performed statistical analysis: Mireia Morgades.
Wrote the manuscript: Maria Huguet, Christelle Ferrà.
Interpretation of the results: Maria Huguet, Marc Boigues, Mireia Morgades, Anna Torrent, Laura Abril, Julià Blanco, Juan-Manuel Sancho, Bibiana Quirant, Christelle Ferrà.
Reviewed the manuscript: Maria Huguet, Marc Boigues, Mireia Morgades, Eudald Felip, Teresa Morán, Edwards Pradenas, Silvia Marfil, Benjamin Trinité, Marc Sorigué, Anna Torrent, Laura Abril, Julià Blanco, Juan-Manuel Sancho, Bibiana Quirant, Christelle Ferrà.
Ethical considerationsAll the patients gave written informed consent before study enrollment.
FundingNone.
Conflict of interestThe authors declare that they have no conflict of interest.
Maria Huguet, Hematology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Spain. Marc Boigues, Immunology Department, Hospital Germans Trias i Pujol, Badalona, Spain. Mireia Morgades, Hematology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Spain. Eudald Felip, Medical Oncology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Badalona Applied Research Group in Oncology, Spain. Teresa Morán, Medical Oncology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Badalona Applied Research Group in Oncology, Spain. Edwards Pradenas, IrsiCaixa AIDS Research Institute, Germans Trias i Pujol Research Institute, Badalona, Spain. Silvia Marfil, IrsiCaixa AIDS Research Institute, Germans Trias i Pujol Research Institute, Badalona, Spain. Benjamin Trinité, IrsiCaixa AIDS Research Institute, Germans Trias i Pujol Research Institute, Badalona, Spain. Marc Sorigué, Hematology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Spain. Anna Torrent, Hematology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Spain. Laura Abril, Hematology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Spain. Julià Blanco, Hematology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Spain. Juan-Manuel Sancho, Hematology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Spain. Bibiana Quirant, Hematology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Spain. Christelle Ferrà, Hematology Department, ICO Badalona – Hospital Germans Trias i Pujol, Badalona, Spain