Diabetic peripheral neuropathy (DPN) is the most dominant cause of neuropathy worldwide, and there has been no specific treatment until now. The aim of the current study was to assess the probable protective effect of empagliflozin in type 2 diabetics who are suffering from DPN.
MethodsFifty eligible type 2 diabetes mellitus (T2DM) cases with diabetic peripheral neuropathy were recruited in this study and classified into 2 groups. Group I (n=25) (control group) received placebo tablets once daily. Group II (n=25) (empagliflozin group) received empagliflozin 25mg once daily for three months. Empagliflozin efficacy was evaluated using electrophysiological studies, and HbA1c levels, the brief pain inventory short-form item (BPI-SF) score, the diabetic neuropathy symptom (DNS) score, the atherosclerotic cardiovascular disease (ASCVD) risk score, and the serum levels of neuron-specific enolase (NSE), malondialdehyde (MDA) and calprotectin (Calpro), lipid profile, and random blood glucose level (RBG).
ResultsAfter three months, comparing the results of the empagliflozin arm to the control arm showed a significant improvement in the electrophysiological studies and a significant decrease in the BPI-SF score and the mean serum levels of NSE and MDA. However, no significant difference was determined in HbA1c, Calpro, lipid profile, and RBG levels. In addition, the DNS and ASCVD risk scores were not significantly different. The NSE and MDA levels were significantly negatively correlated with the electrophysiological parameters. However, the BPI-SF score showed a non-significant difference.
ConclusionsEmpagliflozin may be a promising neuroprotective and therapeutic agent for diabetic peripheral neuropathy.
Trial registration Identifier: NCT05977465.
La neuropatía periférica diabética (NPD) es la causa más dominante de neuropatía en todo el mundo y hasta ahora no ha existido un tratamiento específico. El objetivo del presente estudio fue evaluar el probable efecto protector de la empagliflozina en diabéticos tipo 2 que padecen NPD.
MétodosEn este estudio se reclutaron 50 casos elegibles de diabetes mellitus tipo 2 (DM2) con NPD y se clasificaron en dos grupos. El grupo I (n=25) (grupo de control) recibió comprimidos de placebo una vez al día. El grupo II (n=25) (grupo de empagliflozina) recibió 25mg de empagliflozina una vez al día durante tres meses. La eficacia de la empagliflozina se evaluó mediante estudios electrofisiológicos y los niveles de hemoglobina A1c (HbA1c), la puntuación del ítem breve del inventario de dolor (BPI-SF), la puntuación de los síntomas de neuropatía diabética (DNS), la puntuación de riesgo de enfermedad cardiovascular aterosclerótica (ASCVD) y los niveles séricos de enolasa neuronal específica (NSE), malondialdehído (MDA) y calprotectina (Calpro), perfil lipídico y nivel aleatorio de glucosa en sangre (RBG).
ResultadosDespués de tres meses, la comparación de los resultados del grupo de empagliflozina con los del grupo de control mostró una mejora significativa en los estudios electrofisiológicos y una disminución significativa en la puntuación BPI-SF y los niveles séricos medios de NSE y MDA. Sin embargo, no se determinaron diferencias significativas en los niveles de HbA1c, Calpro, perfil lipídico y RBG. Además, las puntuaciones de riesgo de DNS y ASCVD no fueron significativamente diferentes. Los niveles de NSE y MDA se correlacionaron significativa y negativamente con los parámetros electrofisiológicos. Sin embargo, la puntuación BPI-SF mostró una diferencia no significativa.
ConclusionesLa empagliflozina puede ser un agente neuroprotector y terapéutico prometedor para la NPD.
Diabetic peripheral neuropathy (DPN) is considered one of the most dominant causes of neuropathy all over the world. It consists of a group of heterogeneous disorders due to the destruction of the peripheral nerves.1 The specific pathogenic mechanism of diabetic peripheral neuropathy is still unknown. Prolonged hyperglycemia, oxidative and inflammatory stress, and dyslipidemia in T2DM cases may be the most suggested pathogenesis and disease progression factors.2 It is essential for DM management to prevent or delay the progression of DPN. Precise glucose control is still the most effective approach for primary prevention and delaying the worsening of such complications. However, current antidiabetic treatment cannot achieve the required control of blood glucose levels, and occasional hyperglycemic episodes are encountered. Sodium-glucose co-transporter 2 (SGLT2) inhibitors were introduced to mitigate these hyperglycemic episodes occurring with the current therapies in diabetes mellitus.3
Empagliflozin, an oral SGLT2 inhibitor, acts via blocking glucose reabsorption through the kidney, promoting its excretion and reducing blood glucose concentrations. Empagliflozin is indicated for controlling the glycemic state in T2DM adults and reducing the risk of complications induced by hyperglycemic episodes, such as cardiovascular death in T2DM cases suffering from cardiovascular disease.4 Reportedly, empagliflozin also prevents reactive oxygen species (ROS) production and the transcription of factors involved in inflammatory mediators.5,6 Several studies on animal diabetic models have shown that SGLT2 inhibitors prevent worsening DM complications.7,8 Lee et al. reported that in diabetic rats, empagliflozin can significantly prevent hypersensitivity and the loss of skin intra-epidermal nerve fibers and mesangial matrix expansion.9 Therefore, empagliflozin reportedly decreases remodeling associated with reducing mortality and cardiovascular risks in people with diabetes.10 Also empagliflozin also reportedly to mitigate ischemia reperfusion injury, decrease MI size and microvascular obstruction, improve myocardial salvage in animals.11 Empagliflozin showed improvement in QOL in all patient.12,13 SGLT2 inhibitors may regulate autonomic neuropathy in diabetic patients.14 This clinical study aimed to assess the effect of empagliflozin in cases suffering from T2DM with DPN and not on SGLT2 inhibitor treatment. This study is the 1st prospective clinical study to evaluate the role of empagliflozin in DPN.
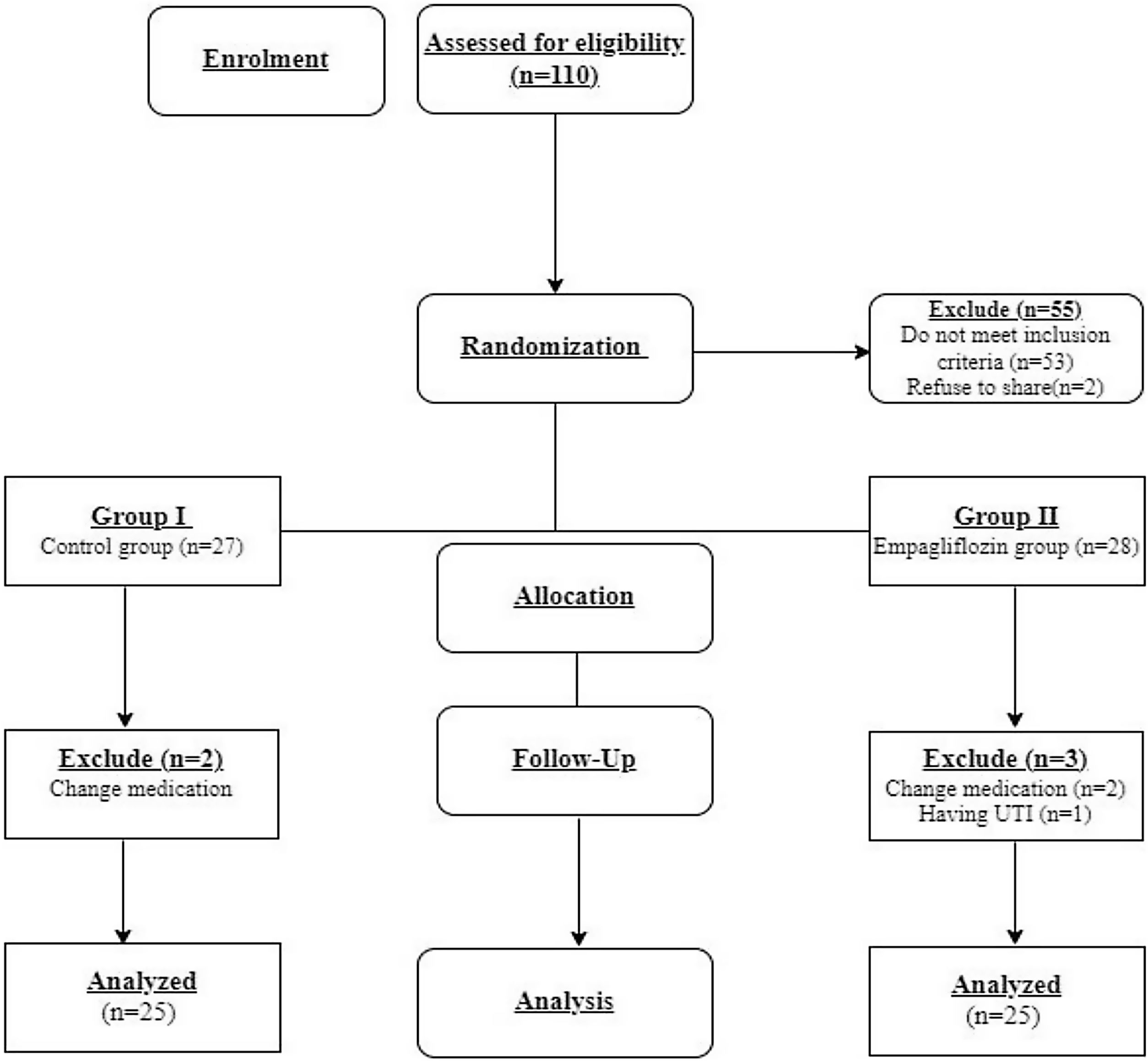
Patients and methodsPatientsFifty eligible patients were enrolled in the study as shown in Fig. 1. Group I (n=25) (control group) consisted of twenty-five patients who had received placebo tablets once daily for three months. Group II (n=25) (empagliflozin group) consisted of twenty-five patients who had received empagliflozin 25mg once daily for three months. The diagnosis was based on the initial clinical examination, especially of the lower extremities, the positivity of the diabetic neuropathy symptom (DNS) score, and the impairment of the electrophysiological studies of the lower limbs either sensory or motor abnormalities. The most common types of abnormalities in motor conduction studies include: Increased motor distal latency, slow motor nerve conduction velocity, relative slowing of motor nerve conduction velocity by comparing segment, decreased amplitude. The most common types of abnormalities seen in sensory nerve conduction study include: Increased sensory latency, slowed conduction velocity of sensory nerves, low amplitude sensory nerve action potential, absent sensory response.
Inclusion criteriaAge: ranged from 18 to 65 y. T2DM cases (HbA1c 7–9%). Patients receiving vildagliptin, metformin±basal insulin. Patients with PN and not on SGLT2 inhibitor treatment.
Exclusion criteriaAge: <18 and >65 years old, breastfeeding, pregnant female, e-GFR<45mL/min/1.73m2, T1DM cases, DKA cases, patients with UTI, dehydrated patients until normalized, lower limb amputation patients, SGLT2 inhibitor hypersensitivity, severe hepatic patients, patients on neuroprotective or neuropathic pain drugs.
Study designThis study was established as a single blind, randomized, parallel, and controlled study conducted on T2DM patients. Sample size was calculated using Openepi with Confidence level 95%, Power of study 80%, Ratio of exposed (empagliflozin group) to unexposed (control group) 1:1, Percent of exposed with outcome 33%, Percent of unexposed with outcome 3%, So sample size was twenty five for each group.15 From January 2022 to May 2022, 110 patients with DM type 2 from Tanta University Hospitals, Tanta, Egypt, were enrolled to be screened for eligibility based on the inclusion and exclusion criteria. Fifty-three patients didn’t meet the criteria, and two didn’t accept to participate. According to the criteria, fifty-five eligible patients (n=55) were selected for the study. They were classified into two groups by randomization. The patients were randomly assigned into two comparable groups using computer-generated random numbers with sequentially numbered closed opaque envelope containing either the empagliflozin group or the control group 27 patients in group I and 28 patients in group II. Two patients in group I had not completed the study due to medication changes. Additionally, three patients in group II had not completed the study, two patients due to changing medications, and one had a UTI. Only 50 patients completed the study (n=25/group). Group I (n=25) (control group) consisted of 25 patients. Group II (n=25) (empagliflozin group) consisted of 25 patients (Fig. 1).
Clinical assessment of peripheral diabetic neuropathyThe clinical assessment of peripheral diabetic neuropathy was confirmed by electrophysiological assessment studies of the lower limbs, which were performed using the Nihon Kohden Neuropack, a six-channel apparatus (Nihon Kohden, Japan) with surface electrodes. Routine nerve conduction studies, including motor conduction studies of the common peroneal and posterior tibial nerves, were performed on both sides, according to Preston and Shapiro 2020.16 The diabetic neuropathy symptom (DNS) score was used as a diagnostic tool and was evaluated at the study baseline and following three months of treatment. The DNS score is a four-item approved score with a high prognostic value in diabetic polyneuropathy (PNP) screening in diabetics.17,18 A score of ≥1 was defined as positive for PNP.19
The brief pain inventory short-form (BPI-SF) scoring was evaluated at the study baseline and after three months to assess the degree of pain. The BPI-SF scale defines pain as follows: Worst Pain Score: 1–4=mild pain, Worst Pain Score: 5–6=moderate pain, and Worst Pain Score: 7–10=severe pain.20
The risk score for atherosclerotic cardiovascular disease (ASCVD) was evaluated at the study baseline and after three months. It calculates the 10-year risk of CVS problems, categorized as low risk (less than 5 percent), borderline risk (5–7.4 percent), intermediate risk (7.5–19.9 percent), and high risk (20 percent or more).21
Study endpointsThe primary endpoints were the electrophysiological assessment, and HbA1c levels.
The secondary endpoints were NSE, MDA, Calpro, lipid profile, and RBG levels. In addition, the BPI-SF score, the DNS score, and the ASCVD score.
Statistical analysisThe data were analyzed using SPSS statistical package version 26.0 (April 2019), IBM corporation software group, USA. The data were normally distributed using the Shapiro–Wilk normality test. Quantitative data were presented as the mean, SD, range, and median (IQR) and were evaluated using the Mann–Whitney U test (2 independent variables) and Wilcoxon signed-rank test (2 dependent groups pre & post-intervention). Qualitative data were displayed as numbers and percentages and evaluated by Fischer's exact test. Statistically significant result was considered when the p-value was below 0.05.
ResultsDemographic characteristicsParticipants’ demographic data, including age, revealed a non-significant differences between the 2 groups (53.4±6.16 versus 52.9±6.29, 95%, p=0.763) in both the control and the empagliflozin groups, respectively.
Clinical assessment of diabetic peripheral neuropathyAt the study baseline, Electrophysiological assessment, BPI-SF score, and ASCVD risk score weren’t significantly different between both arms.
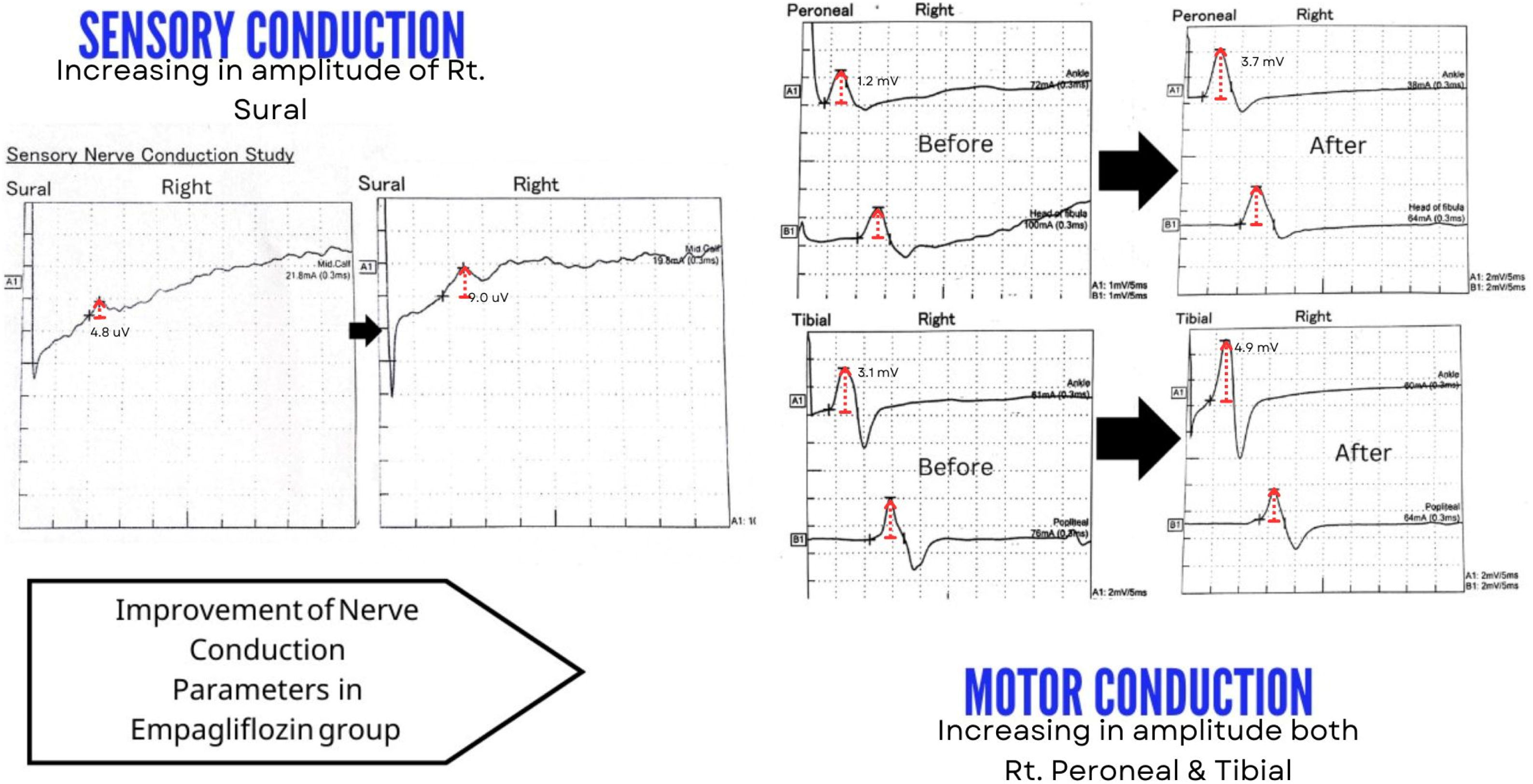
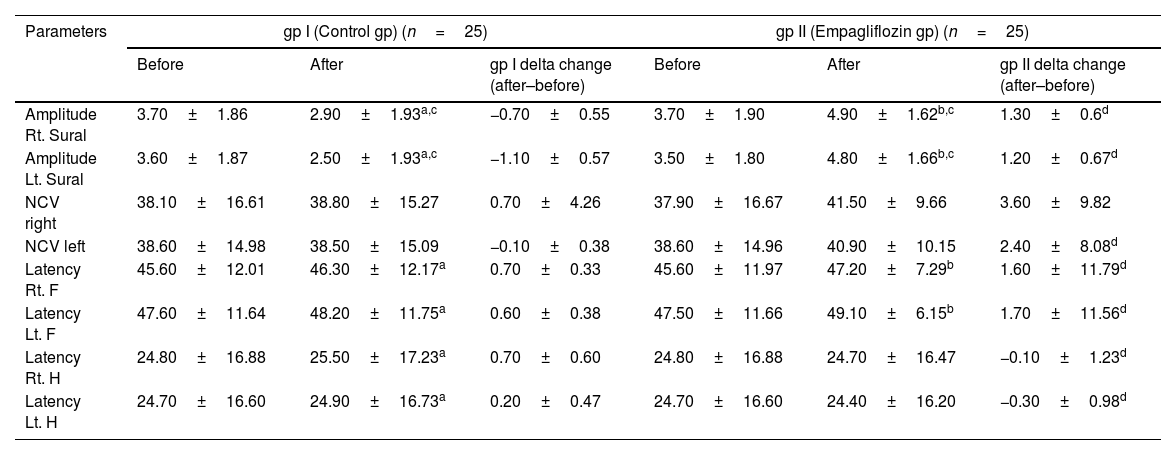
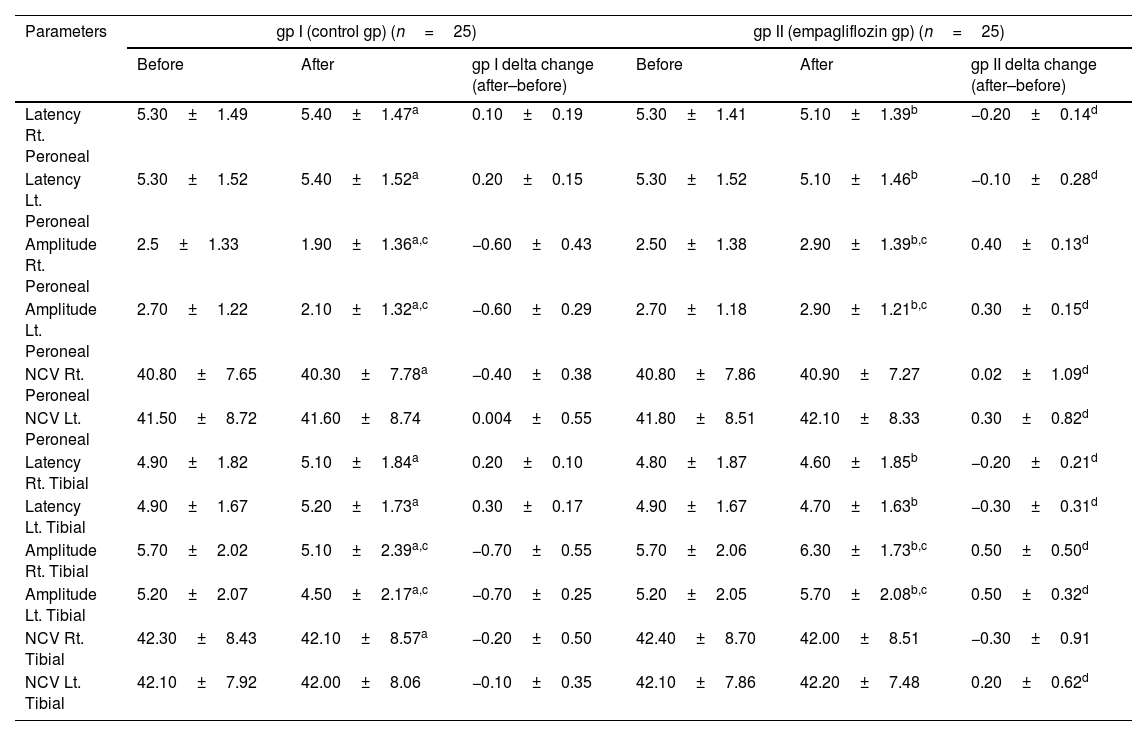
Electrophysiological assessmentComparison of results before and after three months of treatment. Group I had a significant decrease in the amplitude of the sural nerve was determined [(3.70±1.86 versus 2.90±1.93, 95%, p<0.001) (3.60±1.87 versus 2.50±1.93, 95%, p<0.001)]. While a significant increase in the F-wave latency [(45.60±12.01 versus 46.30±12.17, 95%, p<0.001) (47.60±11.64 versus 48.20±11.75, 95%, p<0.001)], and the H-reflex latency [(24.80±16.88 versus 25.50±17.23, 95%, p<0.001) and (24.70±16.60 versus 24.90±16.73, 95%, p=0.032)] in both the right and left extremities, respectively (Table 1). The motor conduction studies also revealed a significant reduction in the common peroneal nerve amplitude [(2.50±1.33 versus 1.90±1.36, 95%, p<0.001) (2.70±1.22 versus 2.10±1.32, 95%, p<0.001)] and the posterior tibial nerve [(5.70±2.02 versus 5.10±2.39, 95%, p<0.001) (5.20±2.07 versus 4.50±2.17, 95%, p<0.001)]. While a significant increase in the latency of the common peroneal nerve [(5.30±1.49 versus 5.40±1.47, 95%, p=0.010) (5.30±1.52 versus 5.40±1.52, 95%, p<0.001)] and the posterior tibial nerves [(4.90±1.82 versus 5.10±1.84, 95%, p<0.001) (4.90±1.67 versus 5.20±1.73, 95%, p<0.001)] in both the right and left extremities, respectively (Table 2). Also a significant reduction in the NCV of both the common peroneal [(40.80±7.65 versus 40.30±7.78, 95%, p<0.001)] and the posterior tibial nerves [(42.30±8.43 versus 42.10±8.57, 95%, p=0.017)] only of the right lower leg. Group II had a significant increase in the amplitude of the sural nerve [(3.70±1.90 versus 4.90±1.62, 95%, p<0.001) and (3.50±1.80 versus 4.8±1.66, 95%, p<0.001)], while there was a significant increase in the F-wave latency [(45.60±11.97 versus 47.20±7.29, 95%, p=0.006) (47.50±11.66 versus 49.10±6.15, 95%, p=0.018)] on both the right and left extremities, respectively (Table 1). The motor conduction studies also revealed a significant elevation in the amplitude of the common peroneal nerve [(2.50±1.38 versus 2.90±1.39, 95%, p<0.001) (2.70±1.18 versus 2.90±1.21, 95%, p<0.001)] and the posterior tibial nerve [(5.70±2.06 versus 6.30±1.73, 95%, p<0.001) (5.20±2.05 versus 5.7±2.08, 95%, p<0.001)]. A significant decrease in the latency of the common peroneal nerve [(5.30±1.41 versus 5.10±1.39, 95%, p<0.001) (5.30±1.52 versus 5.10±1.46, 95%, p<0.001)] and the posterior tibial nerve [(4.80±1.87 versus 4.60±1.85, 95%, p=0.001) (4.90±1.67 versus 4.70±1.63, 95%, p<0.001)] of both the right and left extremities, respectively (Table 2). The results of the two groups following three months of treatments were compared. The sensory conduction studies showed a significant increase amplitude of the sural nerve conduction [(4.90±1.62 versus 2.90±1.93, 95%, p=0.001) and (4.80±1.66 versus 2.50±1.93, 95%, p<0.001)] on both the right and left lower extremities (Table 1). The motor nerve conduction represented by the common peroneal nerve conduction [(2.90±1.39 versus 1.90±1.36, 95%, p=0.001) and (2.90±1.21 versus 2.10±1.32, 95%, p<0.001)] and the posterior tibial nerve conduction [(6.30±1.73 versus 5.10±2.39, 95%, p=0.030) and (5.70±2.08 versus 4.50±2.17, 95%, p=0.028)] measurements showed a significant improvement in the amplitude on both the right and left lower extremities in favor of the empagliflozin arm (Table 2). Nerve conduction velocities and latency revealed no significant differences between the 2 groups. Regarding to delta change between the 2 groups (after three months of treatment - at the baseline of the study) there were also a significant improvement in favor of group II in the amplitude of the sural nerve [(−0.7±0.55 versus 1.3±0.61, 95%, p<0.001) (−1.1±0.57 versus 1.2±0.67, 95%, p<0.001)] and the H-reflex latency [(0.7±0.60 versus −0.1±1.23, 95%, p=0.007) (0.2±0.47 versus −0.3±0.98, 95%, p=0.046)] of both the right and the left lower extremities. Also improvement of the NCV of the left lower extremity [(−0.1±0.38 versus 2.4±8.08, 95%, p=0.019). However, a significant increase in the F-wave latency [(0.7±0.33 versus 1.6±11.79, 95%, p<0.001) (0.6±0.38 versus 1.7±11.56, 95%, p<0.001)] of both the right and the left lower extremities (Table 1). Also increase in the amplitude of the common peroneal nerve [(−0.6±0.43 versus 0.4±0.13, 95%, p<0.001) (−0.6±0.29 versus 0.3±0.15, 95%, p<0.001)] and the posterior tibial nerve [(−0.7±0.55 versus 0.5±0.50, 95%, p<0.001) (−0.7±0.25 versus 0.5±0.32, 95%, p<0.001). A significant decrease in the latency of the common peroneal nerve [(0.1±0.19versus −0.2±0.14, 95%, p<0.001) (0.2±0.15 versus −0.1±0.28, 95%, p<0.001)] and the posterior tibial nerve [(0.2±0.10 versus −0.2±0.21, 95%, p<0.001) (0.3±0.17 versus −0.3±0.31, 95%, p<0.001)]. In addition to increasing the NCV of the common peroneal nerve [(−0.4±0.38 versus 0.02±1.09, 95%, p=0.003) (0.004±0.55 versus 0.3±0.82, 95%, p=0.035)] of both the right and the left extremities, respectively. Also the posterior tibial nerve showed a significant increase in the NCV of the left extremity [(−0.1±0.35 versus 0.2±0.62, 95%, p=0.014)] represented an improvement in motor nerve conduction (Table 2). An example of the improvement in the sensory and motor nerve conduction parameters of a participant in the Empagliflozin group is shown in Fig. 2.
Sensory electrophysiological conduction studies of the two studied groups.
| Parameters | gp I (Control gp) (n=25) | gp II (Empagliflozin gp) (n=25) | ||||
|---|---|---|---|---|---|---|
| Before | After | gp I delta change (after–before) | Before | After | gp II delta change (after–before) | |
| Amplitude Rt. Sural | 3.70±1.86 | 2.90±1.93a,c | −0.70±0.55 | 3.70±1.90 | 4.90±1.62b,c | 1.30±0.6d |
| Amplitude Lt. Sural | 3.60±1.87 | 2.50±1.93a,c | −1.10±0.57 | 3.50±1.80 | 4.80±1.66b,c | 1.20±0.67d |
| NCV right | 38.10±16.61 | 38.80±15.27 | 0.70±4.26 | 37.90±16.67 | 41.50±9.66 | 3.60±9.82 |
| NCV left | 38.60±14.98 | 38.50±15.09 | −0.10±0.38 | 38.60±14.96 | 40.90±10.15 | 2.40±8.08d |
| Latency Rt. F | 45.60±12.01 | 46.30±12.17a | 0.70±0.33 | 45.60±11.97 | 47.20±7.29b | 1.60±11.79d |
| Latency Lt. F | 47.60±11.64 | 48.20±11.75a | 0.60±0.38 | 47.50±11.66 | 49.10±6.15b | 1.70±11.56d |
| Latency Rt. H | 24.80±16.88 | 25.50±17.23a | 0.70±0.60 | 24.80±16.88 | 24.70±16.47 | −0.10±1.23d |
| Latency Lt. H | 24.70±16.60 | 24.90±16.73a | 0.20±0.47 | 24.70±16.60 | 24.40±16.20 | −0.30±0.98d |
Data are presented as the mean±SD, gp I T2DM patients with diabetic PN receiving antidiabetic (dipeptidyl peptidase-4 inhibitors, metformin±basal insulin) plus placebo tablets once daily; gp II: T2DM patients with diabetic PN previous treatment plus empagliflozin 25mg once daily.
Significant difference between the 2 studied groups regarding the delta change (results after three months of therapy – results at study baseline).
Statistically significant (p<0.005).
There was no significant difference before treatment between the two studied groups.
NCV: nerve conduction velocity.
Motor electrophysiological conduction studies of the common peroneal nerve and posterior tibial of the two studied groups.
| Parameters | gp I (control gp) (n=25) | gp II (empagliflozin gp) (n=25) | ||||
|---|---|---|---|---|---|---|
| Before | After | gp I delta change (after–before) | Before | After | gp II delta change (after–before) | |
| Latency Rt. Peroneal | 5.30±1.49 | 5.40±1.47a | 0.10±0.19 | 5.30±1.41 | 5.10±1.39b | −0.20±0.14d |
| Latency Lt. Peroneal | 5.30±1.52 | 5.40±1.52a | 0.20±0.15 | 5.30±1.52 | 5.10±1.46b | −0.10±0.28d |
| Amplitude Rt. Peroneal | 2.5±1.33 | 1.90±1.36a,c | −0.60±0.43 | 2.50±1.38 | 2.90±1.39b,c | 0.40±0.13d |
| Amplitude Lt. Peroneal | 2.70±1.22 | 2.10±1.32a,c | −0.60±0.29 | 2.70±1.18 | 2.90±1.21b,c | 0.30±0.15d |
| NCV Rt. Peroneal | 40.80±7.65 | 40.30±7.78a | −0.40±0.38 | 40.80±7.86 | 40.90±7.27 | 0.02±1.09d |
| NCV Lt. Peroneal | 41.50±8.72 | 41.60±8.74 | 0.004±0.55 | 41.80±8.51 | 42.10±8.33 | 0.30±0.82d |
| Latency Rt. Tibial | 4.90±1.82 | 5.10±1.84a | 0.20±0.10 | 4.80±1.87 | 4.60±1.85b | −0.20±0.21d |
| Latency Lt. Tibial | 4.90±1.67 | 5.20±1.73a | 0.30±0.17 | 4.90±1.67 | 4.70±1.63b | −0.30±0.31d |
| Amplitude Rt. Tibial | 5.70±2.02 | 5.10±2.39a,c | −0.70±0.55 | 5.70±2.06 | 6.30±1.73b,c | 0.50±0.50d |
| Amplitude Lt. Tibial | 5.20±2.07 | 4.50±2.17a,c | −0.70±0.25 | 5.20±2.05 | 5.70±2.08b,c | 0.50±0.32d |
| NCV Rt. Tibial | 42.30±8.43 | 42.10±8.57a | −0.20±0.50 | 42.40±8.70 | 42.00±8.51 | −0.30±0.91 |
| NCV Lt. Tibial | 42.10±7.92 | 42.00±8.06 | −0.10±0.35 | 42.10±7.86 | 42.20±7.48 | 0.20±0.62d |
Data are presented as the mean±SD, gp I T2DM patients with diabetic PN receiving antidiabetic (dipeptidyl peptidase-4 inhibitors, metformin±basal insulin) plus placebo tablets once daily; gp II T2DM patients with diabetic PN previous treatment plus empagliflozin 25mg once daily.
Significant difference between the 2 studied groups regarding the delta change (results after three months of therapy – results at study baseline).
Statistically significant (p<0.005).
There was no significant difference before treatment between the two studied groups.
NCV: nerve conduction velocity.
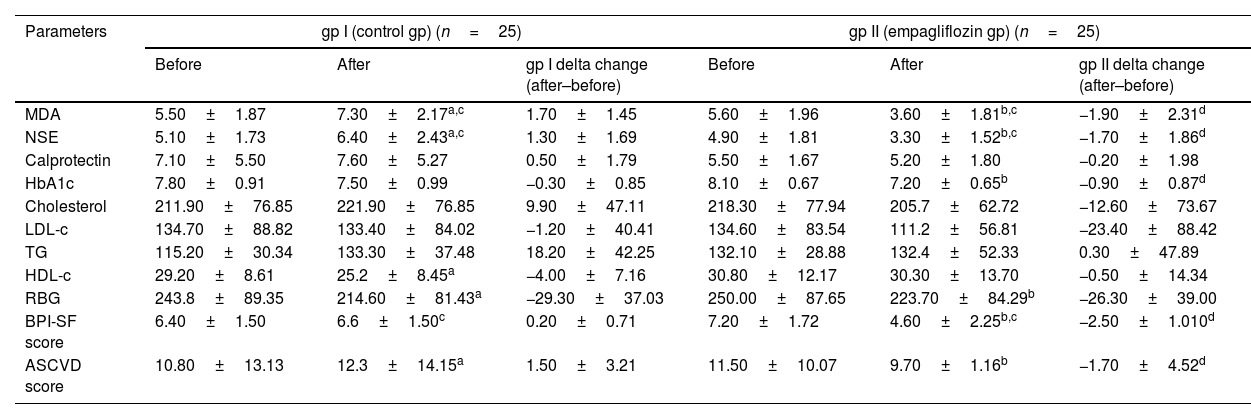
Comparison of results before and after three months. Group I showed a non-significant differences. While group II showed a significant decrease (7.20±1.72 versus 4.60±2.25, 95%, p value of less than 0.001. Comparison of the results of the two study groups following three months. There was a significant decrease in favor of the empagliflozin arm (4.60±2.25 versus 6.60±1.50, 95%, p=0.002). Regarding to delta change between the two studied groups, there were also a significant decrease (0.2±0.71versus −2.5±1.01, 95%, p value of less than 0.001) (Table 3).
Biological markers, ASCVD risk score, and BPI-SF score of the two studied groups.
| Parameters | gp I (control gp) (n=25) | gp II (empagliflozin gp) (n=25) | ||||
|---|---|---|---|---|---|---|
| Before | After | gp I delta change (after–before) | Before | After | gp II delta change (after–before) | |
| MDA | 5.50±1.87 | 7.30±2.17a,c | 1.70±1.45 | 5.60±1.96 | 3.60±1.81b,c | −1.90±2.31d |
| NSE | 5.10±1.73 | 6.40±2.43a,c | 1.30±1.69 | 4.90±1.81 | 3.30±1.52b,c | −1.70±1.86d |
| Calprotectin | 7.10±5.50 | 7.60±5.27 | 0.50±1.79 | 5.50±1.67 | 5.20±1.80 | −0.20±1.98 |
| HbA1c | 7.80±0.91 | 7.50±0.99 | −0.30±0.85 | 8.10±0.67 | 7.20±0.65b | −0.90±0.87d |
| Cholesterol | 211.90±76.85 | 221.90±76.85 | 9.90±47.11 | 218.30±77.94 | 205.7±62.72 | −12.60±73.67 |
| LDL-c | 134.70±88.82 | 133.40±84.02 | −1.20±40.41 | 134.60±83.54 | 111.2±56.81 | −23.40±88.42 |
| TG | 115.20±30.34 | 133.30±37.48 | 18.20±42.25 | 132.10±28.88 | 132.4±52.33 | 0.30±47.89 |
| HDL-c | 29.20±8.61 | 25.2±8.45a | −4.00±7.16 | 30.80±12.17 | 30.30±13.70 | −0.50±14.34 |
| RBG | 243.8±89.35 | 214.60±81.43a | −29.30±37.03 | 250.00±87.65 | 223.70±84.29b | −26.30±39.00 |
| BPI-SF score | 6.40±1.50 | 6.6±1.50c | 0.20±0.71 | 7.20±1.72 | 4.60±2.25b,c | −2.50±1.010d |
| ASCVD score | 10.80±13.13 | 12.3±14.15a | 1.50±3.21 | 11.50±10.07 | 9.70±1.16b | −1.70±4.52d |
Data are presented as the mean±SD, gp I T2DM patients with diabetic PN receiving antidiabetic (dipeptidyl peptidase-4 inhibitors, metformin±basal insulin) plus placebo tablets once daily; gp II: T2DM patients with diabetic PN previous treatment plus empagliflozin 25mg once daily.
Significant difference between the 2 studied groups regarding the delta change (results after three months of therapy – results at study baseline).
Statistically significant (p<0.005).
There was no significant difference before treatment between the two studied groups.
MDA: malondialdehyde enzyme; NSE: neuron-specific enolase enzyme.
LDL-c: low-density lipoprotein cholesterol. TGs: triglycerides.
HDL-c: high-density lipoprotein cholesterol. RBG: random blood glucose.
BPI-SF score: The brief pain inventory short-form item score.
ASCVD score: The atherosclerotic cardiovascular disease risk score.
Comparing the results before and after three months of the study. Both group I and group II showed a non-significant difference, also by comparing the results of the two study groups after three months of the study, and regarding to delta change between the two studied groups, there was a non-significant difference in the DNS score.
The ASCVD risk scoreComparison of the results of before and after three months of the study. Group I showed a significant increase (10.80±13.13 versus 12.30±14.15, 95%, p=0.044). While group II showed a significant decrease (11.50±10.07 versus 9.70±11.16, 95%, p=0.041). The results of the two study were compared after three months, there was a non-significant difference in the ASCVD score between the two groups. Regarding to delta change between the two studied groups, there were also a significant decrease in the ASCVD score (1.5±3.21versus −1.7±4.52, 95%, p=0.003) (Table 3).
Biological markersAt the study baseline, human HbA1c%, NSE, MDA, and Calpro serum levels, lipid profile, and RBG level weren’t significantly different between both arms (Table 3).
HbA1c levelComparing the results before and after three months. Group I showed a non-significant difference. While group II showed a significant decreased HbA1c level (8.10±0.67 versus 7.20±0.65, 95%, p<0.001). The results of the HbA1c of the two study groups after three months were compared. HbA1c exhibited a non-significant difference. However, regarding to delta change between the two studied groups, there were also a significant decrease in the HbA1c (−0.3±0.85versus −0.9±0.87, 95%, p=0.003) (Table 3).
Random blood glucose (RBG) levelsComparing the results before and after three months. Group I showed a significant decrease (243.80±89.35 versus 214.6±81.43, 95%, p=0.001). Group II showed a significant decrease (250.0±87.65 versus 223.70±84.29, 95%, p=0.002). Comparing the results of the RBG levels of the two study groups after three months and regarding to delta change between the two studied groups there was a non-significant difference (Table 3).
Neuron-specific enolase (NSE) serum levelComparing the results before and after three months. Group I revealed a significant increase in NSE levels (5.10±1.73 versus 6.40±2.43, 95%, p=0.002). Group II revealed a significant decrease in NSE levels (4.90±1.81 versus 3.30±1.52, 95%, p<0.001). The NSE levels of the two study groups were compared after three months. The mean serum levels of NSE were significantly decreased in the empagliflozin group in comparison with the control one (3.30±1.52 versus 6.40±2.43, 95%, p<0.001). In addition, regarding to delta change between the two studied groups, there were also a significant decrease in the NSE levels (1.3±1.69 versus −1.7±1.86, 95%, p value less than 0.001) (Table 3).
Malondialdehyde (MDA) serum levelComparing the results before and after three months. Group I showed a significant elevation in MDA level (5.50±1.87 versus 7.30±2.17, 95%, p<0.001). Group II showed a significant decrease (5.60±1.96 versus 3.60±1.81, 95%, p<0.001). The MDA levels of the two study groups were compared after three months. The mean serum levels of MDA exhibited significant decrease (3.60±1.81 versus 7.30±2.17, 95%, p<0.001). In addition, regarding to delta change between the two studied groups, there were also a significant decrease in MDA (1.7±1.45versus −1.9±2.31, 95%, p value less than 0.001) (Table 3).
Calpro serum levelThe results of calpro serum levels revealed non-significant difference at all (Table 3).
Lipid profile (HDL-c, LDL-c, triglycerides, and total cholesterol) levelsThe results of lipid profile revealed non- significant difference at all, except for comparing the results of group I before and after three months. HDL-c levels revealed a significant decrease (29.20±8.61 versus 25.20±8.45, 95%, p=0.014) (Table 3).
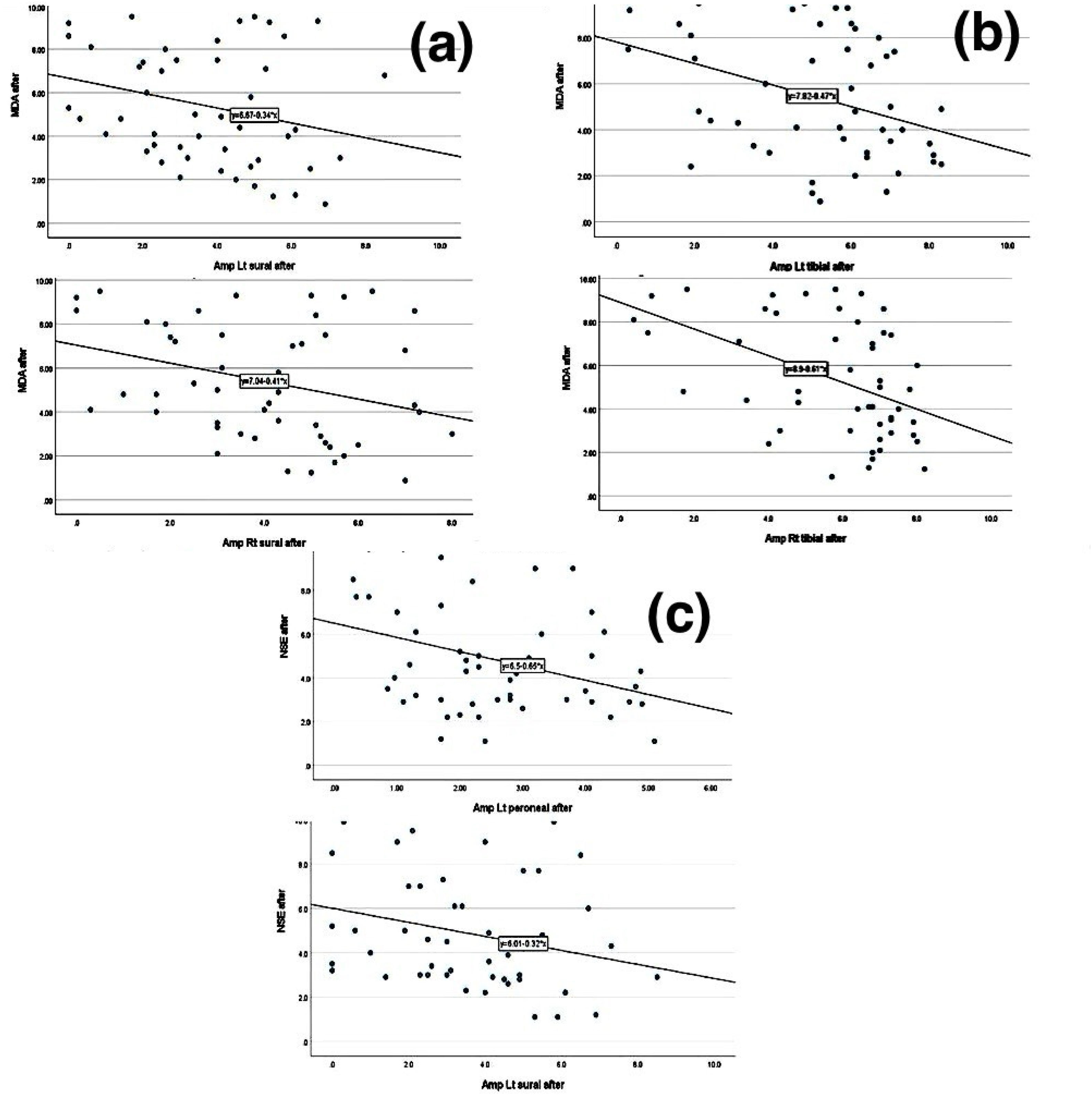
Correlation between measured parametersThe correlation between the measured biological markers and the BPI-SF score and the electrophysiological parameters as clinical parameters for neuropathy was performed after three months of the study. Spearman's correlation analysis revealed that the MDA level exhibited a significant negative correlation with the electrophysiological parameters of both the right and left sural nerve amplitudes (Fig. 3a) and the amplitudes of both the right and left posterior tibial nerve (Fig. 3b). The NSE level showed a significant negative correlation with the electrophysiological parameters of the amplitude of the left sural nerve and the amplitude of the left common peroneal nerve (Fig. 3c). However, the BPI-SF score correlation with the electrophysiological parameters showed no significant difference.
(a) Correlation between NSE levels and electrophysiological parameters at the end of the study. (b) Correlation between MDA levels and sensory electrophysiological parameters at the end of the study. (c) Correlation between MDA levels and motor electrophysiological parameters at the end of the study.
This study can be considered the 1st prospective clinical trial that investigated the effect of empagliflozin in T2DM patients with DPN who were not on SGLT2 inhibitor treatment. Preclinical studies have proven that SGLT2 inhibitors improve nerve conduction with amelioration of in neuropathic pain in DM rats, so this study showed agreement with this preclinical study.9,22
In the present study, the efficiency of empagliflozin as a neuroprotective agent in patients with T2DM with DPN was evaluated using electrophysiological assessment studies as a primary endpoint. After three months of treatment, the electrophysiological studies showed a significant improvement in favor of the empagliflozin arm compared to the control arm in both the sensory and the motor conduction studies. The study was conducted in a short period, and prolonging the study may improve the results; however, this short period was due to financial reasons.
The results of the BPI-SF score were used as a secondary endpoint of this study to evaluate the degree of improvement in the degree of pain, which also reflects QOL. After three months of treatment, a significant decrease in the BPI-SF score in favor of the empagliflozin arm in comparison with the control.
Respecting the results of the ASCVD risk score as a secondary endpoint showed a non-significant difference at the beginning of the study between the 2 groups, nor after three months of therapy; however, when comparing the results of the empagliflozin arm at the study baseline and after three months of treatment, a significantly improved ASCVD risk score was detected, while it worsened in the control group. This effect showed agreement with Lui et al., a study of the mechanism of the protective effect of SGLT2 inhibitors in CVS diseases and kidney dysfunction.23
Until now, there has been no specific biomarker for neural damage, but in the Bönhof et al. study and LI et al., NSE might be considered one of the novel biomarkers for DPN.24,25 In our study, the mean serum level of NSE showed a significant improvement in favor of the empagliflozin arm, regardless of the HbA1c % or the RBG levels, which demonstrated a non-significant difference after three months between the control arm and empagliflozin arm. This may prove that the improvement in the electrophysiological studies was not a result of strict blood glucose levels.
Malondialdehyde, was significantly lower in the empagliflozin arm in comparison with the control arm, which proves the antioxidant effect of empagliflozin and shows agreement with the preclinical study of Ahmed et al.22
Calpro serum level as a marker of anti-inflammatory effect wasn’t significantly different in both groups after three months of the study.26
The study's data were not normally distributed because of the small sample size, which may be improved in further studies; however, the small sample size was due to financial problems as the research had no fund and was self-funding.
ConclusionsIn summary, this study was carried out to detect whether empagliflozin has a neuroprotective effect in T2DM cases and those having DPN, in addition to its impact on glycemic control. The drug ameliorated neuropathy and systemic oxidative stress with minimal to no side effects. Therefore, empagliflozin has been shown to improve DPN and the patient's QOL. After more studies support this concept, empagliflozin may be considered a 1st-line of treatment in DN cases.
Authors’ contributionsSahar Mohamed El-Haggar and Maha Khalifa developed the study concept. Maha Khalifa and Amira Mohamed El Sharkawy performed the study. Sahar Mohamed El-Haggar, Yasser Mostafa Hafez, and Maha Khalifa analyzed the data. All authors participated in writing and reviewing the main manuscript, and they all read and approved the final manuscript.
Ethical approvalThe study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments, Approval letter was given by The National Research Ethics Committee (Tanta University Ethical Committee; approval code: 35200/1/22, and by The Faculty of Pharmacy Tanta University – Research Ethics Committee; approval code: TP/RE/01-22-P-001).
Clinical trial registrationThe study was registered at clinicaltrials.gov (identifier: NCT05977465).
Consent for publicationNot applicable.
Availability of data and materialsThe datasets used and/or analyzed in the present study are available from the corresponding author upon request.
Informed consentWritten informed consent was obtained from all the participants.
FundingThis research didn’t receive grants from any funding organizations in the public, commercial, or not-for-profit sector.
Conflict of interestThe authors declared no conflict of interest.
Not applicable.