Scarce data on Fas, one of the main receptors that activates the apoptosis extrinsic pathway, in septic patients exists. Higher blood soluble Fas (sFas) concentrations in non-survivor septic patients compared with survivors have been found in small studies; however, the association of blood sFas concentrations with mortality controlling for sepsis severity has not been stablished due to this small sample size in those studies. Thus, our main objective study was to determine whether an association between blood sFas concentrations and sepsis mortality controlling for sepsis severity exists.
MethodsWe included septic patients in this observational and prospective study carried out in three Spanish Intensive Care Units. We obtained serum samples at sepsis diagnosis sepsis for sFas levels determination.
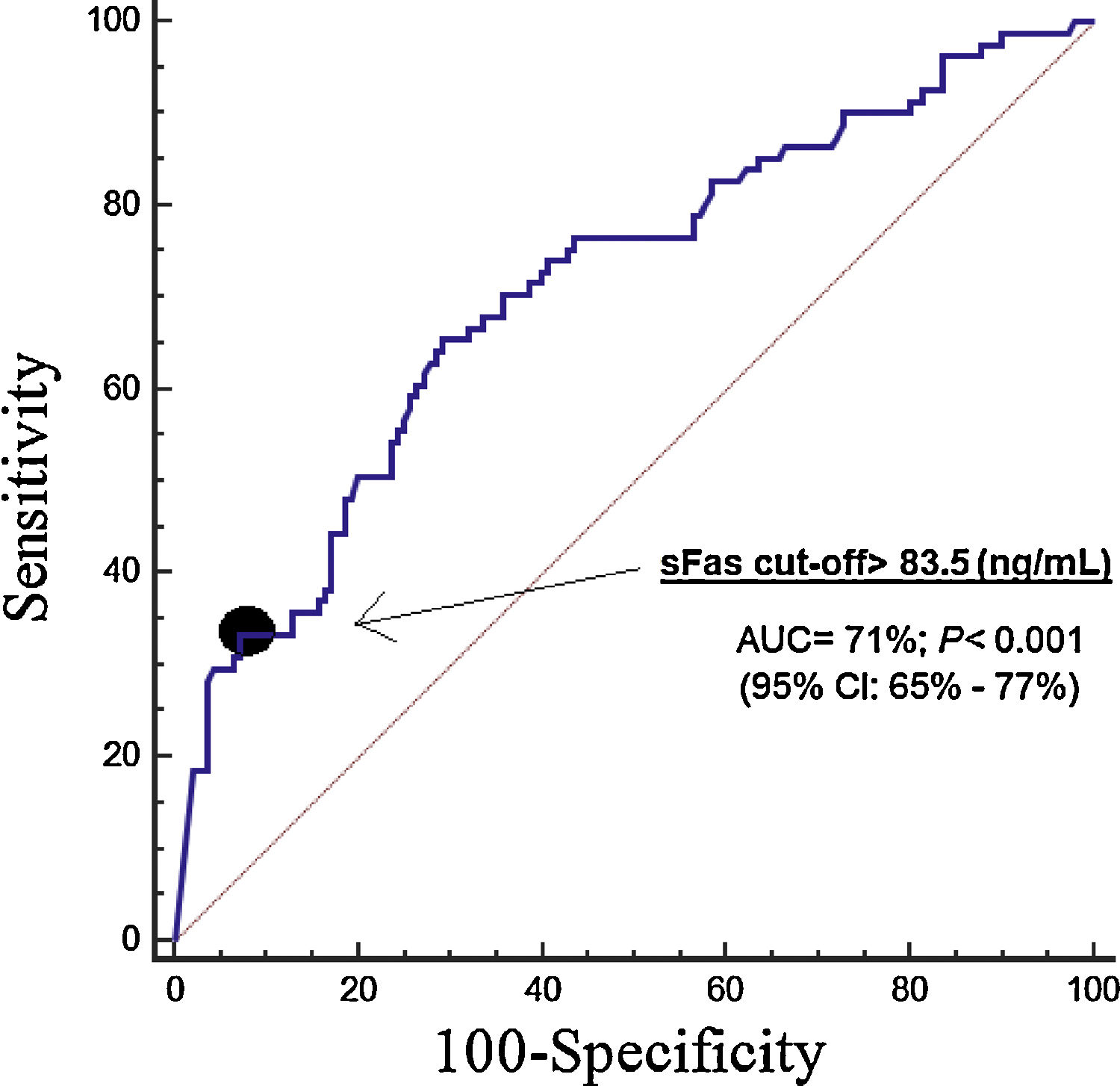
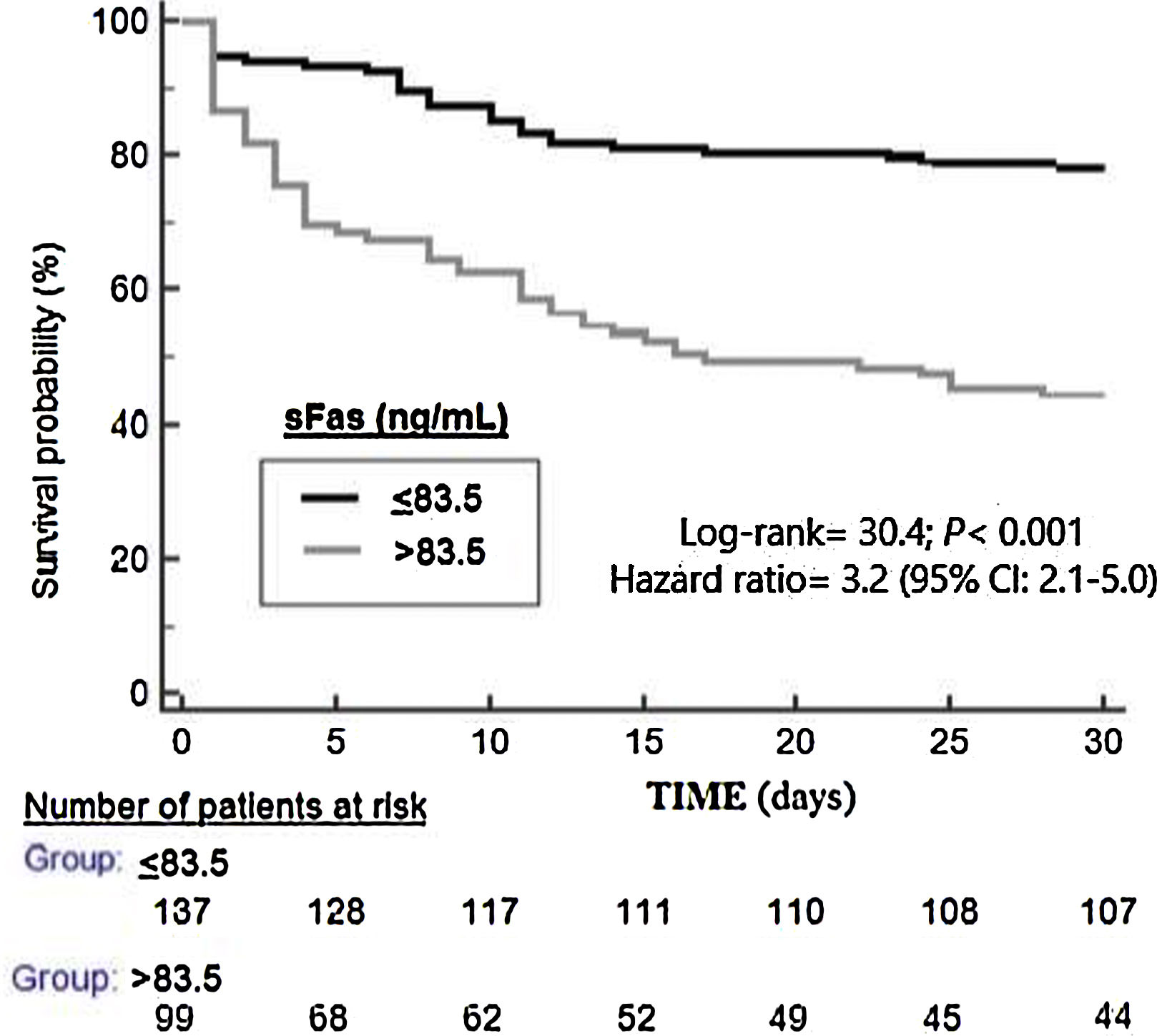
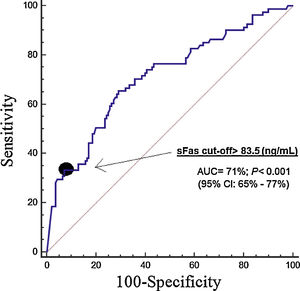
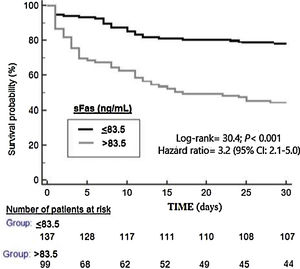
ResultsThirty-day non-surviving patients (n=85) compared to surviving patients (n=151) had higher serum sFas levels (p<0.001). We found in multiple logistic regression analysis an association of serum sFas levels with mortality controlling for age and SOFA (OR=1.004; 95% CI=1.002–1.006; p<0.001), and for age and APACHE-II (OR=1.004; 95% CI=1.002–1.006; p<0.001). Serum sFas levels showed and area under the curve for mortality prediction of 71% (95% CI=65–71%; p<0.001). Kaplan–Meier analysis showed higher mortality rate in patients with serum sFas levels>83.5ng/mL (Hazard ratio=3.2; 95% CI=2.1–5.0; p<0.001).
ConclusionsThat an association between blood sFas concentrations and sepsis mortality controlling for sepsis severity exists was our main new finding study.
Existen pocos datos sobre Fas, uno de los principales receptores que activan la vía extrínseca de la apoptosis, en pacientes septicos. En estudios de pequeño tamaño muestral se han encontrado altas concentraciones sanguíneas de soluble Fas (sFas) en pacientes sépticos fallecidos en comparación con supervivientes; sin embargo, no ha sido establecida la asociación de concentraciones sanguíneas de sFas con mortalidad controlando por la gravedad de la sepsis. Por lo tanto, el principal objetivo de nuestro estudio fue determinar si existe una asociación entre concentraciones sanguíneas de sFas y la mortalidad en sepsis controlando por la gravedad.
MétodosIncluímos pacientes sépticos en este estudio observacional y prospectivo realizado en tres Unidades de Cuidados Intensivos españolas. Obtuvimos muestras de suero en el momento del diagnóstico de la sepsis para la determinación de concentraciones de sFas.
ResultadosLos pacientes fallecidos durante los primeros treinta días (n=85) comparados con los supervivientes (n=151) tuvieron mayores concentraciones séricas de sFas (p<0,001). Se encontró una asociación de concentraciones séricas de sFas con mortalidad controlando por edad y SOFA (OR=1,004; 95% IC=1,002-1,006; p < 0,001), y por edad y APACHE-II (OR=1,004; 95% IC=1,002-1,006; p < 0,001). Las concentraciones séricas de sFas mostraron un área bajo la curva para la predicción de mortalidad del 71% (IC 95%=65%-71%; p < 0,001). El análisis Kaplan-Meier mostró una mayor mortalidad en los pacientes con concentraciones séricas de sFas > 83,5 ng/mL (Hazard ratio=3,2; 95% IC=2,1-5,0; p < 0,001).
ConclusionesLa asociación entre concentraciones sanguíneas de sFas y la mortalidad en sepsis controlando por la gravedad fue el principal nuevo hallazgo de nuestro estudio.
Great healthcare costs and many deaths are produced annually by sepsis.1,2 In physiological processes (as morphogenesis and tissue remodeling) and in different diseases exists cell death by apoptosis.3–5 In addition, increased apoptosis has been found in septic animal models.3–5 Two main pathways exist of apoptosis cell death, the intrinsic or mitochondrial pathway and the extrinsic or death receptor pathway. The extrinsic pathway is activated when binding a tumor necrosis factor ligand superfamily (TNFSF) with its tumor necrosis factor membrane receptors superfamily (TNFRSF). The main ligands and receptors of this extrinsic pathway are the Fas ligand and its Fas receptor, and the TNF-related apoptosis-inducing ligand (TRAIL) and its TNF-related apoptosis-inducing ligand receptors (TRAILR1 to 4). After binding some TNFSF ligand with its TNFRSF is generated a death signal that activates pro-caspase-8 in caspase-8. Afterwards, this initiator caspase-8 activates the effector caspase-3 producing the cellular damage by apoptotic.3–5
Scarce data on Fas in septic patients exists.6–14 Increased expression of Fas in white blood cells in healthy volunteers after to receive intravenous endotoxin6 and in septic patients compared to control subjects7 has been found. Higher blood soluble Fas (sFas) concentrations have been found in septic patients than in healthy subjects,8,9 and in patients with higher sepsis severity.10 In addition, higher blood sFas concentrations in non-survivor septic patients compared with survivors have been found in small studies11–14; however, the association of blood sFas concentrations with mortality controlling for sepsis severity has not been stablished due to this small sample size in those studies. Thus, our main objective study was to determine whether an association between blood sFas concentrations and sepsis mortality controlling for sepsis severity exists.
Materials and methodsDesign and subjectsThree Spanish Intensive Care Units recruited patients between 2013 and 2014 in this prospective and observational study. The Ethics Committee of each hospital approved the study. The informed and signed consent from patients or their relatives was obtained for the participation in the study.
We included septic patients with Sepsis-3 Consensus criteria.15 We excluded patients with radiation therapy, hematological tumor, immunosuppressive therapy, blood cell count <1000/μl, white solid tumor, steroid agents, human immunodeficiency virus, age <18 years, pregnancy or breastfeeding.
Epidemiological and clinical variables were collected at moment of sepsis diagnosis. Sex, age, history of chronic obstructive pulmonary disease, ischemic heart disease, diabetes mellitus and chronic renal failure were registered. Bilirubin, creatinine, leukocytes, platelets, activated partial thromboplastin time (aPTT), international normalized ratio (INR), lactic acid, pressure arterial of oxygen, fraction inspired of oxygen, site of infection, microorganism responsible, Sepsis-related Organ Failure Assessment [SOFA] score,16 septic shock, Acute Physiology and Chronic Health Evaluation (APACHE)-II score17 and bloodstream infection were also recorded. Thirty-days survival was the endpoint study.
Determination of serum sFas concentrationsSerum of patients at moment of sepsis diagnosis were frozen at −80°C until the serum sFas concentration determinations. Human Fas ELISA Kit (Elabscience, Houston, Texas, United States), which had an assay detection limit of 19pg/mL and coefficient of variation of intra-assay and inter-assays lower than 6%, were used.
Statistical methodsMedians (percentile 25–75), frequencies (percentages), Mann–Whitney U test and chi-square test were used for description and comparison of continuous and categorical variables between surviving and non-surviving patients. A receiver operating characteristic analysis was performed using serum sFas concentrations and 30-days survival. Thirty-day survival Kaplan–Meier curves with the cut-off of serum sFas concentration that correspond to Youden J index were carried out. Multiple logistic regression analysis was performed to stablish whether an association of serum sFas concentrations and 30-day mortality controlling for sepsis severity exist. We reported Odds ratio (OR) and its 95% confidence interval (CI) of each variable. NCSS 2000 (Kaysville, Utah) and SPSS 17.0 (SPSS Inc., Chicago, IL, USA) were used for analyses. The point p<0.05 was used to establish significant differences.
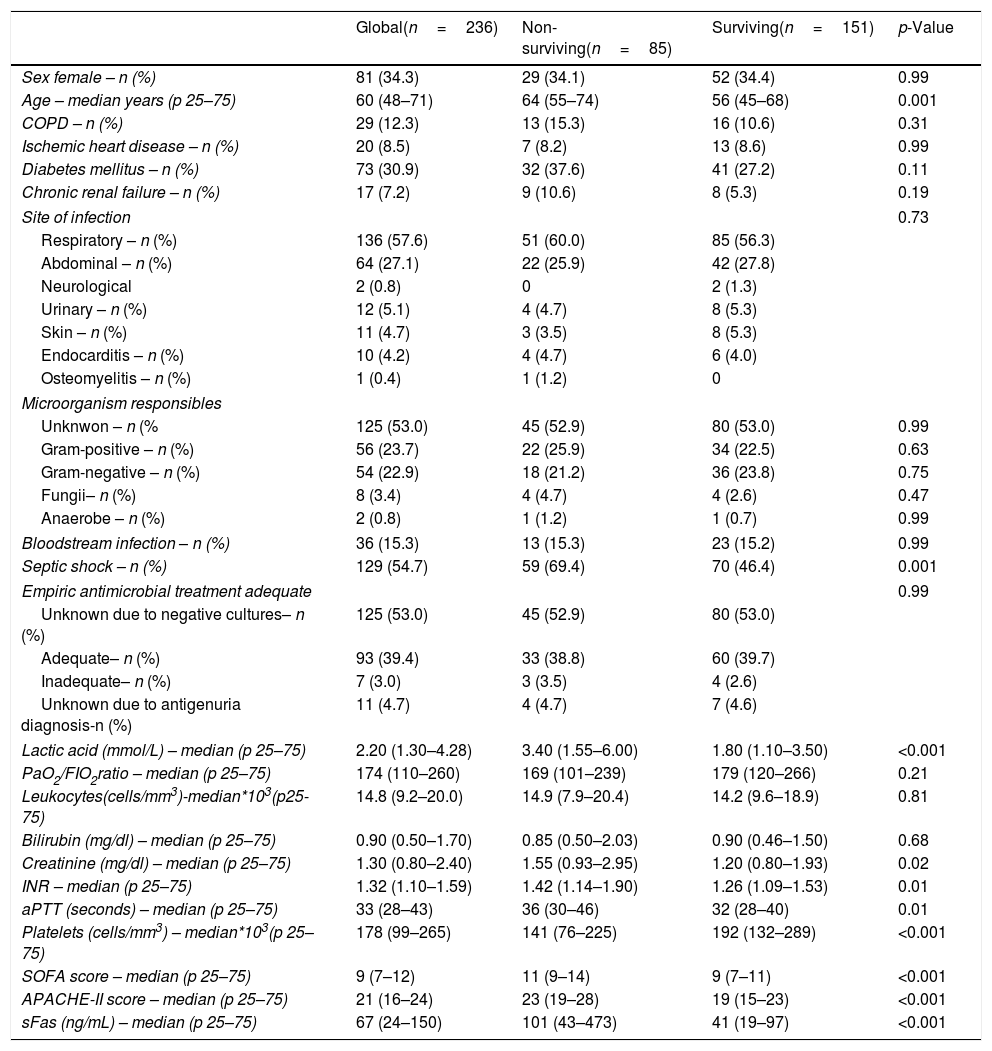
ResultsThirty-day non-surviving patients (n=85) compared to surviving patients (n=151) had higher serum sFas levels (p<0.001), age, APACHE-II, SOFA, creatinine, INR, aPTT, lactate and septic shock rate, and lower platelet count (Table 1). A total of 127, 74 and 35 patients were included in each hospital participating, and no significant differences in the mortality rate between hospitals were found (37.0%, 35.1% and 34.3%; p=0.94).
Demographic and clinical characteristics at moment of sepsis diagnosis in non-surviving and surviving patients.
| Global(n=236) | Non-surviving(n=85) | Surviving(n=151) | p-Value | |
|---|---|---|---|---|
| Sex female – n (%) | 81 (34.3) | 29 (34.1) | 52 (34.4) | 0.99 |
| Age – median years (p 25–75) | 60 (48–71) | 64 (55–74) | 56 (45–68) | 0.001 |
| COPD – n (%) | 29 (12.3) | 13 (15.3) | 16 (10.6) | 0.31 |
| Ischemic heart disease – n (%) | 20 (8.5) | 7 (8.2) | 13 (8.6) | 0.99 |
| Diabetes mellitus – n (%) | 73 (30.9) | 32 (37.6) | 41 (27.2) | 0.11 |
| Chronic renal failure – n (%) | 17 (7.2) | 9 (10.6) | 8 (5.3) | 0.19 |
| Site of infection | 0.73 | |||
| Respiratory – n (%) | 136 (57.6) | 51 (60.0) | 85 (56.3) | |
| Abdominal – n (%) | 64 (27.1) | 22 (25.9) | 42 (27.8) | |
| Neurological | 2 (0.8) | 0 | 2 (1.3) | |
| Urinary – n (%) | 12 (5.1) | 4 (4.7) | 8 (5.3) | |
| Skin – n (%) | 11 (4.7) | 3 (3.5) | 8 (5.3) | |
| Endocarditis – n (%) | 10 (4.2) | 4 (4.7) | 6 (4.0) | |
| Osteomyelitis – n (%) | 1 (0.4) | 1 (1.2) | 0 | |
| Microorganism responsibles | ||||
| Unknwon – n (% | 125 (53.0) | 45 (52.9) | 80 (53.0) | 0.99 |
| Gram-positive – n (%) | 56 (23.7) | 22 (25.9) | 34 (22.5) | 0.63 |
| Gram-negative – n (%) | 54 (22.9) | 18 (21.2) | 36 (23.8) | 0.75 |
| Fungii– n (%) | 8 (3.4) | 4 (4.7) | 4 (2.6) | 0.47 |
| Anaerobe – n (%) | 2 (0.8) | 1 (1.2) | 1 (0.7) | 0.99 |
| Bloodstream infection – n (%) | 36 (15.3) | 13 (15.3) | 23 (15.2) | 0.99 |
| Septic shock – n (%) | 129 (54.7) | 59 (69.4) | 70 (46.4) | 0.001 |
| Empiric antimicrobial treatment adequate | 0.99 | |||
| Unknown due to negative cultures– n (%) | 125 (53.0) | 45 (52.9) | 80 (53.0) | |
| Adequate– n (%) | 93 (39.4) | 33 (38.8) | 60 (39.7) | |
| Inadequate– n (%) | 7 (3.0) | 3 (3.5) | 4 (2.6) | |
| Unknown due to antigenuria diagnosis-n (%) | 11 (4.7) | 4 (4.7) | 7 (4.6) | |
| Lactic acid (mmol/L) – median (p 25–75) | 2.20 (1.30–4.28) | 3.40 (1.55–6.00) | 1.80 (1.10–3.50) | <0.001 |
| PaO2/FIO2ratio – median (p 25–75) | 174 (110–260) | 169 (101–239) | 179 (120–266) | 0.21 |
| Leukocytes(cells/mm3)-median*103(p25-75) | 14.8 (9.2–20.0) | 14.9 (7.9–20.4) | 14.2 (9.6–18.9) | 0.81 |
| Bilirubin (mg/dl) – median (p 25–75) | 0.90 (0.50–1.70) | 0.85 (0.50–2.03) | 0.90 (0.46–1.50) | 0.68 |
| Creatinine (mg/dl) – median (p 25–75) | 1.30 (0.80–2.40) | 1.55 (0.93–2.95) | 1.20 (0.80–1.93) | 0.02 |
| INR – median (p 25–75) | 1.32 (1.10–1.59) | 1.42 (1.14–1.90) | 1.26 (1.09–1.53) | 0.01 |
| aPTT (seconds) – median (p 25–75) | 33 (28–43) | 36 (30–46) | 32 (28–40) | 0.01 |
| Platelets (cells/mm3) – median*103(p 25–75) | 178 (99–265) | 141 (76–225) | 192 (132–289) | <0.001 |
| SOFA score – median (p 25–75) | 9 (7–12) | 11 (9–14) | 9 (7–11) | <0.001 |
| APACHE-II score – median (p 25–75) | 21 (16–24) | 23 (19–28) | 19 (15–23) | <0.001 |
| sFas (ng/mL) – median (p 25–75) | 67 (24–150) | 101 (43–473) | 41 (19–97) | <0.001 |
COPD=Chronic Obstructive Pulmonary Disease; PaO2/FIO2=pressure of arterial oxygen/fraction inspired oxygen; INR=International normalized ratio; aPTT=Activated partial thromboplastin time; SOFA=Sepsis-related Organ Failure Assessment; APACHE=Acute Physiology and Chronic Health Evaluation.
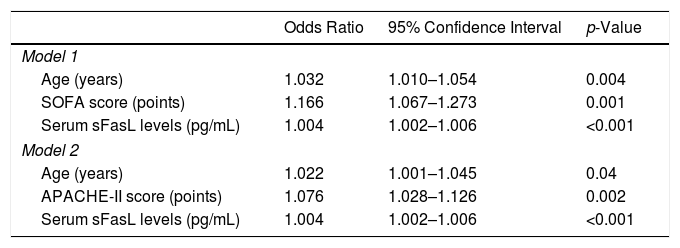
We found in multiple logistic regression analysis an association of serum sFas levels with mortality controlling for age and SOFA (OR=1.004; 95% CI=1.002–1.006; p<0.001), and for age and APACHE-II (OR=1.004; 95% CI=1.002–1.006; p<0.001) (Table 2). Other variables as INR, aPTT, platelets, septic shock, lactic acid, creatinine and adequate empiric antimicrobial treatment were not statistically significant when were introduced in the model and were finally removed.
Multiple logistic regression analyses to predict mortality at 30 days.
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Model 1 | |||
| Age (years) | 1.032 | 1.010–1.054 | 0.004 |
| SOFA score (points) | 1.166 | 1.067–1.273 | 0.001 |
| Serum sFasL levels (pg/mL) | 1.004 | 1.002–1.006 | <0.001 |
| Model 2 | |||
| Age (years) | 1.022 | 1.001–1.045 | 0.04 |
| APACHE-II score (points) | 1.076 | 1.028–1.126 | 0.002 |
| Serum sFasL levels (pg/mL) | 1.004 | 1.002–1.006 | <0.001 |
SOFA=Sepsis-related Organ Failure Assessment; APACHE=Acute Physiology and Chronic Health Evaluation.
Serum sFas levels showed and area under the curve for mortality prediction of 71% (95% CI=65–71%; p<0.001) (Fig. 1). The mortality prediction but the cutt-point of serum sFas levels>83.5ng/mL had sensitivity of 65% (54–75%), specificity of 71% (63–78%), positive likelihood ratio of 2.2 (1.7–3.0), negative likelihood ratio of 0.5 (0.4–0.7), positive predictive value of 56% (48–63%) and negative predictive value of 78% (72–83%). Kaplan–Meier analysis showed higher mortality rate in patients with serum sFas levels>83.5ng/mL (Hazard ratio=3.2; 95% CI=2.1–5.0; p<0.001) (Fig. 2).
No significant differences were found in serum sFas levels in respect to site of infection (p=0.32), microbiological documentation of sepsis, development of bloodstream infection (p=0.78), and that the microorganism responsible was Gram-positive (p=0.27) or Gram-negative (p=0.57).
DiscussionThat an association between blood sFas concentrations and sepsis mortality controlling for sepsis severity exists was our main new finding study. Previously, higher blood sFas concentrations in non-survivor septic patients compared with survivors had been found in small studies11–14; therefore, our results findings are in consonance with those found in previous studies. However, the association of blood sFas concentrations with mortality controlling for sepsis severity regarding to the results of the multiple logistic regression analysis is a novel finding of our study that there was not previously reported due to the small sample size of previous studies (total of patients included in those studies were between 14 and 100 septic patients, and the number of non-survivor patients varied between 8 and 17).11–14 We found in multiple logistic regression analysis an association of serum sFas levels with mortality controlling for age and SOFA, and for age and APACHE-II. However, we did not found this association with other variables as INR, aPTT, platelets, septic shock, lactic acid, creatinine and adequate empiric antimicrobial treatment. Besides, that serum soluble Fas concentrations could be used for mortality prediction according to the findings of the receiver operating characteristic analysis is another new finding of our study.
Fas is one of the main death receptors that activates the apoptosis extrinsic pathway when its ligand binding with it. At this moment is generated a death signal that will activate caspase-8 leading to activation of caspase-3, which is the main initiator caspase in this apoptosis extrinsic pathway, that will produce the cellular damage by apoptosys.3–5 We think that the findings of our study about the association between high serum sFas and mortality could mean that non-surviving patients have higher apoptosis activation by extrinsic pathway leading to higher cell death by apoptosis.
In a study by Paunel-Görgülü et al. including patients with major trauma patients, some with development of sepsis during the following days after trauma, were found higher neutrophil apoptosis and serum sFas levels in the patients developing sepsis compared with those that no developed sepsis.18 Besides, in that study the authors found a reduction of Fas-mediated neutrophil apoptosis in cells cultured with the use of agonistic anti-Fas antibodies.18 In addition, the use of small interfering RNA (siRNA) against Fas to block extrinsic apoptosis pathway blocking Fas/FasL systrem in septic animals has been associated with apoptosis reduction and survival increase.19–22
Some limitations must be recognized in our study. First, the percentage of patients that were excluded and the causes for exclusion were not registered. Second, although we found that serum sFas concentrations could be used for mortality prediction; however, the statistical values were not high. Third, the rate of bloodstream infection reach even 40% in other series23 and in our series was quite lower. Fourth, the delay time to empirical antibiotic administration was not registered. Fifth, no serum sFas levels were determined in healthy subjects; however, whether serum sFas levels could add for sepsis diagnosis was not the objective of our study, but whether serum sFas levels could add for sepsis mortality prediction. Sixth, some assays as terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) or Annexin V were not performed to assess cellular damage by apoptosis. Therefore, in spite of those limitations, researching about the potential role of blood sFas concentrations in mortality prediction of septic patients could be interesting due to that its determination is easy and cheap.
ConclusionsThat an association between blood sFas concentrations and sepsis mortality controlling for sepsis severity exists was our main new finding study.
FundingThis study was supported by a grant from Instituto de Salud Carlos III (PI-18-00500) (Madrid, Spain) and co-financed with Fondo Europeo de Desarrollo Regional (FEDER).
Conflicts of interestThe authors declare that they have no competing interests.