The standard treatment for locally advanced rectal cancer is total mesorectal excision. However, organ preservation has been proposed for tumours with good response to neoadjuvant treatment. The aim of this study was to evaluate the oncologic results of this strategy.
MethodsThis is a retrospective cohort study (2005–2014) including a consecutive series of patients with rectal adenocarcinoma with complete or almost complete clinical response after preoperative chemo-radiotherapy, that were treated according to a strategy of preservation of the rectum.
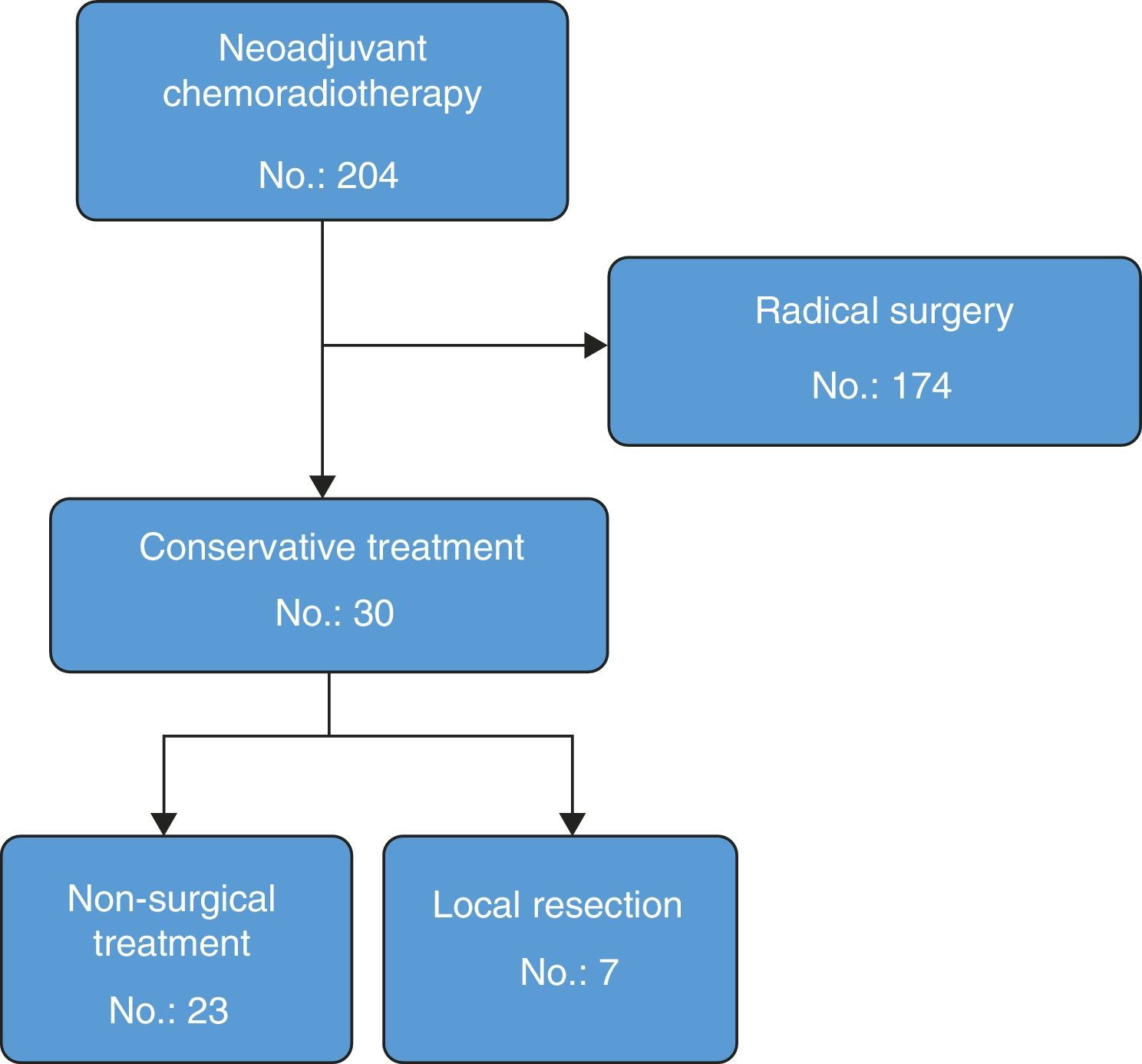
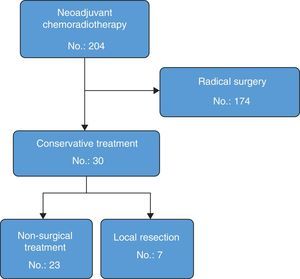
ResultsA total of 204 patients with rectal cancer received neoadjuvant therapy. Thirty (14.7%) had a good response and were treated with rectal preservation (23 “Watch and wait” and 7 local resections). Median follow-up was 46 months (interquartile range: 30–68). In the group of “Watch & Wait”, 4 patients had local recurrence before 12 months (actuarial local recurrence rate=18.5%). All of them underwent salvage surgery (2 with radical surgery and 2 local resections) without any further recurrence. Disease-free survival actuarial rate at 3 years follow-up was 94.1% (95% CI 82.9–100). None of the 7 patients that were treated by local excision had local recurrence. The organ preservation rate for the whole group was 93%.
ConclusionThe strategy of organ preservation in locally advanced rectal cancer is feasible in cases with good response to neoadjuvant therapy. When implemented in a highly selected group of patients this strategy is associated with satisfactory oncologic results.
El estándar de tratamiento del cáncer de recto localmente avanzado es la escisión total del mesorrecto. Sin embargo, la preservación del órgano ha sido propuesta para los tumores con buena respuesta al tratamiento neoadyuvante. El objetivo de este estudio es investigar los resultados oncológicos de esta estrategia.
MétodosSe realizó un estudio de cohorte retrospectivo, en el que se analizó a los pacientes con adenocarcinoma de recto tratados con intención curativa entre 2005 y 2014 que, después de recibir quimiorradioterapia neoadyuvante, presentaron una respuesta clínica completa o casi completa y fueron tratados con preservación del recto.
ResultadosDurante el periodo de estudio, 204 pacientes con cáncer del recto recibieron neoadyuvancia. Treinta (14,7%) presentaron una respuesta clínica completa o casi completa y se trataron según una estrategia de preservación de órgano (23 watch & wait y 7 resecciones locales). La mediana de seguimiento fue de 46 meses (rango intercuartil: 30-68). En el grupo de watch & wait, 4 casos presentaron recurrencia local antes del año (tasa actuarial 18,5%). Todos pudieron ser rescatados (2 con cirugía radical y 2 con resecciones locales) sin presentar nuevas recurrencias. El índice de supervivencia libre de enfermedad a distancia a 3 años fue de 94,1% (IC 95%: 82,9-100). De los 7 casos que se trataron mediante resección local, ninguno presentó recurrencia local. Considerando toda la muestra, la proporción de conservación de órgano fue del 93%.
ConclusionesLa estrategia de preservación de órgano en el cáncer rectal localmente avanzado es factible en casos con buena respuesta a la neoadyuvancia. Implementada en un grupo altamente seleccionado de pacientes, se asocia con resultados oncológicos satisfactorios.
Total mesorectal excision (TME) is currently the only accepted curative treatment for locally advanced rectal cancer. However, some surgical groups1,2 have reported oncologically satisfactory results with local resection after neoadjuvant treatment in select patients who were not apt for surgery. Meanwhile, Habr-Gama et al. have described acceptable long-term results using a non-surgical watch-and-wait approach in patients who presented complete clinical response after neoadjuvant chemoradiotherapy (CRTx).3–5 Although the intention to preserve the rectum has demonstrated oncological results similar to radical surgery in selected cases,6 the role of this strategy is still controversial.
The objective of the present study is to report the long-term oncological results of a rectum-preserving strategy after neoadjuvant CRTx.
MethodsWe carried out a retrospective, observational cohort study based on a prospective database of colorectal cancer at a tertiary referral hospital (the Hospital Italiano in Buenos Aires). Patients analysed in this study had received neoadjuvant therapy for tumours of the middle or lower rectum (less than 11cm from the anal margin) that were locally advanced (extrarectal invasion detected during rectal examination, sphincter invasion, or magnetic resonance imaging [MRI] results showing compromised circumferential resection margin [≤2mm] or tumour invasion of the mesorectum greater than 5mm) between January 2005 and June 2014. The patients included for analysis had complete or almost complete clinical response as defined by the Habr-Gama et al. criteria.7 Complete clinical response was defined by the disappearance of the lesion with or without residual scarring, whitish mucosa or telangiectasia. Near-complete clinical response was defined by the clinical or radiological persistence of a lesion smaller than 2cm that was mobile and non-ulcerated.
Preoperative staging was determined by rectal examination, colonoscopy, computed tomography (CT) scans of the thorax, abdomen and pelvis, and high-resolution diffusion MRI and CEA. The tumour distance to the anal margin was measured by rectal examination and rigid rectoscopy. Neoadjuvant therapy involved 5040cGy divided into 180cGy per day, 5 days per week and 6 cycles of chemotherapy with 5-fluorouracil (1000mg/m2/day) as well as leucovorin (25mg/m2/day). The evaluation of the clinical response took place between 8 and 12 weeks after the end of radiotherapy and included rectal examination and rectoscopy.
Patients with complete clinical response were re-evaluated with rectoscopy, CEA, MRI and CT scan. When the study results were negative, patients were offered the non-surgical treatment protocol. Patients with near-complete clinical response were treated with radical surgery or local resection, depending on the height of the lesion or patient/surgeon preference.
The degree of tumour regression was reported in accordance with the Dworak grading system8: grade 0=no tumour regression; grade 1=predominantly tumour; grade 2=predominantly fibrosis; grade 3=scattered tumour cells; and, grade 4=no tumour cells.
Follow-up included digital rectal examination and endoscopic evaluation with rigid or flexible rectoscopy monthly for the first year, every 2 months the second year, and every 6 months the third year. Complete colonoscopy was performed after 12 months, as well as high-resolution diffusion MRI every 3–6 months and CT scan of the thorax, abdomen and pelvis every 3–6 months. In accordance with the findings, in recent years PET/CT has been included in the follow-up. No biopsies were taken of scar tissue. Local recurrence was defined as the reappearance of tumour lesions after complete clinical response or local resection, regardless of the time of its presentation.
All the cases were discussed in a multidisciplinary committee of oncologists, radiologists and surgeons. The study was carried out in accordance with the guidelines of the Declaration of Helsinki, while respecting data confidentiality and the regulations of the local Ethics Committee.
Statistical AnalysisThe continuous variables were expressed as median and interquartile range. The categorical data were expressed as number of patients and percentage. Survival rates were calculated with the Kaplan–Meier method.
ResultsDuring the study, 204 rectal cancer patients received neoadjuvant CRTx with curative intent. Out of these, 174 (85%) were treated with post-neoadjuvant radical surgery: 131 anterior resections (75%) and 43 abdominoperineal resections (25%). Seven patients with radical resection had complete clinical response and 4 had complete pathological response; another 7 had a near-complete clinical response and, out of these, 2 had a complete pathological response. In total, the rate of complete pathological response in the patients who were treated with radical surgery was 13% (23 cases, ypT0 ypN0). In 30 of the 204 patients (14.7%), an organ-preserving strategy was used. These patients comprise the population analysed in this present study (Fig. 1). Average age was 71 (interquartile range: 45–76), and 56% were males. Mean tumour distance from the lower edge of the anal margin was 4cm (range: 2–8). In 29 patients (93%), the lesion was located in the lower rectum. Mean follow-up was 46 months (interquartile range: 30–68). Twenty-three patients (76%) had complete clinical response and initiated the non-surgical treatment strategy. In 7 patients (24%) with near-complete clinical response, it was decided to perform local resection.
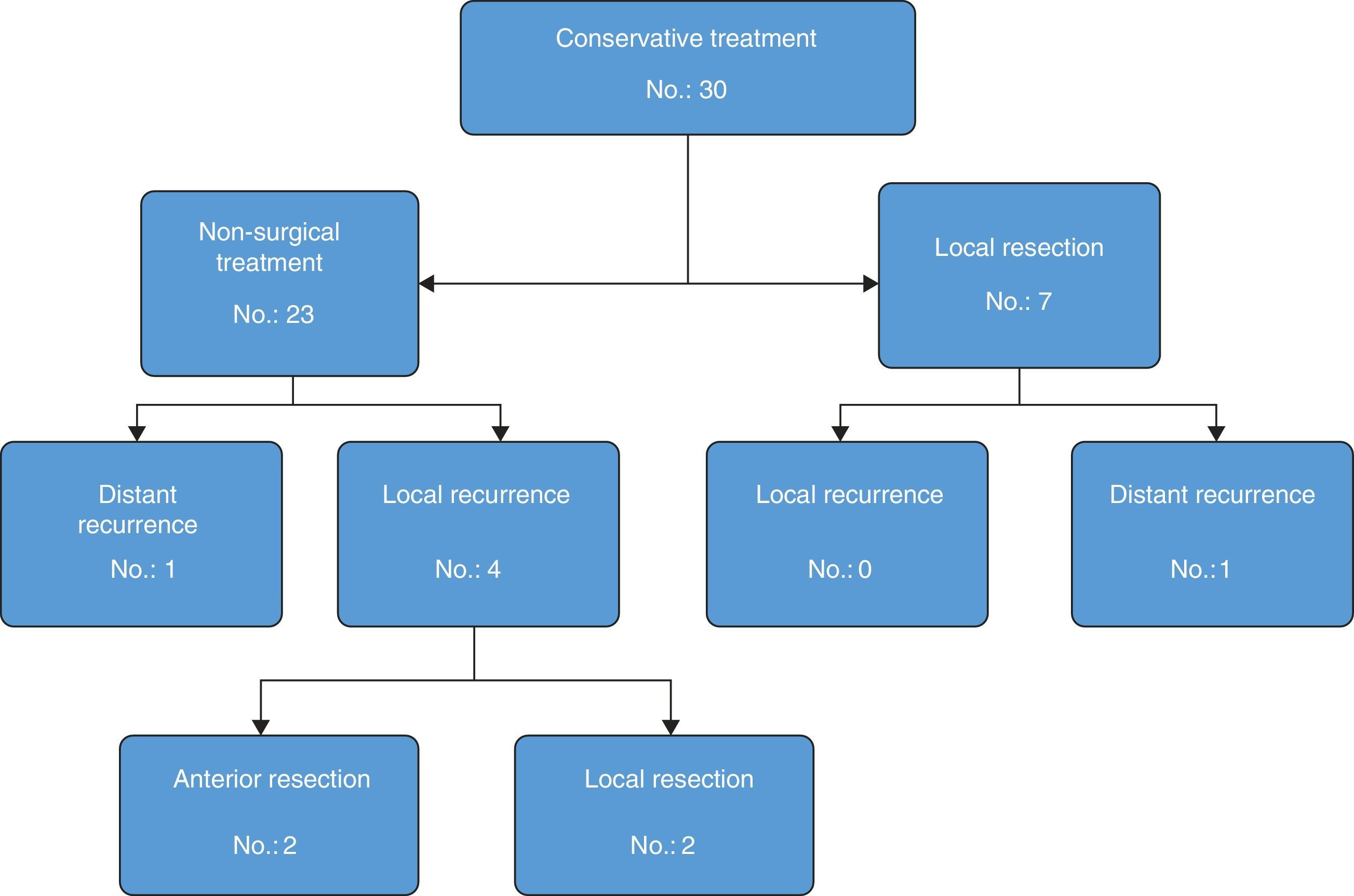
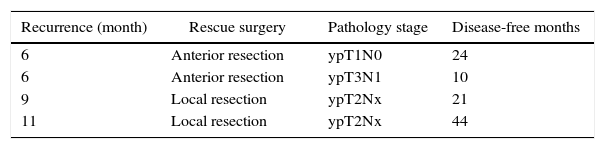
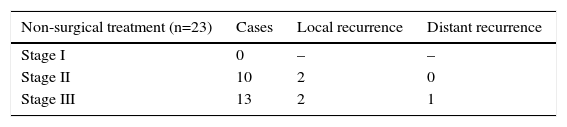
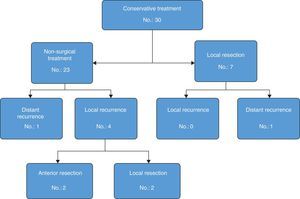
Out of the 23 patients who initiated non-surgical treatment, 4 cases presented local recurrence within one year (actuarial rate of local recurrence: 18.5%). These recurrences were detected by rectal examination, MRI and, in certain cases, PET/CT (7 out of 23 patients); the results of the MRI and PET/CT correlated in all cases except one (one patient had negative rectal exam and MRI, positive PET/CT–CT and confirmed recurrence). These patients were rescued with radical surgery in 2 cases and local resection in the remaining 2 because they were tumours with sphincter invasion and the patients had rejected definitive colostomy (Table 1). The organ preservation rate was 91% (21/23 patients). The 19 remaining patients with sustained complete clinical response presented no local recurrence after one year. One patient, however, presented liver metastasis after 5 months, which was resected by segmentectomy: the patient died after 30 months due to progression of the liver disease. The total 3-year disease-free survival rate was 94.1% (95% CI: 82.9–100).
Out of the 7 cases with near-complete clinical response treated with local resection, the pathology studies revealed ypT1 lesions in 2 cases (both Dworak 3) and ypT2 in the remaining 5 cases (Dworak 2). None of the patients presented local recurrence. One patient presented pulmonary and hepatic metastases, which were treated by means of lobectomy and radiofrequency after 6 and 15 months, respectively. The patient was disease-free after 85 months of follow-up. Fig. 2 shows the evolution of the patients who had been treated with the organ-preservation strategy.
Tables 2 and 3 present the recurrence types according to initial tumour stage on MRI in patients who had agreed to non-surgical treatment but then later underwent local resection. It was possible to preserve the rectum in 28 patients of the population analysed, and only 2 needed to be treated with radical surgery. Hence, the total organ preservation percentage was 93%.
The interest in conservative treatment for rectal cancer as a therapeutic alternative to radical surgery has increased in the last decade. At our institution, we started to implement this strategy after the publication of the Habr-Gama et al. study in 2004,3 which included 71 patients and reported local, distant and overall recurrence rates of 2.8, 4.2 and 7%, respectively. This study was questioned, however, due to the lack of clarity of its inclusion criteria and the exclusion of relapses within the first year. In 2013, this same group published a prospective study of 70 patients with a higher dose of radiotherapy (5400cGy),4 which provided a complete clinical response rate of 68%, an early local recurrence rate of 17% (8 cases) and a late recurrence rate of 10% (4 cases). Three-year overall survival and disease-free survival rates in patients with sustained complete clinical response were 94 and 75%, respectively.
In a systematic review of 9 series with 650 patients, Glynne-Jones et al.9 concluded that the justification of the non-surgical treatment strategy is based on retrospective series, and that the good results observed in small-sized tumours should not be indiscriminately extrapolated to more advanced tumours.
In 2011, Maas et al.10 compared patients treated without surgery with those who presented complete pathologic response in the radical resection surgical specimen (ypT0 ypN0). In the non-surgical group (21 patients), mean follow-up was 25 months and local recurrence was 4% (one case was successfully treated after 22 months with local resection). In the control group (20 patients), there were no local recurrences. The 2-year disease-free survival rates were 89 and 93% (P=.77) and overall survival rates were 100 and 91%, respectively (P=.2). In 2012, the group from Memorial Sloan-Kettering Cancer Center6 published a similar study: out of 32 patients treated without surgery, 4 presented early local recurrence (12%) and 2 late recurrence (6%). The sustained complete clinical response rate was 87% (28 cases). All patients with local recurrence were able to be treated with anterior resection. Out of the 57 patients of the control group (radical surgery with complete pathological response), none developed local recurrence, while 3 patients developed distant metastasis. The disease-free survival rates between the patients who entered the non-surgical treatment protocol and those who underwent surgery were 88 and 98%, respectively (P=.27), and overall survival rates were 97 and 100%, respectively (P=.56). The results of our series are within the rates reported by the previously mentioned groups, especially for the relatively high rate of relapse within the first year. Although there were 4 local recurrences during the first year of follow-up, all patients were able to be rescued without developing new recurrences. This phenomenon was compensated, as in the other publications, by the possibility to perform curative rescue surgeries (even with sphincter preservation) thanks to the strict follow-up protocol implemented. In this regard, Habr-Gama et al.11 recently published a retrospective study that demonstrated the feasibility and long-term oncological results of patients who had presented local recurrence. In 93% of these cases, rescue surgery was able to be done, and in 89% the surgery was R0. One case was treated with brachytherapy, and the 2 remaining cases presented non-resectable local recurrences.
When persistence of the primary tumour is observed after local resection, the safest recommendation is anterior resection with TME. This is justified by the high rates of lymph node metastases reported in ypT1 and ypT2 lesions, which reach 17 and 21%, respectively.12 However, it could be argued that those percentages were observed without considering the degree of clinical response and in patients operated on less than 8 weeks after finalising CRTx. As for post-neoadjuvant local resection, Oliva-Pérez et al.13 retrospectively analysed 27 patients who underwent transanal endoscopic resection and observed that the rate of local recurrence of the series was 15% after a mean follow-up of 15 months. This high rate of local recurrence has led the authors to the conclusion that local resection after neoadjuvant therapy should only be limited to patients with complete clinical response of the primary tumour, to very select cases of patients with residual tumours in whom TME involves abdominoperineal resection, or when the patient is high risk. Our position concurs with these criteria. In the patients of our series who presented residual tumour after local resection, we did not perform a TME since those tumours were only treatable by abdominoperineal resection and the patients had refused to have a definitive colostomy (5 cases), were very high risk (1 case) or had distant progression of the disease (1 case).
We also believe that it is important to consider the degree of tumour remission when it is not complete. In this direction, Berho et al.14 found in a series of 86 patients that those patients with complete or near-complete remission (Dworak 3 or 4) had a rate of lymph node metastasis that was significantly lower than those who did not have such a definite response (14% vs 37%). In a recent analysis of our series reported at the American Society of Colon & Rectal Surgeon Meeting in 2015,15 the degree of tumour regression was directly related with the lymph node metastasis rate: complete, near-complete and minimal response, presenting percentages of 8, 27.6 and 49.2%, respectively (P<.05).
The main limitation of the present study is the lack of uniformity in the selection of cases. The final treatment decision was based not only on the degree of clinical response but also the criteria of the treating surgeon, while taking into account the informed decision of the patients. Changes in rectal cancer treatment that have been proposed over the years by reference groups, as well as new evidence, all had an influence on the selection of treatment for our patients, which means that the group analysed with conservative treatment was not homogenous.
Based on our results, we can conclude that the organ preservation strategy after neoadjuvant CRTx is feasible and that, when implemented in a highly select group of patients, it is associated with very satisfactory oncologic results. Although these results are promising, we feel it must also be considered that there are currently no reports from randomised studies evaluating this strategy. Likewise, since no diagnostic study is able to identify with high sensitivity and specificity which cases will present complete pathologic response, patients should be selected by an interdisciplinary colorectal surgery committee within the framework of a defined protocol, and a strict follow-up programme is essential.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Vaccaro CA, Yazyi FJ, Ojra Quintana G, Santino JP, Sardi ME, Beder D, et al. Cáncer de recto localmente avanzado: resultados preliminares de la preservación del recto después de quimiorradioterapia neoadyuvante. Cir Esp. 2016;94:274–279.