To analyze the predictors of pCR in NSCLC patients who underwent anatomical lung resection after induction therapy and to evaluate the postoperative results of these patients.
MethodsAll patients prospectively registered in the database of the GE-VATS working group undergone anatomic lung resection by NSCLC after induction treatment and recruited between 12/20/2016 and 3/20/2018 were included in the study. The population was divided into two groups: patients who obtained a complete pathological response after induction (pCR) and patients who did not obtain a complete pathological response after induction (non-pCR). A multivariate analysis was performed using a binary logistic regression to determine the predictors of pCR and the postoperative results of patients were analyzed.
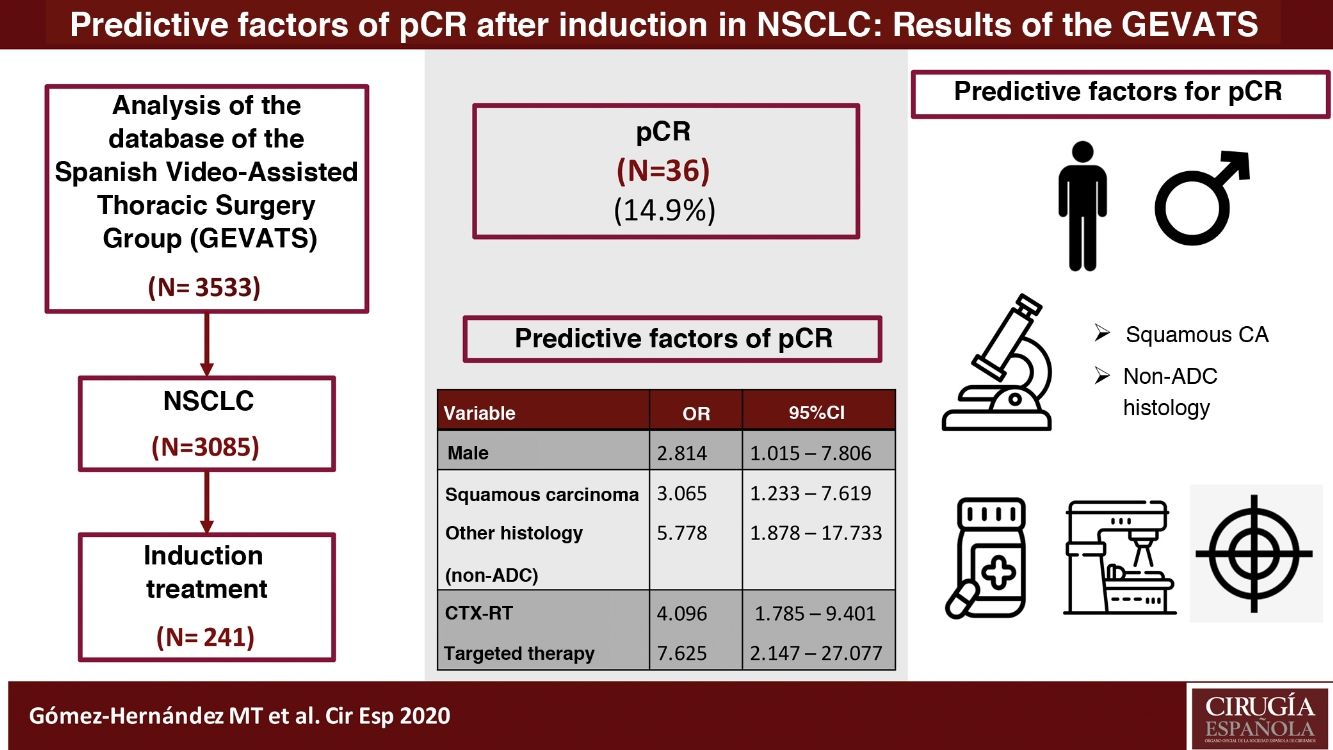
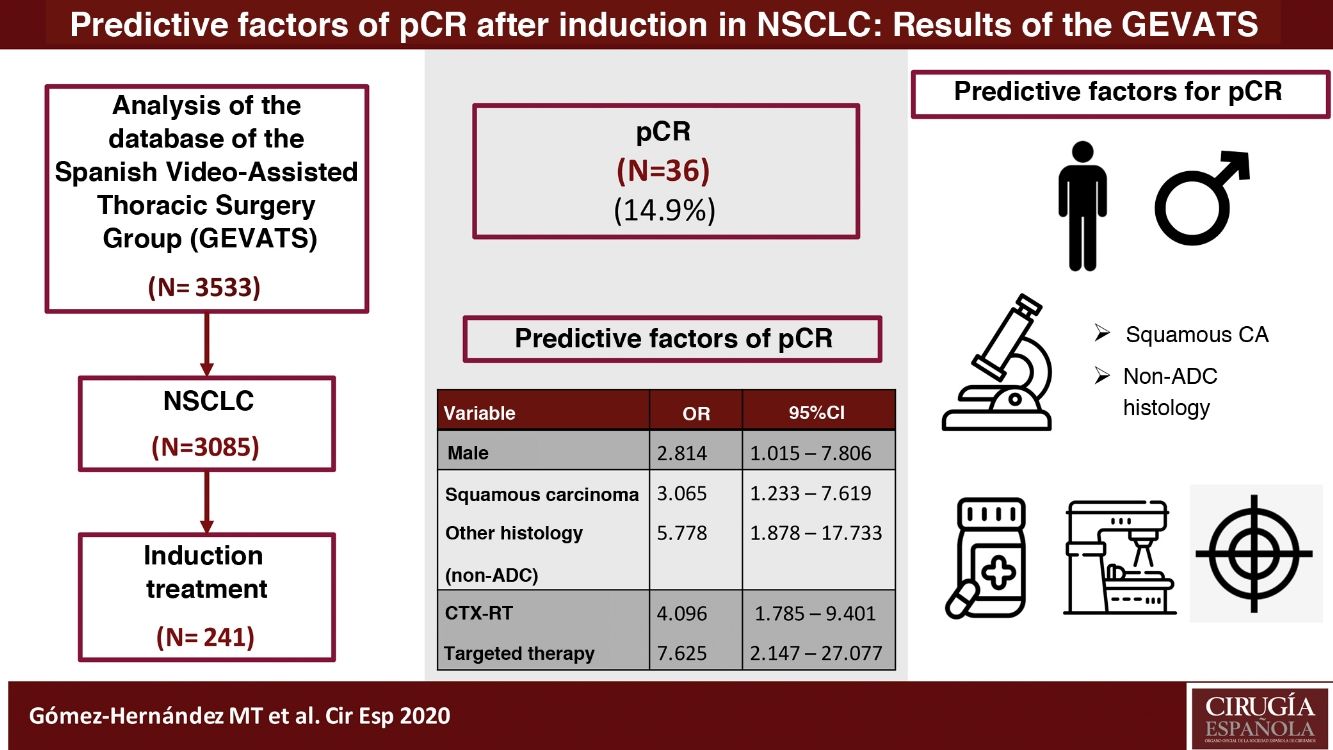
ResultsOf the 241 patients analyzed, 36 patients (14.9%) achieved pCR. Predictive factors for pCR are male sex (OR: 2.814, 95% CI: 1.015−7.806), histology of squamous carcinoma (OR: 3.065, 95% CI: 1.233−7.619) or other than adenocarcinoma (OR: 5.788, 95% CI: 1.878−17.733) and induction therapy that includes radiation therapy (OR: 4.096, 95% CI: 1.785−9.401) and targeted therapies (OR: 7.625, 95% CI: 2.147−27.077). Prevalence of postoperative pulmonary complications was higher in patients treated with neoadjuvant chemo-radiotherapy (p = 0.032).
ConclusionsMale sex, histology of squamous carcinoma or other than ADC, and induction therapy that includes radiotherapy or targeted therapy are positive predictors for obtaining pCR. Induction chemo-radiotherapy is associated with a higher risk of postoperative pulmonary complications.
Analizar los factores predictores de RCp en pacientes con CPNM sometidos a resección pulmonar anatómica tras terapia de inducción y evaluar los resultados postoperatorios de estos pacientes.
MétodosSe incluyeron en el estudio todos los pacientes registrados de forma prospectiva en la base de datos del grupo de trabajo GE-VATS reclutados entre el 20/12/2016 y el 20/3/2018 sometidos a resección pulmonar anatómica por CPNM tras tratamiento de inducción. La población se dividió en dos grupos: pacientes que obtuvieron respuesta completa patológica tras inducción (RCp) y pacientes que no obtuvieron una respuesta patológica completa tras inducción (no-RCp). Se realizó un análisis multivariante mediante una regresión logística binaria para determinar los factores predictores de RCp y se analizaron los resultados postoperatorios de los pacientes.
ResultadosDe los 241 pacientes analizados, 36 pacientes (14.9%) alcanzaron RCp. Los factores predictores de RCp son el sexo varón (OR: 2.814, IC 95%: 1.015−7.806), la histología de carcinoma escamoso (OR: 3.065, IC 95%: 1.233−7.619) u otra distinta de adenocarcinoma (OR: 5.788, IC 95%: 1.878−17.733) y la terapia de inducción que incluya radioterapia (OR: 4.096, IC 95%: 1.785−9.401) y terapias dirigidas (OR: 7.625, IC 95%: 2.147−27.077). La ocurrencia de complicaciones respiratorias postoperatorias fue superior en los pacientes que recibieron quimio-radioterapia de inducción (p = 0.032).
ConclusionesEl sexo varón, la histología de carcinoma escamoso o distinta de ADC y la terapia de inducción que incluya radioterapia o terapia dirigida son factores predictores positivos para la obtención de RCp. La quimio-radioterapia de inducción se asocia con un mayor riesgo de complicaciones respiratorias postoperatorias.
Induction therapy followed by surgical resection and lymphadenectomy is one of the treatment options for patients diagnosed with locally advanced non-small-cell lung cancer (NSCLC)1. The goals of neoadjuvant therapy are to control and eliminate occult metastases, while reducing the size of the primary tumor and mediastinal lymph node metastases. The results of this therapeutic strategy vary considerably, from unintended disease progression to pathological complete response (pCR). When pathological complete response is obtained, defined as the absence of tumor cells in all resection samples (ypT0N0M0) after induction therapy, the long-term results are very favorable2–4. According to published data, the 5-year survival in this group of patients ranges between 53% and 67%5–11, which is similar to patients with stage Ib disease12. pCR is therefore a good prognostic factor in patients with locally advanced NSCLC treated with induction therapy and surgery.
According to different published studies, the pCR rates achieved after induction therapy vary widely, from 8% to 48%5,6,13–18. However, the studies mentioned include a small number of patients recruited for long periods in a single institution and are mainly focused on long-term results. Furthermore, significant progress has been made in recent years in the treatment of NSCLC, such as improved surgical techniques, perioperative patient management, chemotherapy, targeted therapies, and immunotherapy, which have been able to positively influence obtaining pCR and in the short- and long-term results of these patients.
Currently, however, the response rate to neoadjuvant protocols cannot be predicted in advance, and predictors of pCR have not been extensively studied to date. According to the results of Kayawake et al.18, only squamous cell carcinoma histology is positively associated with obtaining pCR.
The objectives of this present study are to identify the predictors of pCR in patients with NSCLC undergoing anatomical lung resection after induction therapy and to evaluate the postoperative results of these patients by analyzing the data of the patients registered prospectively in the multicenter database created by the Spanish Group of Video-Assisted Thoracic Surgery (GE-VATS), belonging to the Spanish Society of Thoracic Surgery (SECT). The results obtained will provide an updated view of the most relevant predictive factors for obtaining pCR in a national cohort of patients with NSCLC treated with induction therapy.
MethodsStudy populationThe study included all patients who had been prospectively registered in the GE-VATS database and had undergone anatomical lung resection for NSCLC after receiving induction treatment. The patients were recruited during the period between December 20, 2016 and March 20, 2018 (15 months) by 33 Spanish thoracic surgery departments. The study was approved by the ethics committees of all participating hospitals, and specific informed consent was obtained for this study. The methodology, audit and initial results of the study have been recently published by Embún et al.19
Clinical staging of NSCLC before induction therapy was performed based on computed tomography (CT) and positron emission tomography (PET) findings, in accordance with the staging protocol proposed in the 8th edition of the TNM classification for lung cancer20. Invasive staging methods to determine lymph node status were not performed routinely in all participating hospitals, so clinical staging was based on imaging tests.
The indication for induction treatment and the type of therapy administered were determined by the multidisciplinary oncology committees of each participating hospital. Basically, induction therapy was considered in cases of suspected N2 lymph node involvement, centrally located tumors, and tumors with suspected invasion of adjacent organs to ensure free surgical margins.
The population was divided into two groups: patients who obtained pathological complete response after induction (pCR) and patients who did not obtain a pathological complete response after induction (non-pCR).
Statistical analysisFirst, we analyzed the predictive factors for pCR in all patients undergoing anatomical resection after induction therapy.
The variable selected as the result was the achievement of pCR, defined as the absence of tumor cells in all resection samples (ypT0N0M0).
The baseline demographic, oncological, and surgical variables of the patients were evaluated to detect a possible association with obtaining pCR. The variables were initially assessed using a bivariate analysis. Only statistically significant variables were used as independent predictor variables in the logistic regression analysis. Data for continuous quantitative variables were expressed as mean ± standard deviation. The normal distribution of the numerical variables was previously evaluated with the Kolmogorov-Smirnov normality test. Numerical variables with normal distribution were analyzed with the Student’s t-test for independent data, while those without normal distribution were analyzed with the Mann-Whitney U test Categorical variables were expressed as frequencies and percentages and were analyzed with the chi-squared or Fisher’s exact test if the expected frequency was less than 5. The statistically significant variables in the bivariate analysis were used as independent variables in the multivariate analysis performed using binary logistic regression. Results are presented as odds ratio (OR) with 95% confidence interval (CI) and P-value.
Secondly, we analyzed the occurrence of global postoperative morbidity (reoperation, wound infection, respiratory complications, cardiovascular complications, etc.), hospital mortality, and 90-day mortality in the overall series and according to the induction treatment received using the chi-squared test.
For all analyses, a P-value <.05 was considered statistically significant. The data analysis was performed using SPSS® version 26 (IBM Corp, Chicago, Illinois, 2019).
ResultsDuring the study period, 3085 patients were diagnosed with lung cancer, 261 (8.46%) of whom received induction treatment prior to surgery. Twenty patients were excluded due to incomplete data (7.7%). Out of the 241 patients analyzed, 36 patients (14.9%) achieved pCR.
Table 1 shows the main demographic and clinical characteristics of the patients included in each group.
Demographic and clinical characteristics of the population.
| Variable | pCR (n = 36) | No pCR (n = 205) | P value |
|---|---|---|---|
| Age (years) | 61.98 ± 8.27 | 62.13 ± 9.2 | 0.523 |
| Sex, male, n (%) | 30 (83.3) | 132 (64.4) | 0.026 |
| BMI | 27.07 ± 4.36 | 26.08 ± 3.96 | 0.174 |
| Smoking, n (%) | 0.580 | ||
| Never-smoker | 1 (2.8) | 14 (6.8) | |
| Ex-smoker <12 months | 15 (41.7) | 100 (48.8) | |
| Ex-smoker >12 months | 10 (27.8) | 45 (22) | |
| Active smoker | 10 (27.8) | 46 (22.4) | |
| Ischemic heart disease, n (%) | 4 (11.1) | 14 (6.8) | 0.321 |
| Creatinine >2 mg/dL, n (%) | 0 (0) | 8 (3.9) | 0.610 |
| VEF1ppo% | 62.8 ± 14.42 | 66.03 ± 17.49 | 0.242 |
| DLCOppo% | 60.06 ± 19.07 | 57.84 ± 17.31 | 0.494 |
The results are expressed as mean ± standard deviation, except when otherwise specified. The data in bold indicate statistically significant values.
63.9% of patients received chemotherapy (CTx) as the only induction therapy, while 28.6% were treated with induction chemo-radiotherapy (CTX-RT), and 7.5% of patients targeted therapies, either associated or not with CTx.
Table 2 describes the oncological and surgical characteristics of the patients included in each group.
Oncological and surgical characteristics.
| Variable | pCR (n = 36) | No pCR (n = 205) | P value |
|---|---|---|---|
| Tumor size >3 cm, n (%) | 12 (35.3) | 93 (45.6) | 0.263 |
| Tumor density, n (%) | 0.481 | ||
| Solid | 34 (94.4) | 186 (90.7) | |
| Mixed | 1 (2.8) | 16 (7.8) | |
| Ground glass | 1 (2.8) | 3 (1.5) | |
| Central location, n (%) | 25 (69.4) | 111 (54.1) | 0.088 |
| Clinical N stage (PET), n (%) | 0.265 | ||
| N0 | 9 (25) | 75 (36.6) | |
| N1 | 5 (13.9) | 17 (8.3) | |
| N2 | 22 (61.1) | 106 (51.7) | |
| N3 | 0 (0) | 7 (3.4) | |
| Histology, n (%) | 0.001 | ||
| ADC | 9 (29) | 114 (58.8) | |
| Squamous cell carcinoma | 18 (58.1) | 73 (37.6) | |
| Other | 4 (12.9) | 7 (3.6) | |
| Induction therapy, n (%) | 0.000 | ||
| CTx | 12 (33.3) | 142 (69.3) | |
| CTX-RT | 18 (50) | 51 (24.9) | |
| Targeted therapy | 6 (16.7) | 12 (5.9) | |
| Resection type, n (%) | 0.053 | ||
| Lobectomy/bilobectomy | 30 (83.3) | 170 (82.9) | |
| Segmentectomy | 1 (2.8) | 0 (0) | |
| Pneumonectomy | 5 (13.9) | 35 (17.1) | |
| Extended resection, n (%) | 9 (25) | 30 (14.6) | 0.119 |
| Approach, n (%) | 0.773 | ||
| Thoracotomy | 27 (75) | 149 (72.7) | |
| VATS | 9 (25) | 56 (27.3) |
ADC: adenocarcinoma; CTx: chemotherapy; CTX-RT: chemoradiotherapy; pCR: pathologic complete response; VATS: video-assisted thoracoscopic surgery. The data in bold indicate statistically significant values.
The predictor variables associated with obtaining pCR in the logistic regression model were male sex, histology of squamous cell carcinoma (or other than adenocarcinoma), and induction therapy that included radiotherapy or targeted therapy. The results are shown in Table 3.
Multivariate analysis of predictive factors associated with pCR in patients with NSCLC (expressed in odds ratio with 95% confidence interval).
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Sex | |||
| Female | 1 | ||
| Male | 2.814 | 1.015−7.806 | 0.047 |
| Histology | |||
| ADC | 1 | ||
| Squamous carcinoma | 3.065 | 1.233−7.619 | 0.016 |
| Other | 5.778 | 1.878−17.733 | 0.002 |
| Type of induction | |||
| CTx | 1 | ||
| CTX-RT | 4.096 | 1.785−9.401 | 0.001 |
| Targeted therapy | 7.625 | 2.147−27.077 | 0.002 |
ADC: adenocarcinoma; 95% CI: 95% confidence interval; OR: odds ratio; CTx: chemotherapy; CTX-RT: chemoradiotherapy. Data in bold indicate statistically significant values.
Regarding the postoperative results, 34% of the patients in the global series presented postoperative complications, and the 30-day readmission rate was 9.5%. In-hospital and 90-day mortality rates were 0.8% and 3.3%, respectively. No significant differences were detected in postoperative adverse effects depending on the induction treatment received, except in the occurrence of postoperative pulmonary complications, which were significantly higher in patients treated with induction CTX-RT (Table 4).
Postoperative results of the overall series and depending on the induction treatment received.
| Result | Total (n = 241) | CTx (n = 154) | CTX-RT (n = 69) | Targeted therapy (n = 18) | P-value |
|---|---|---|---|---|---|
| Total complications, n (%) | 82 (34) | 49 (31.8) | 30 (43.5) | 3 (16.7) | 0.064 |
| Re-operation | 12 (5) | 5 (3.2) | 6 (8.7) | 1 (5.6) | 0.223 |
| Wound infection | 5 (2.1) | 1 (1.3) | 3 (4.3) | 0 (0) | 0.274 |
| Respiratory complications | 47 (19.5) | 26 (16.9) | 30 (29) | 1 (5.6) | 0.032 |
| Cardiovascular complications | 32 (13.3) | 17 (11) | 13 (18.8) | 2 (11.1) | 0.273 |
| Other complications | 12 (5) | 9 (5.8) | 3 (4.3) | 0 (0) | 0.537 |
| Readmission, n (%) | 21 (9.5) | 13 (9) | 6 (9.8) | 2 (12.5) | 0.894 |
| Hospital mortality, n (%) | 2 (0.8) | 0 (0) | 2 (2.9) | 0 (0.0) | 0.081 |
| 90-day mortality, n (%) | 8 (3.3) | 3 (1.9) | 4 (5.8) | 1 (5.6) | 0.286 |
CTx: chemotherapy; CTX-RT: chemoradiotherapy. Data in bold indicate statistically significant values.
The most relevant findings of our study reveal that almost 15% of patients who underwent anatomical lung resection achieved pCR after induction therapy based on different regimens (CTx, CTX-RT and targeted therapy). This figure is consistent with the results of previously published studies5,6,13–18.
Second, the factors that were positively associated with obtaining pCR were male sex, histology of squamous cell carcinoma or types other than adenocarcinoma, and induction therapy that included radiotherapy or targeted therapies.
According to the results of our study, men are 2.81 times more likely to obtain pCR after induction than women. There are studies that suggest that the effect of CTx may be greater in women21, although the cause has not been clearly defined. However, a meta-analysis analyzing 11 randomized clinical trials (n = 2288) did not find clear evidence that sex was associated with a greater or lesser benefit of preoperative CTx22.
Also, similar to the report by Kayawake et al.18, we identified squamous cell carcinoma histology and other histological variants other than adenocarcinoma as positive predictive factors to achieve pCR. The probability to obtain pCR is 3 times higher in patients with squamous cell carcinoma compared to patients with adenocarcinoma. The mechanism underlying the association between squamous cell carcinoma and pCR is uncertain, but it is likely that clinicopathological and immunological differences between adenocarcinoma and other histological types may play an important role23,24. Similarly, the histological subtype has been considered a prognostic factor in patients with NSCLC, showing better survival results in patients with squamous cell histology25. However, a meta-analysis by the NSCLC Meta-analysis Collaborative Group (mentioned above)22, which analyzed the results of 14 clinical trials (n = 2359) did not identify clear evidence that the effect of preoperative CTx on survival differed depending on the histological subtype (squamous cell carcinoma versus adenocarcinoma).
The association of CTx and RT is a positive predictive factor for pCR in our analysis. The probability of obtaining pCR after preoperative treatment with CTX-RT is four times higher than with CTx alone. Among the 69 patients treated with induction CTX-RT, 18 (26.1%) achieved pCR, compared to 7.8% of the patients who received induction with CTx alone. Previous studies in which induction therapy was based on CTX-RT describe similar pCR rates, ranging from 22 %–46 %5,9–11,26. In addition, although Kayawake et al.18 did not identify RT as a positive predictive factor for obtaining pCR (OR 2.14 [0.85–5.95], P = .107), a recent study by Haque et al.27 (1750 patients treated with neoadjuvant CTX-RT and lobectomy recruited between 2004 and 2015) found that the radiation dose >54 Gy independently predicted achieving pCR.
Regarding targeted therapies, more than 30% of patients undergoing induction with this regimen achieved pCR, and the probability of obtaining pCR was almost 8 times higher in patients treated with targeted therapies like induction versus CTx alone. Therapies directed against specific molecular markers are associated with response rates that exceed 50%, with less toxicity than cytotoxic chemotherapy agents28. However, their role as induction therapy has not yet been extensively evaluated. Some case series have shown that this protocol is feasible and that surgical resection in patients treated with epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) inhibitors is not associated with new toxicities or higher incidence of perioperative complications29. However, long-term results from ongoing clinical trials will be needed to define the role of targeted therapies, such as induction agents.
Lastly, surgical resection after neoadjuvant therapy can be performed safely, with acceptable postoperative results. The surgical results of our series are slightly better than those published by Cerfolio et al.17, who described an overall morbidity rate of 37% and a hospital mortality rate of 2.3%, while Kayawake et al.18 reported a hospital mortality rate of 2.6%. However, our study reveals a higher prevalence of postoperative pulmonary complications in patients treated with induction CTX-RT, so the risk-benefit of this treatment strategy should be assessed individually, especially in patients with associated pulmonary pathology.
The main limitation of this study is that the data was obtained from a prospective multicenter database, whose main objective was to determine the degree of current implementation of the VATS approach for anatomical lung resections in Spain, as well as to determine the main results of this approach. Thus, we do not have certain data that could be relevant, such as the results of invasive staging, restaging after induction therapy, chemotherapy drugs used, dose of radiotherapy administered, and CTX-RT regimen used (sequential, concurrent, etc.) as neoadjuvant therapy. Likewise, we have not been able to analyze factors such as the SUV of the tumor or the degree of tumor differentiation due to the lack of complete data in the series.
Our series includes a total of 36 patients who achieved pCR after induction therapy. Considering the short duration of the recruitment period, it is one of the longest series published to date. In addition, given the prospective and multicenter nature of the study, we feel that the results reflect current clinical practice in Spain. Furthermore, we believe that this study opens the door to the creation of multidisciplinary, multi-institutional working groups and the development of joint research projects, which could resolve the main limitations of our analysis.
In conclusion, 15% of the patients we treated with induction therapy and surgery obtained pCR. In addition, we identified male sex, squamous cell carcinoma or non-ADC histology, and induction therapy (including radiation therapy or targeted therapy) as positive predictors for achieving a complete pathologic response. Lastly, we found no differences in early postoperative results between patients who achieved pCR versus those who did not.
These findings could be potentially relevant and very useful for the development of future therapeutic algorithms aimed at decision-making and treatment planning in patients with locally advanced NSCLC.
FundingAll the expenses associated with the creation and maintenance of the GEVATS database have been absorbed by Ethicon, Johnson & Johnson.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Gómez Hernández MT, Novoa Valentín NM, Fuentes Gago MG, Embún Flor R, Gómez de Antonio D, Jiménez López MF, et al. Factores predictores de respuesta completa patológica tras inducción (ypT0N0M0) en cáncer de pulmón no microcítico y resultados a corto plazo: resultados del Grupo Español de Cirugía Torácica Videoasistida (GE-VATS). Cir Esp. 2022;100:345–351.