Laparoscopy has not been as widely used in hepatic surgery as in other areas due to the complexity of this type of surgery and the lack of surgical teams with experience in both fields.
Material and methodsThe aim of this work is to present the technique used in our centre to perform left lateral sectionectomy (LLS) using laparoscopy. A total of 70 patients have been operated on using laparoscopy due to both benign and malignant liver between February 2000 and July 2010. An LLS was performed on twenty-one cases using the technique described. The surgical technique is described, highlighting aspects such as, the arrangement of the trocars, the mobilisation of the liver or hepatic transection. The morbidity and mortality associated with the procedure are analysed.
ResultsLLS was performed on 12 women and nine men, with ages between 35 and 89 years. The mean number of lesions was 1.4 (between 1 and 4), with a mean size of 3.5cm. The mean surgical time was 142min (between 90 and 210). There was one conversion to laparotomy. Complications were recorded in 3 (14%) patients. There were no repeat surgery, and one patient required a transfusion. The mean hospital stay was 4.3 days.
ConclusionsThe best techniques and the wide experience in laparoscopy have enabled this technique to become established, with a morbidity of less than 15% and zero mortality. LLS is a safe and effective technique in selected patients. The detailed description of this procedure may stimulate other surgery groups to perform this approach.
En cirugía hepática, la laparoscopia no ha conseguido la difusión obtenida en otras áreas debido a la complejidad de este tipo de cirugía y a la falta de equipos quirúrgicos con experiencia en ambos campos.
Material y métodosEl objetivo de este trabajo es presentar la técnica utilizada en nuestro centro para realizar la seccionectomía lateral izquierda por laparoscopia (SLI). Entre febrero de 2000 y julio de 2010, 70 pacientes han sido intervenidos por laparoscopia por patología hepática, tanto benigna como maligna. En veintiún casos se realizó una SLI según la técnica descrita. Se describe la técnica quirúrgica, destacando aspectos como la disposición de los trocares, la movilización del hígado o la transección hepática. Se analiza la morbimortalidad relacionada con el procedimiento.
ResultadosSe ha realizado la SLI en 12 mujeres y 9 hombres con edades comprendidas entre los 35 y los 89 años. El número de lesiones fue de 1,4 (entre 1 y 4), con un tamaño de 3,5cm. El tiempo operatorio fue de 142 min (entre 90 y 210). Hubo una conversión a laparotomía. Se registraron complicaciones en 3 pacientes (14%). No hubo reintervenciones y un paciente requirió una transfusión. La estancia media hospitalaria fue de 4,3 días.
ConclusionesLas mejoras técnicas y la mayor experiencia en laparoscopia han permitido plantear la realización de este procedimiento con una morbilidad inferior al 15% y una mortalidad nula. La SLI es una técnica segura y efectiva en pacientes seleccionados. La descripción detallada de este procedimiento puede estimular a otros grupos de cirugía hepática a realizar este abordaje.
The success obtained by laparoscopic abdominal surgery is unquestionable. However, hepatic surgery has not seen a widespread use of laparoscopic techniques, mostly due to the complexity inherent in these procedures and the lack of surgical teams specialised in both fields (hepatic and laparoscopic surgery).1–5
The first laparoscopic anatomical liver resection was performed in 1996, and in Spain the first procedure was performed in 2000.6 From its onset, the majority of authors have coincided in defining certain favourable liver segments that are more accessible for a laparoscopic approach, these being the left lateral and right anterior segments.7–9
The anatomy of the left lateral sector and the configuration of the portal and supra-hepatic pedicles facilitate a feasible and safe laparoscopic approach in this area, with oncological results similar to those achieved using open surgery when performing R0 resections with tumour-free margins. The laparoscopic approach for lesions on the left lateral segment should be considered the method of choice for experienced centres,10–12 with superior results in terms of blood losses, operational time, and hospital stay, when compared to the laparotomy approach.13–19
The aim of our study was to describe the surgical technique used for a left lateral sectionectomy (LLS) as performed by our surgical team, and to analyse the morbidity and mortality rates associated with this procedure.
Patients and MethodPatientsIn 2000, our institution started performing laparoscopic liver surgery. The first cases were for benign pathologies, but indications were later expanded to include laparoscopic resections for malignant ones. A total of 70 procedures have been performed using laparoscopic techniques for hepatic pathologies: 17 cases of cystic pathologies (6 simple cysts, 4 hydatid cysts, 6 cases of polycystic liver disease, and 1 cystadenoma), 10 cases of solid benign tumours (8 adenomas, 1 sclerosing cholangitis, and 1 haemangioma), and 43 cases of malignant tumours (21 metastases from colorectal cancer, 10 hepatocellular carcinomas [HCC], 5 metastases from breast cancer, 2 metastases from lung cancer, 2 cholangiocarcinomas, 1 lymphoma, 1 metastasis from a haemangioendothelioma, and 1 metastasis from a melanoma). Since 2005, laparoscopic resection of lesions in segments II and III has been well established.
Surgical TechniqueOur surgical protocol involved balanced general anaesthesia, using propofol for induction and sevoflurane and remifentanil on continuous perfusion as maintenance therapy. Blood pressure and central venous pressure were monitored throughout the procedure. During the operation, we also administered the necessary liquids to maintain diuresis at 0.5mg/kg/h, without exceeding a central venous pressure (CVP) of 6mm Hg, administering the necessary vasoactive drugs according to patient tolerance to clamping and unclamping of the hepatic hilum.
The patient was placed in the supine decubitus position with the legs spread (“French position”). The operating table was placed in the anti-Trendelenburg position. We used six trocars (three 10–12mm and three 5mm). Pneumoperitoneum was achieved using a Veress needle at the umbilical level. We used a pressure of 10–12mmHg, and CO2 for the pneumoperitoneum.
We used a 30° optic placed at the umbilical level. The 10–12mm working trocars were placed on each side of the median line, approximately 5cm above the navel. We then placed a 5mm trocar in the left hypochondrium and another in a very lateral position on the right hypochondrium, which was then used for the Pringle manoeuvre.
Finally, we placed the last 5mm trocar in the epigastric region for the traction of the round ligament of the liver during the transection. The surgeon was situated between the patient's legs, the first assistant to the left of the patient, and the second assistant to the right.
The abdominal cavity was then examined to rule out the presence of widespread disease in the case of malignant pathology.
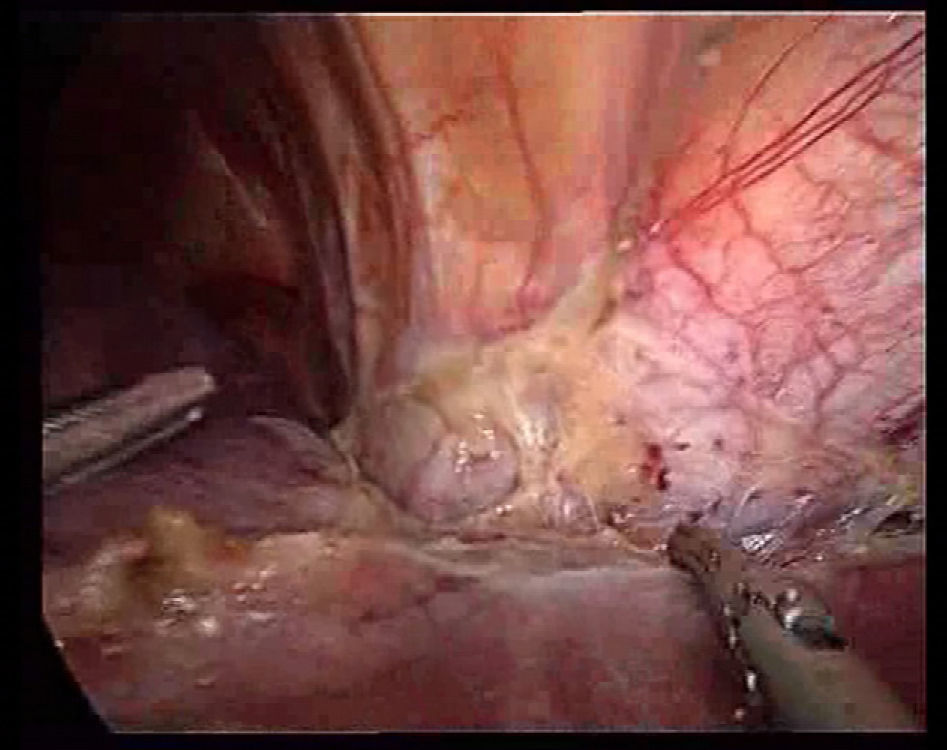
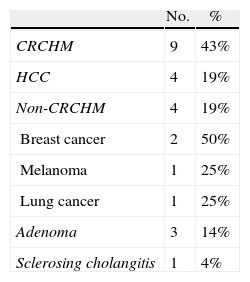
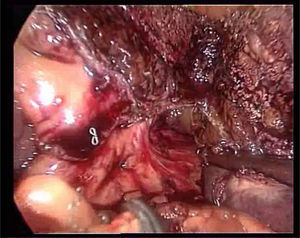
We proceeded with a dissection of the round ligament using Ligasure® V (Valleylab, Tyco Healthcare) or Ultracision® (Ethicon EndoSurgery, Johnson & Johnson Ltd., Cincinnati, OH, USA), cutting as close as possible to the abdominal wall for traction during the transection and to prevent it from obstructing the view. Then we dissected the falciform ligament until nearing the suprahepatic veins, since dissection of these vessels is favoured by the pneumoperitoneum (Fig. 1). This manoeuvre facilitates quick access to the suprahepatic veins for clamping, should it be necessary.
We then made an incision in the left triangular ligament, taking special care to avoid the left diaphragmatic vein, which passes very close to the ligament. Damage to this vein produces bleeding similar to the suprahepatic vein, which is very difficult to control and causes the risk of gas embolism. Complete mobilisation of segments II and III is essential since this allows for separating them from the diaphragm, facilitating the hepatic transection and the placement of endostaples at the proper angle.
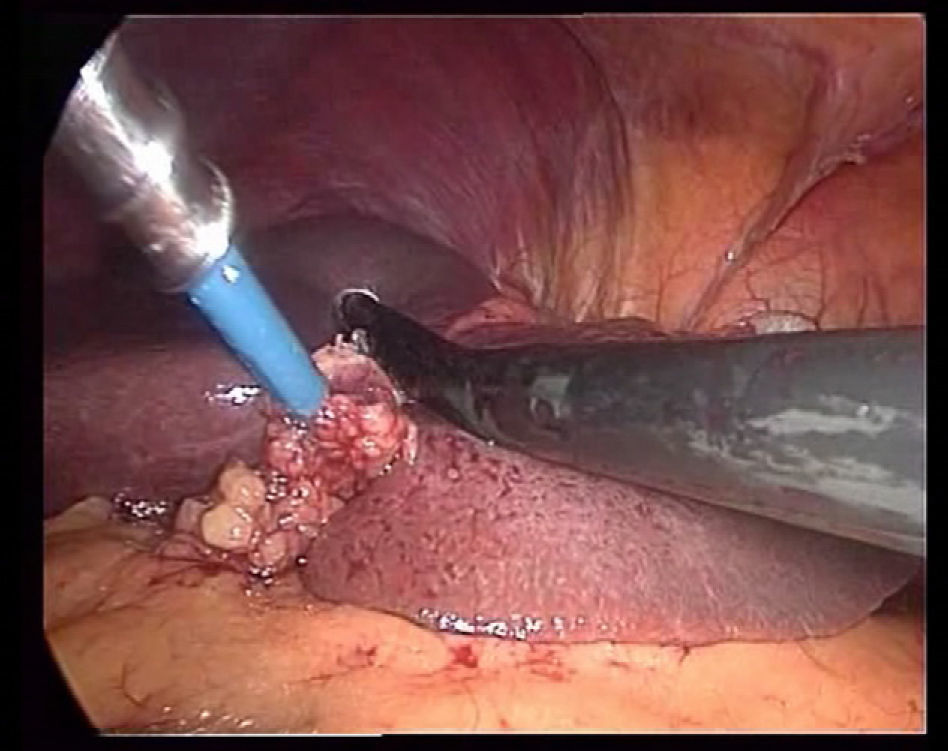
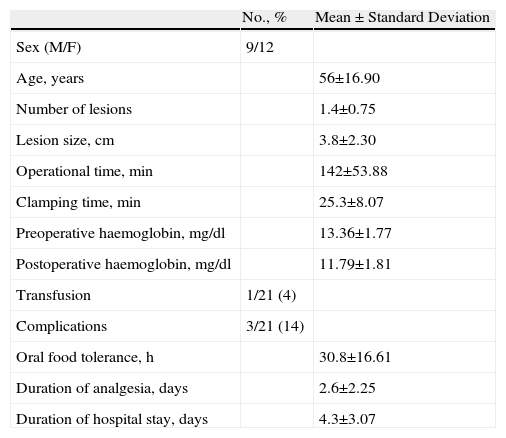
The use of intraoperative ultrasound (IOUS) (Fig. 2) is a standard procedure of this surgery since it allows us to: (a) rule out lesions that were not diagnosed before the procedure; (b) ensure a transection line with proper margins, and (c) diagnose variants of the primary pedicles in order to prevent haemorrhage during the transection.
The traction of the round ligament and the elevation of segment IV using a clamp allow for the exposure of the hepatic hilum and pars flaccida of the lesser omentum to be dissected. Using a clamp through the trocar situated on the lateral side of the right hypochondrium, a 10cm non-elastic band is passed under the hilum, through the foramen of Winslow, and out the opening we have made in the lesser omentum, encircling the hepatic hilum. The two ends are passed through a 2cm-long silastic tube that will serve to clamp the hilum using grasping forceps situated in the right lateral trocar. If the patient had undergone a previous operation on the hepatic hilum, passing the band through the foramen of Winslow can be impeded, and the surgeon can opt for the use of a laparoscopic clamp on the anterior face of the hepatic hilum.
It is of paramount importance during the hepatic transection to maintain CVP as low as possible, since this minimises blood loss. Due to the effect of the pneumoperitoneum on the CVP transducer, readings are not completely reliable, and so it is best to take these measurements before achieving pneumoperitoneum.
Several different devices can be used for the transection of normal hepatic parenchyma. The superficial part of the hepatic parenchyma has little vascularisation, facilitating the use of a monopolar scalpel down to a depth of 0.5–1cm. From this point, the underlying vessels are dissected using a laparoscopic ultrasonic dissector, with coagulation achieved using 5mm Ligasure Atlas® or Ultracision® combined with laparoscopic Tissuelink® (Salient Surgical Technologies). Tissuelink® provides superficial coagulation of the hepatic parenchyma and small vessels, while the Ligasure Atlas® and Ultracision® coagulate and divide the major vessels and parenchyma.
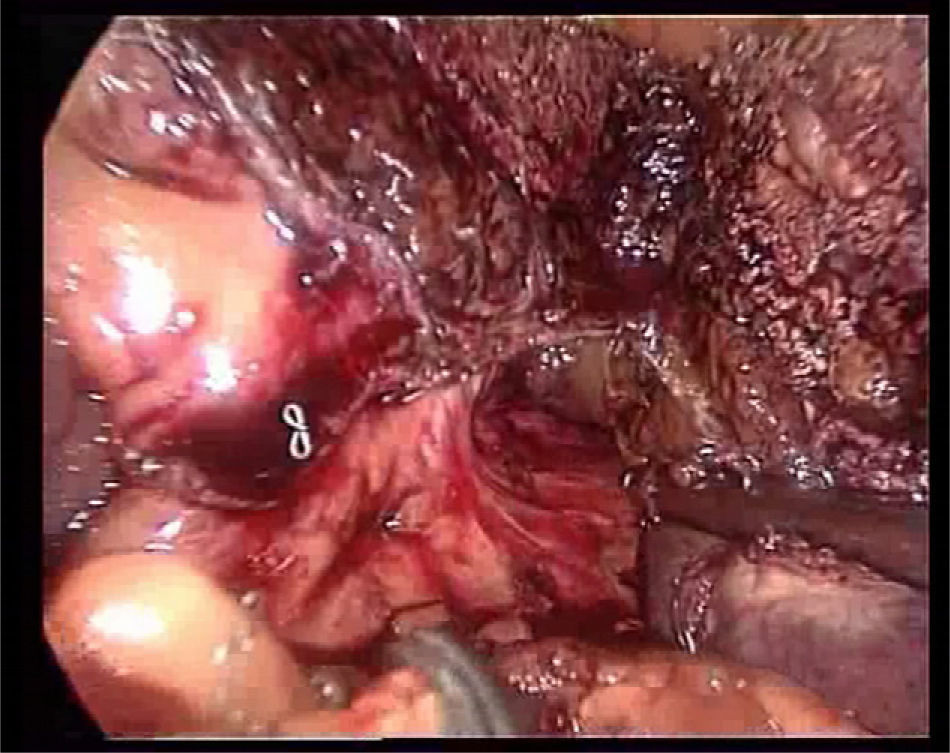
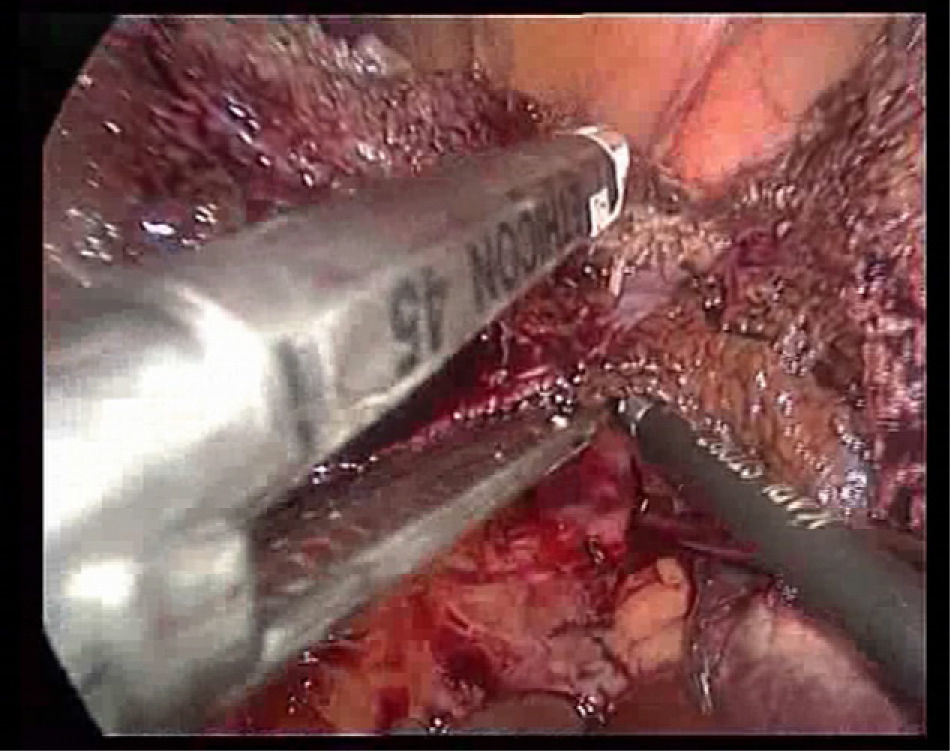
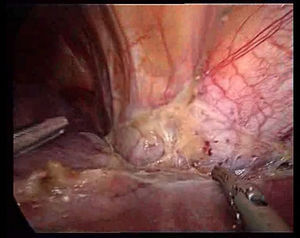
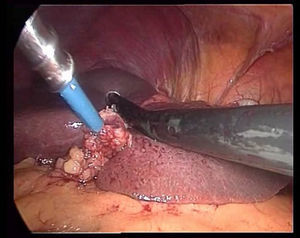
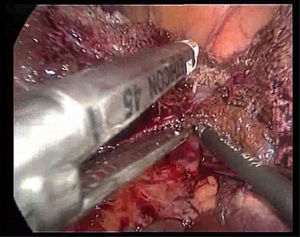
The transection is performed under continuous occlusion of the hepatic pedicle (Pringle manoeuvre). During the transection, the round ligament of the liver is under traction to the right using a clamp through the epigastric trocar, a line is marked for transection on the surface of the hepatic parenchyma by means of Tissuelink® surface coagulation, and then the transection of the superficial parenchyma performed using the two previously described devices until reaching the area of the main portal pedicles of the segment. Generally, one staple is needed for the segment III portal pedicle, another for the segment II portal pedicle (Fig. 3), and another for the sectioning of the left suprahepatic vein (Fig. 4).
With the goal of ensuring haemostasis and bile stasis, we applied a sponge made of a haemostatic-sealant material of collagen with fibrin and thrombin (Tachosil®, Nycomed) over the surface of the hepatic transection.
The whole surgical specimen is extracted through an accessory incision, within a bag. If the tumour was small, usually one of the 10–12mm trocar holes must be widened by 3–4cm. In patients with larger tumours, we used a Pfannenstiel incision. A suction drain is placed beneath the resection bed through the 5mm trocar in the right flank. The accessory incision and 10–12mm trocars are then closed using absorbable sutures.
ResultsBetween May 2005 and July 2010, 21 patients were operated upon using the procedure described here (Table 1). Nine of these patients were men and 12 were women, with ages ranging between 35 and 89 years.
Aetiology of the Laparoscopic LLS Performed at the Hospital Mútua de Terrassa (n=21). Time period: 2005–2010.
| No. | % | |
| CRCHM | 9 | 43% |
| HCC | 4 | 19% |
| Non-CRCHM | 4 | 19% |
| Breast cancer | 2 | 50% |
| Melanoma | 1 | 25% |
| Lung cancer | 1 | 25% |
| Adenoma | 3 | 14% |
| Sclerosing cholangitis | 1 | 4% |
HCC: hepatocellular carcinoma; CRCHM: colorectal cancer hepatic metastasis; non-CRCHM: non-colorectal cancer hepatic metastasis.
The surgery was for a malignant pathology in 17 patients (9 colorectal cancer metastases, 4 HCC, 2 breast cancer metastases, 1 melanoma metastasis, and 1 primary lung metastasis), and for benign pathologies in 4 cases (3 adenomas and 1 case of sclerosing cholangitis) (Table 1).
The mean number of lesions found per patient was 1.4 (range: 1–4), with a mean size of 3.8cm (range: 1–5cm). The mean duration of the procedure was 142min (range: 90–120min). In most cases (76%), we used a Pringle manoeuvre during the hepatic transection, with a mean duration of 26min (range: 10–60min).
We had to switch to a laparotomy in one case in order to complete the resection of a lesion that went undiagnosed in prior intraoperative ultrasound examinations. Postoperative complications were produced in 3 patients (14%): one infection of the surgical wound (grade I on the Dindo-Clavien scale), one pulmonary thromboembolism in a patient operated on for a metastasized (M1) melanoma, with little clinical repercussions (grade II), and one intra-abdominal collection in the resection bed that required a percutaneous drain (grade III). There were no second operations for any patients. We provided intraoperative blood transfusions in one patient with M1 breast cancer that had intraoperative bleeding from a lesion on an epiploic vessel that was controlled using laparoscopy.
The patients started oral tolerance at a mean 30h after the operation (range: 16–72h). The duration of analgesic requirements was 2.6 days (range: 2–4 days). The mean hospital stay was 4.3 days (range: 2–7 days). The histological analysis of the resection margins was negative in all cases (Table 2).
Results from Laparoscopic LLS Performed at the Hospital Mútua de Terrassa (n=21). Time period: 2005–2010.
| No., % | Mean±Standard Deviation | |
| Sex (M/F) | 9/12 | |
| Age, years | 56±16.90 | |
| Number of lesions | 1.4±0.75 | |
| Lesion size, cm | 3.8±2.30 | |
| Operational time, min | 142±53.88 | |
| Clamping time, min | 25.3±8.07 | |
| Preoperative haemoglobin, mg/dl | 13.36±1.77 | |
| Postoperative haemoglobin, mg/dl | 11.79±1.81 | |
| Transfusion | 1/21 (4) | |
| Complications | 3/21 (14) | |
| Oral food tolerance, h | 30.8±16.61 | |
| Duration of analgesia, days | 2.6±2.25 | |
| Duration of hospital stay, days | 4.3±3.07 |
Hepatic resection of segments II and III is a feasible and safe technique. The current results reproduce and even improve upon those from open surgery in selected cases, with a mortality rate of <5% and morbidity of <20%.20–24
LLS should be considered as the technique of choice in benign or malignant lesions located on segments II and III in patients that do not have severe comorbidities that would contraindicate laparoscopy. A patient history involving previous open surgical procedures is not uncommon in our field (laparotomy for colon resection), but this does not contraindicate the laparoscopic approach, although it may increase the technical difficulty of the procedure due to adherences.
A few groups support the use of hand-assisted laparoscopic surgery in the case of large lesions,25–27 but since the transection line to the left of the round and falciform ligaments of the liver is always the same, we believe that the size of the tumour alone is not an exclusion criteria for performing a completely laparoscopic procedure, if it is possible to achieve adequate mobilisation of the liver.
The trocars used in this procedure should be placed following a concave line with respect to the lesion, placing one 5mm trocar on the right flank in the most posterior and superior position possible, in order to facilitate the Pringle manoeuvre with a better angle for access to the foramen of Winslow.
A complete mobilisation of the left hepatic lobe facilitates transection and allows for proper access to the suprahepatic veins. The dissection of the anterior face of the suprahepatic veins allows for directly clamping these vessels in the case of haemorrhage, and avoids damaging the left diaphragmatic vein when separating the liver from the diaphragm. Better control of the intrahepatic left suprahepatic vein is best when the left liver is mobilised, since the vein is then in a perpendicular position with respect to the endostapler.
Laparoscopic intraoperative ultrasound must always be used in order to rule out lesions that may have gone unnoticed during the preoperative analysis. This point is of vital importance when dealing with a malignancies, especially colorectal cancer hepatic metastases (CRCHM), since in 20% of cases, IOUS diagnoses hepatic lesions that were not identified using other diagnostic techniques.28–30 This occurred in one of the patients from our study, in which the IOUS showed a deep metastatic lesion in segment V that had gone undiagnosed, requiring conversion to a laparotomy in order to safely complete the oncological resection.
Clamping the hepatic hilum during transection is a highly debated issue. Several different possible postoperative complications are associated with prolonged clamping (longer than 1h).2,3 In our experience, we have not registered any postoperative complications attributable to the Pringle manoeuvre. Additionally, continuous clamping does not tend to last for more than 25minutes, and is usually well tolerated by most patients both during the procedure and postoperatively.
Using ischaemic preconditioning of the area with a 10-min clamping and 10-min unclamping could provide a beneficial effect on liver cells through various mechanisms, including the inhibition of apoptosis and the local release of proinflammatory cytokine antagonists, protecting the liver from later ischaemia-reperfusion damage.31–33
In spite of the fact that several groups do not support a systematic clamping of the hilum in laparoscopic LLS,34 we used hilar clamping with ischaemic preconditioning as a standard practice because: (a) no secondary side effects were observed in our study after continuous clamping, both in laparoscopic and open surgeries; (b) this provides as a blood-free operative field as possible (the presence of blood in the surgical area absorbs light, darkening the image and making it difficult to identify key structures during the hepatic transection); and (c) blood losses and consequent transfusion in patients that undergo hepatic resection due to metastatic colon cancer can be a prognostic factor against long-term survival.35–37 In our study, one patient required a blood transfusion due to damage to a gastroepiploic vessel during the mobilisation manoeuvre, causing severe haemorrhage due to the increased splenic pressure caused by the hilar clamping, although this was controlled without the need for conversion to open surgery.
We find it best to work with a low CO2 pressure in the pneumoperitoneum, around 10–12mmHg, in order to minimise the possibility of gas entering the veins in the case of damage to a suprahepatic vein. Also, in contrast to the standard procedures used for open surgery, i.e. a central venous pressure close to 0mmHg in order to minimise haemorrhage, it is preferable to maintain this pressure close to 6mmHg in order to decrease the gradient between the intra-abdominal pressure and CVP, thus reducing the risk of gas embolism.3,38–40
For the treatment of hepatic malignancies, LLS is indicated in selected cases as long as the principles of oncological resection are respected, with adequate tumour-free margins. Doubts exist regarding the risk of facilitating intra-abdominal dissemination of the tumour and metastases in the trocar access ports during laparoscopic resections for malignancies.41–44 The results obtained in studies focusing on colon cancer appear to contradict this theory. In our cohort, all cases of malignancies were resected with tumour-free margins greater than 1cm, and to this date we have not registered any cases of recurrence in the abdominal wall in any patients.
Laparoscopic resections of HCC located in the left lateral sector of the liver offer several advantages over open surgery. It is a less invasive approach that preserves collateral circulation of the abdominal wall and minimises the possibility of ascitic decompensation in the postoperative period. For this reason, the surgical indications for this procedure could possibly be expanded to include selected patients with hepatic cirrhosis and portal hypertension.45–48
In conclusion, laparoscopic LLS is a feasible and reproducible technique. The anatomical positioning of segments II and III and their portal and suprahepatic pedicles facilitates a laparoscopic approach. Additionally, this technique has been proven effective and safe for the treatment of both benign and malignant pathologies in selected patients.49 Finally, standardising this procedure could facilitate widespread acceptance of this method for the majority of liver surgeons with laparoscopic experience.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please, cite this article as: Herrero Fonollosa E, et al. Seccionectomía lateral izquierda por laparoscopia. Presentación de nuestra técnica. Cir Esp. 2011;89:650–6.