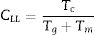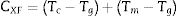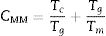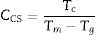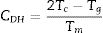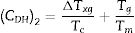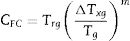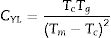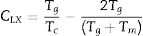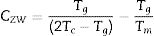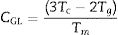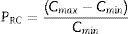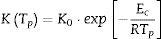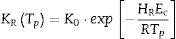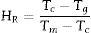In this endeavor, various glass stability criteria have been discussed by using two ternary alloys of SeTePb glassy system. These criteria can be obtained based on the interrelationship between the characteristic temperatures. Differential Thermal Analysis measurements under the non-isothermal conditions can be used for this purpose. Two groups of these criteria according to the onset and peak crystallization temperaturas have been studied as a function of composition. The heating rate and composition dependences of all criteria concluded that the C ZW and C LX criteria are not suitable to discuss the glass stability (GS) for the studied compositions. The extracted data from various GS criteria and their relative change parameters reveal that the criterion C YL of Yuan et al is best and it exhibits the best ability for apprising the GS.
En este esfuerzo se han discutido varios criterios de estabilidad del vidrio utilizando dos aleaciones ternarias del sistema vítreo SeTePb. Estos criterios pueden obtenerse en función de la interrelación entre las temperaturas características. Las mediciones de análisis térmico diferencial en condiciones no isotérmicas se pueden utilizar para este propósito. Se han estudiado dos grupos de estos criterios según las temperaturas de inicio y pico de cristalización en función de la composición. La tasa de calentamiento y las dependencias de composición de todos los criterios concluyeron que el criterio Zhang-Wei CZW y el criterio Long-Xie CLX no son adecuados para discutir la estabilidad del vidrio (GS) para las composiciones estudiadas. Los datos extraídos de varios criterios de estabilidad del vidrio y sus parámetros de cambio relativo revelan que el criterio CYL de Yuan-Lu es el mejor y exhibe la mejor capacidad para conocer el GS.
Glass stability (GS) or thermal stability (TS) represents the resistance of the glass to the devitrification of glassy alloys through the nucleation and growth processes [1–3]. To determine thermal stability for glasses, several parameters must be determined which are known as the glass stability criteria. Glass stability criteria can be estimated based on the determination of the characteristic temperature values, which determined using standard Differential Thermal Analysis (DTA) or Differential Scanning Calorimetry (DSC) methods. In the present work, the DTA technique was used to determine the characteristic temperatures at different heating rates.
The first endothermic peak in the DTA thermogram represented the peak glass transition temperature Tg, Another peak is an exothermic peak that manifests the crystallization process and it appears due to an abrupt increase in the specific heat of the sample. This exothermic peak has three characteristic points; the first point is the onset temperature of crystallization Tc. The second is the peak temperature of crystallization Tp, The third is the finish temperature of crystallization Tf. At higher temperatures in DTA pattern an endothermic peak appeared corresponds to the melting temperature Tm.
The advantages of the glass stability criteria are that they are an easy and fast estimation based upon the above mentioned characteristic temperatures. Moreover, the thermal stability of glass (which is the indicator of resistance against crystallization) and its tendency of glass formation (i.e., glass-forming ability) play an important role to determine the utility of chalcogenide alloys as the recording materials because that the phase-change optical recording and erasing technique based on laser-induced crystallization of chalcogenide glasses [4]. Therefore, the study of glass stability can be considered a great interest subject [5]. On the other hand, the study of the composition dependence on the thermal stability for recording materials is very important because the recording materials must remain stable in the amorphous state so that the life-time of the memory devices is large. Further, if we consider the rewritable memory devices, then the crystallization rate of recording materials must be fast for erasing of the recorded spots so that a laser pulse of short duration can complete the erasing of data in a very short duration (∼ nano-second to the pico-seconds range).
In chalcogenide glasses, it is important to be aware of the knowledge of thermal stability because this parameter is very useful for the specific technological applications [6–9]. Thermal stability is the direct measurement of stability against crystallization and so the aging effects are found less dominant in the glasses having high thermal stability [10,11]. Consequently, it helps us in determining the optimized conditions for the durability of the optical devices made from chalcogenide glasses [12,13].
In the present paper, we determined and reported the glass stability of glassy SeTePb system, by determining their glass stability criteria. We can say that the main target of this work is related to the delimiting of the more stable alloy and both of the more suitable and sensitive GS criteria for the studied compositions and the less one. Such investigations are very important for determining the utility of the chalcogenide glasses and these studies are found closely related to the durability of the devices fabricated from these materials [14–19].
Experimental detailsFor the thermal investigation of aforesaid glass stability criteria, the samples were prepared by using a cost-effective and conventional melt-quench technique [20–22]. Appropriate amounts of 99.999% purity Se, Te, and Pb elements were weighted according to their atomic percentage and loaded in two silica tubes (length 15cm, internal diameter 12mm) for two compositions (Se90Te8Pb2 and Se90Te4Pb6) which then sealed under the vacuum of 10−5Torr. The contents of the tubes were heated gradually in an oscillatory furnace with rising temperature up to a suitably high value. The tubes were kept at these temperatures for 10–12h with frequent rocking to ensure the homogeneity of the melt.
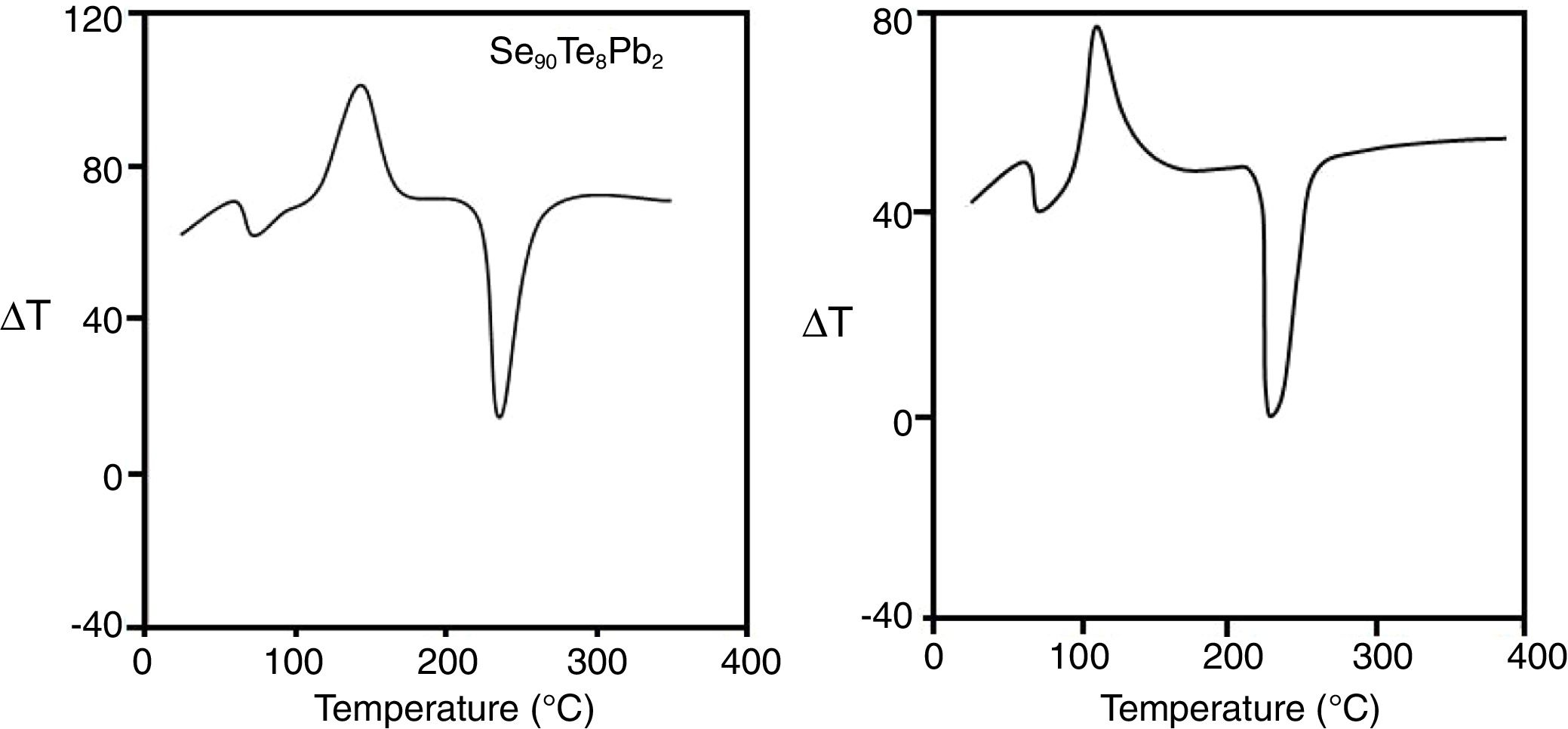
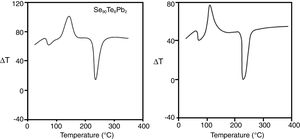
The elements (Se, Te, and Pb) present in the sealed tube tend to have significant effects on the melting point of the collective system during the process of alloying. Thus, the melting point of the alloy is significantly lower as compared to the melting points of constituent elements. Though the melting points of Se (220°C), Te (449.5°C), and Pb (327.5°C) are different, but when they are mixed for alloying then the resulting alloy have a lower melting point. Therefore, we have chosen the maximum temperature ∼470°C in the melt-quench technique. After sufficient heating and rocking of the tube at this appropriate temperature, the molten materials were quenched in ice-cooled water to have glassy forms. The bulk samples were taken out by breaking the silica ampoules. These ingots were then ground into a fine powder using a pestle and mortar. The grain size of the powdered samples was ∼5–10μm. The thermal behavior was investigated using a differential thermal analyzer (Shimadzu DTA-50). Typically, almost the same amount (∼5mg) of both samples in fine powder form were crimped in standard aluminum sample pans then, heated at different heating rates. The DTA analyses were carried out under non-isothermal conditions. The temperature range covered in DTA was raised from room temperature 303 to 773K. DTA traces have been studied at five different heating rates (from 10 to 50K/min). The accuracy of the heat flow is ±0.01mW. Fig. 1 shows the DTA scans of the samples at a particular heating rate (50Kmin−1). Each DTA trace shows an endothermic peak and an exothermic corresponding to well-defined glass transition and crystallization. The on-set temperature Tx of the glass transition peak is followed by glass transition temperature Tg. Similarly, the crystallization peak starts with the onset of crystallization temperature Tc and reaches to maximize value at peak crystallization temperature Tp. At a higher temperature in the obtained DTA thermograms, another endothermic peak represented the melting temperature Tm. The temperature precision of this equipment is ±1K with an average standard error of about ±0.03 in the measured values of characteristic temperatures Tg, TxTc, Tp, and Tm). Similar results were obtained at other heating rates. The homogeneity of our samples was checked by Energy dispersive X-ray (EDX) composition analysis. From DTA scans, it is evident that well-defined single endothermic and exothermic peaks appear during the glass transition and crystallization phenomenon in the samples. Thus, there is no sign of phase separation which also ensures the homogeneity of your samples.
Results and discussionDifferential Thermal Analysis measurements (DTA)Differential thermal analysis DTA measurements have been carried out under non-isothermal conditions for glassy Se90Te8Pb2 and Se90Te4Pb6 compositions at different heating rates of 10, 20, 30, 40, and 50Kmin−1. The characteristic temperatures, the glass transition Tg, the onset crystallization Tc, the peak crystallization Tc, and melting Tm temperatures were extracted from the DTA thermograms for the studied compositions at different heating rates in the studied range. These values and their interrelationship between them will be used to determine the glass stability criteria values according to different theories, models, and authors. The change of these characteristic temperatures with the heating rate helps us to study and analyze the heating rate dependence of the glass stability criteria which play an important role to examine and evaluate them.
Estimation of the glass stability criteria for the investigated compositionsVarious glass stability criteria related to the characteristic temperatures have been investigated, in recent years, according to several workers depending on a different theory.
Based on the thermal analysis of the crystallization process during cooling and reheating of the super-cooled liquid, Lu and Liu [23] proposed a new criterion that is defined as Lu-Li criterion CLL. This criterion can be expressed according to the following equation:
It is necessary to mention that glass-forming ability and glass thermal stability are independent properties but they are empirically related to each other. Therefore, we have examined CLL parameter as a glass stability criterion though, originally, this parameter was proposed by Lu and Liu as a new glass-forming ability criterion.
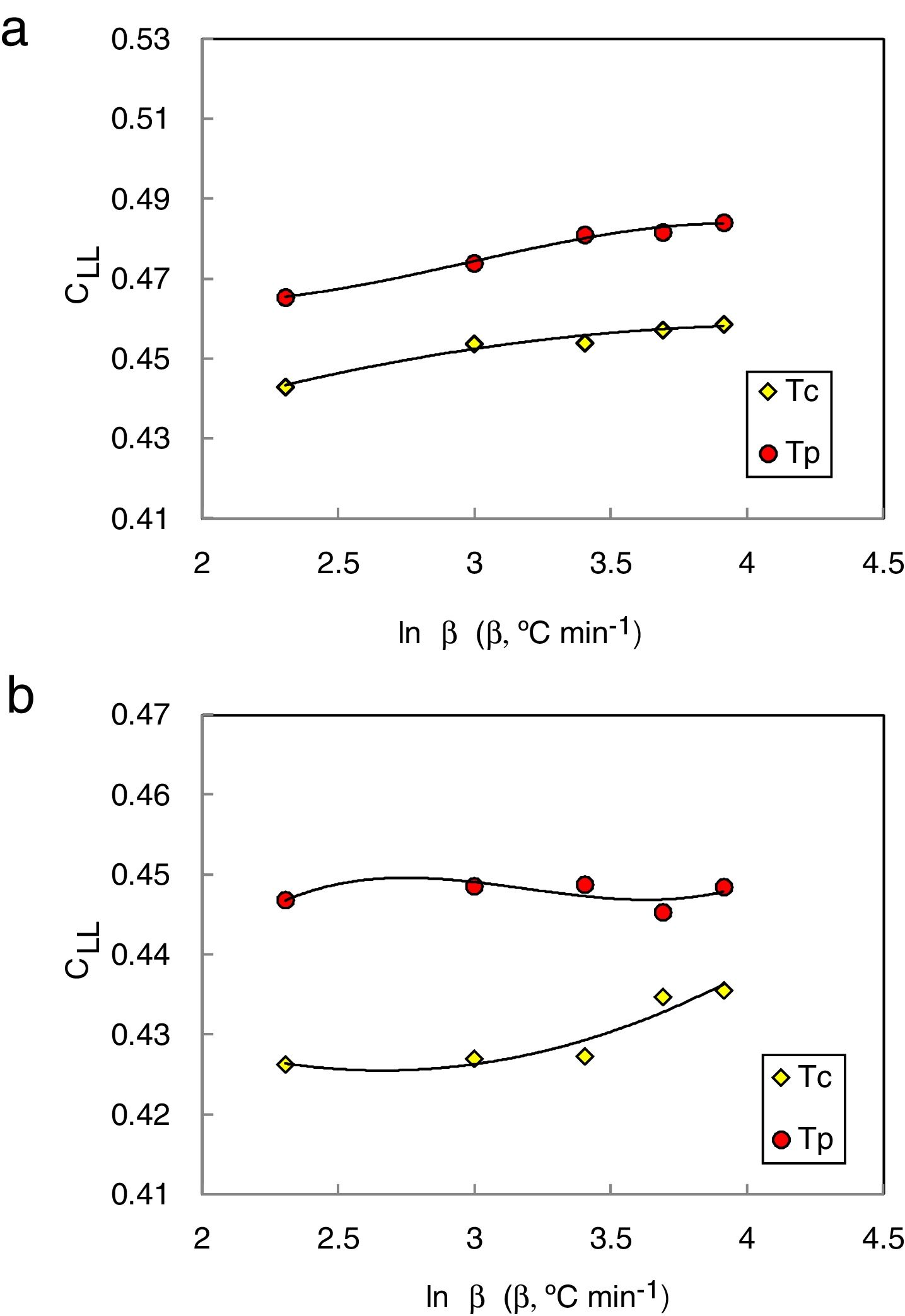
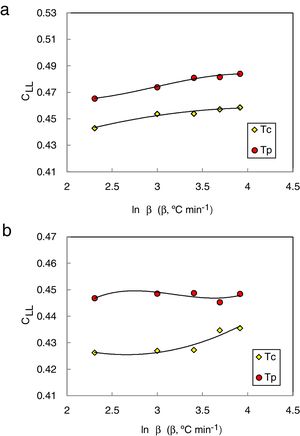
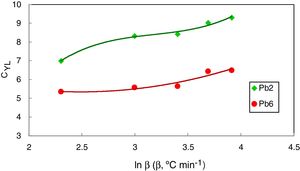
They [23] also suggested that the interrelationship between this new criterion and the critical cooling rate of critical section thickness is elaborated and discussed in comparison with two other parameters such as the reduced glass transition temperature Trg which is given as (Trg=Tg/Tm) [24]. In general, Trg values are almost constant which reflects the (two-third rule) for almost glassy alloys. Turnbull [25] gave more details about the case of larger values above 2/3 of Trg, the homogeneous crystal nucleation will be essentially suppressed due to the sluggishness of crystallization kinetics. Furthermore, Trg parameter has been considered an earlier parameter to evaluate the glass stability and glass formation ability. Lu and Liu criterion CLL can be recalculated using the maximum (peak) crystallization temperature Tp instead of the onset crystallization temperature Tc in the last equation [26]. The heating rate dependence of CLL criterion is shown in Fig. 2 for glassy Se90Te8Pb2 and Se90Te4Pb6 alloys respectively.
In (2004), Xioa et al. [27] give an expression for a new criterion for the glass stability and glass formation ability as follows:
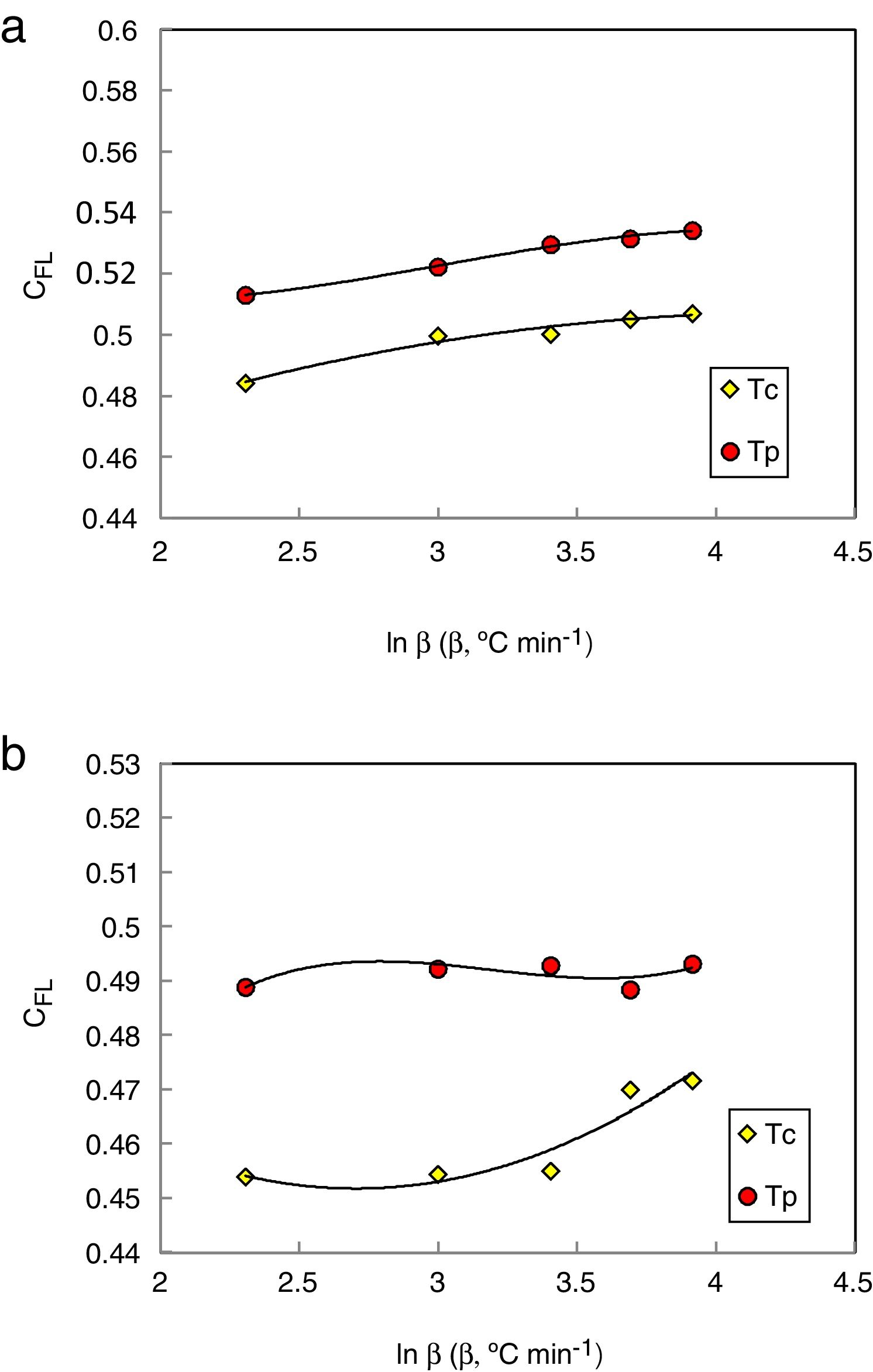
Mondal and Murty [28] proposed a new criterion to evaluate the glass stability for glasses using the following equation:
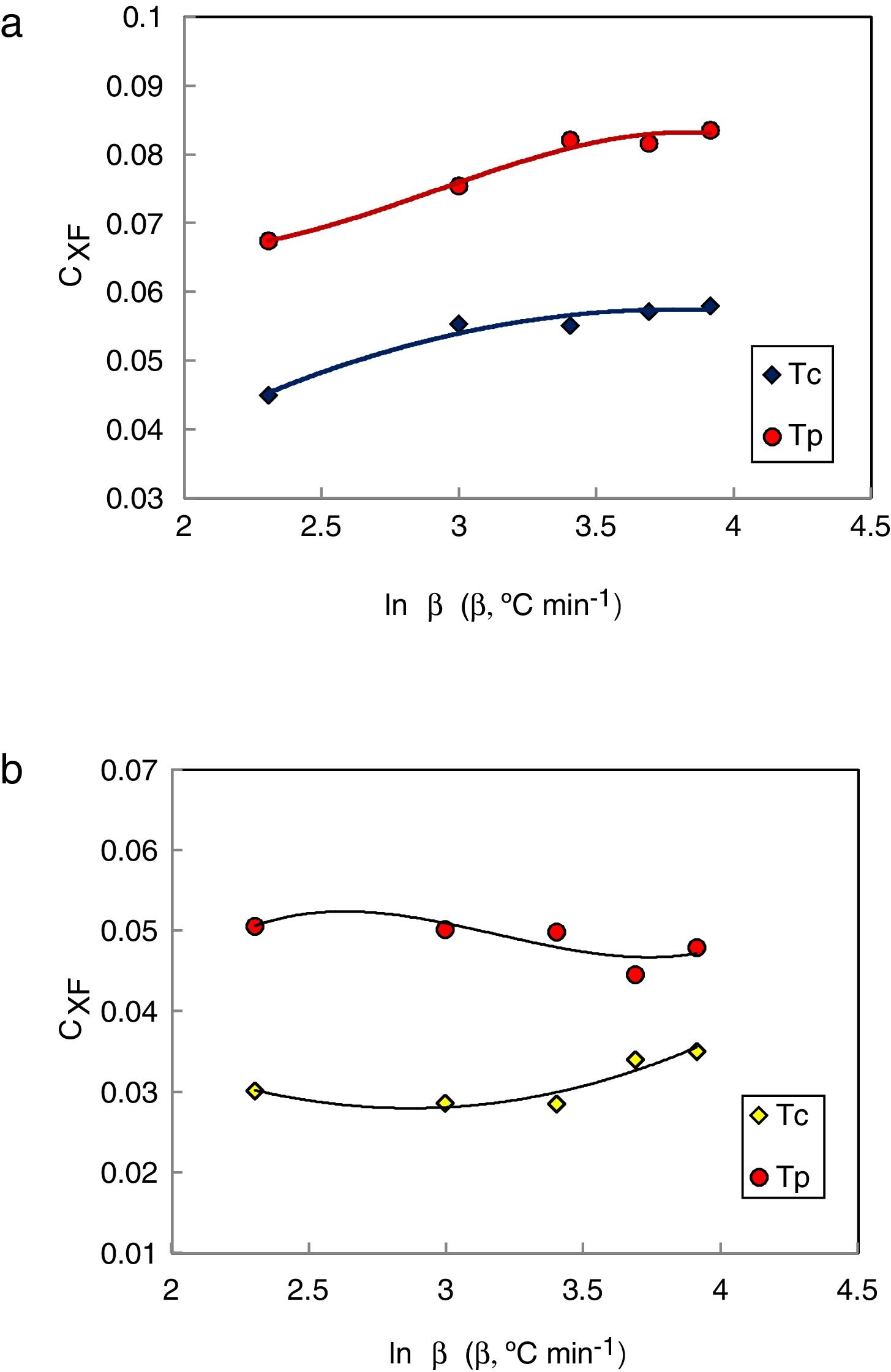
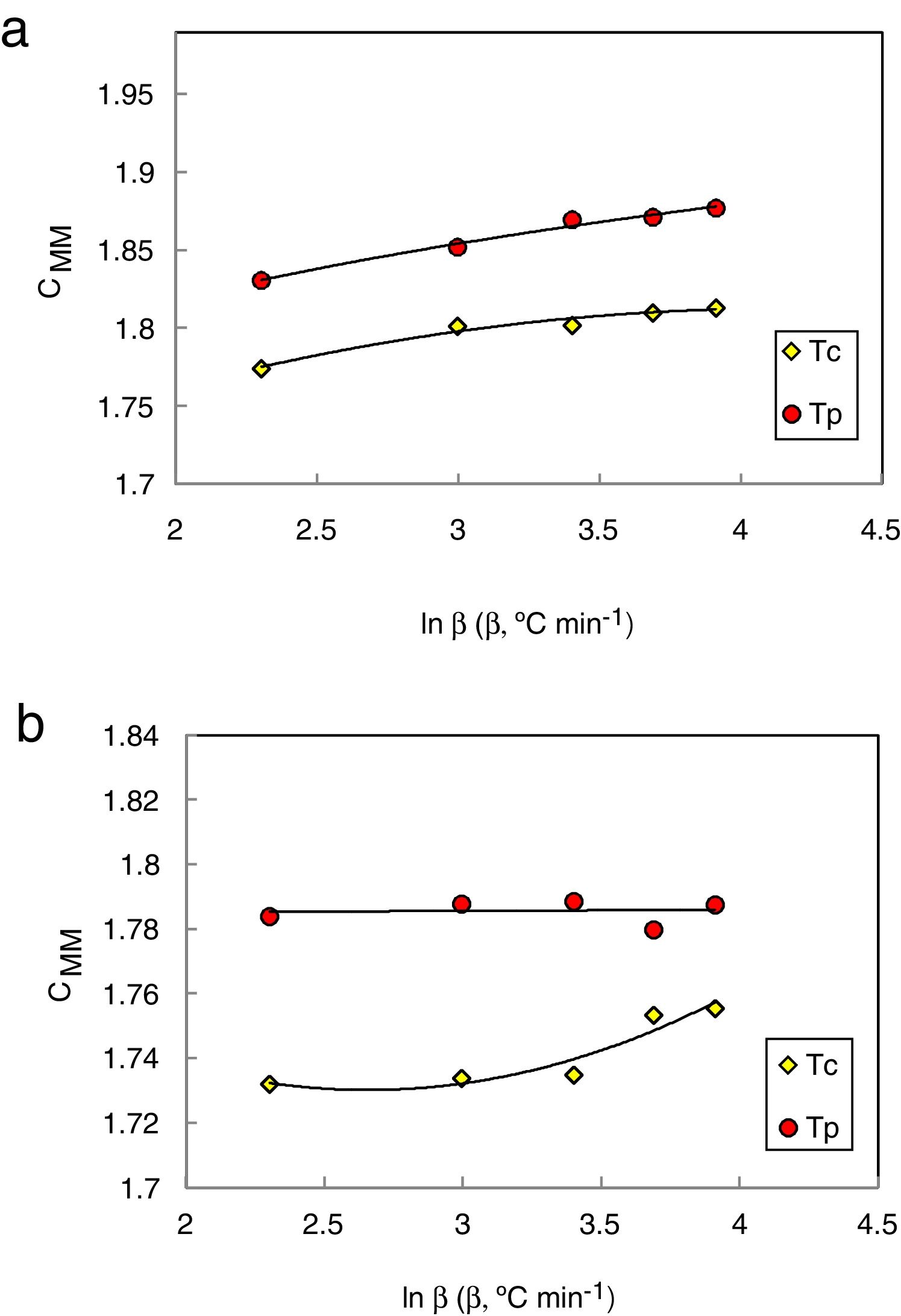
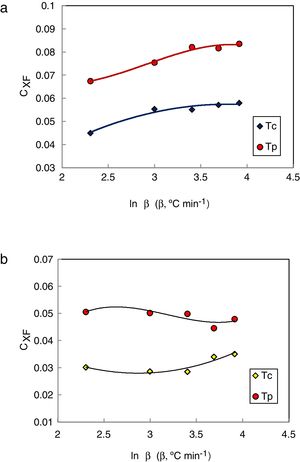
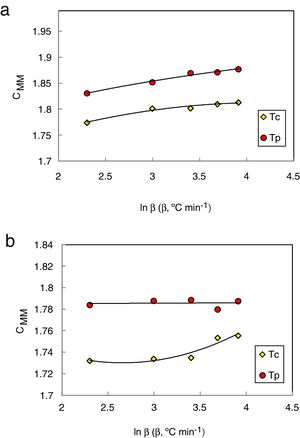
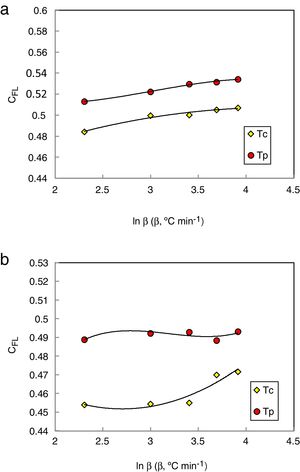
The values of CXF and CMM were calculated according to equations (2 and 3) with Tc as well as Tp and have been plotted versus the heating rate as shown in Figs. 3 and 4 respectively for the studied compositions.
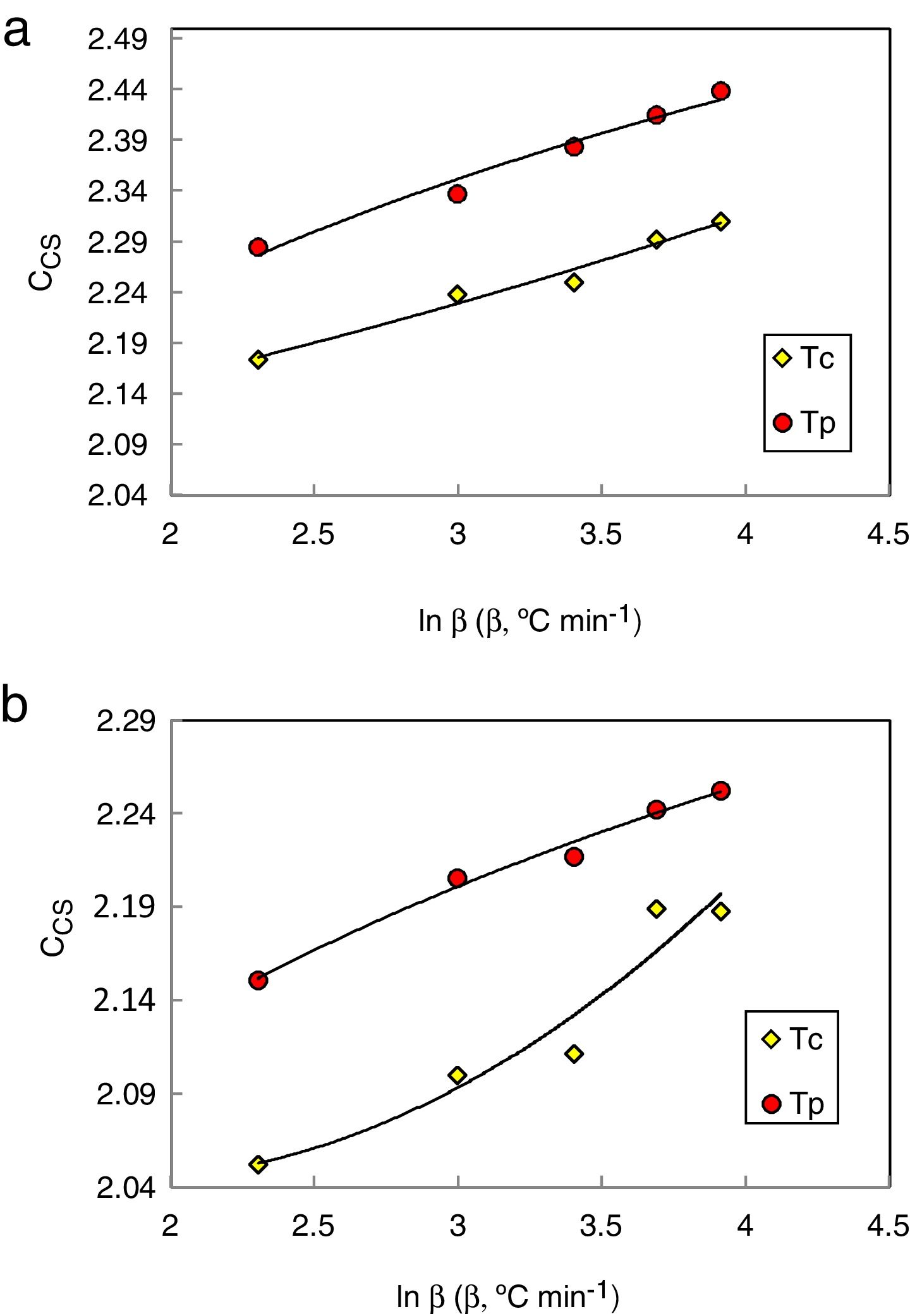
Chen et al. [29] shown that both Trg and CLL criteria cannot in many cases reflect the glass stability. So they developed a new criterion CCS, given by equation (4), based on the classical nucleation, growth theory, and respective of phase transformation kinetics. Moreover, Chen [29] used this new criterion to evaluate the glass stability and glass formation ability of bulk glasses in a wide range:
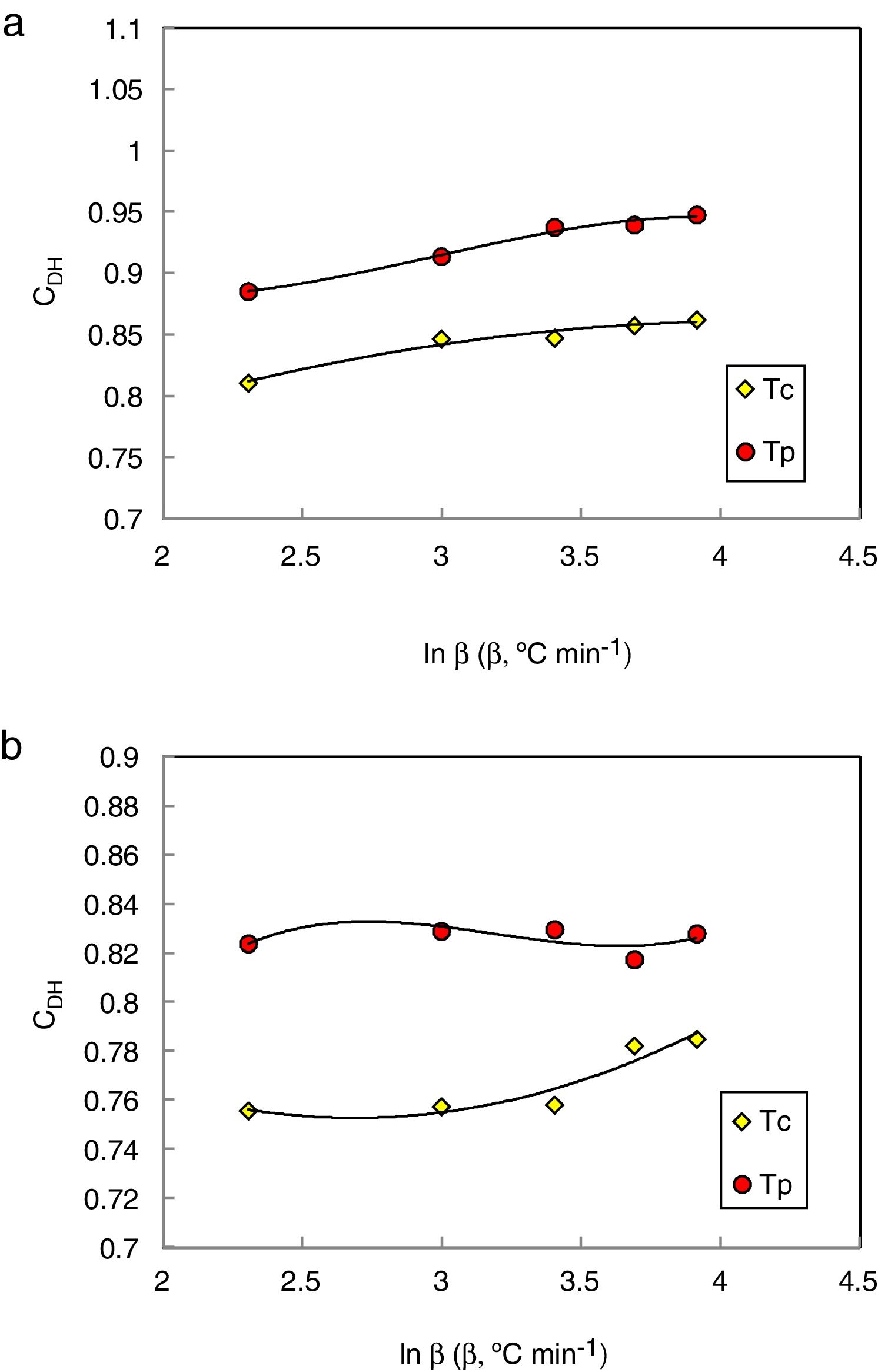
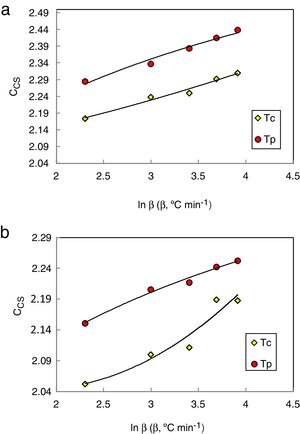
Considering that the glass stability related to crystallization resistance not only the liquid phase stability a modified CDH criterion was reported by Du et al. [30] as follows:
Moreover, the above criterion shows a good correlation with the glass stability of different bulk glasses with the statistical correlation factor.
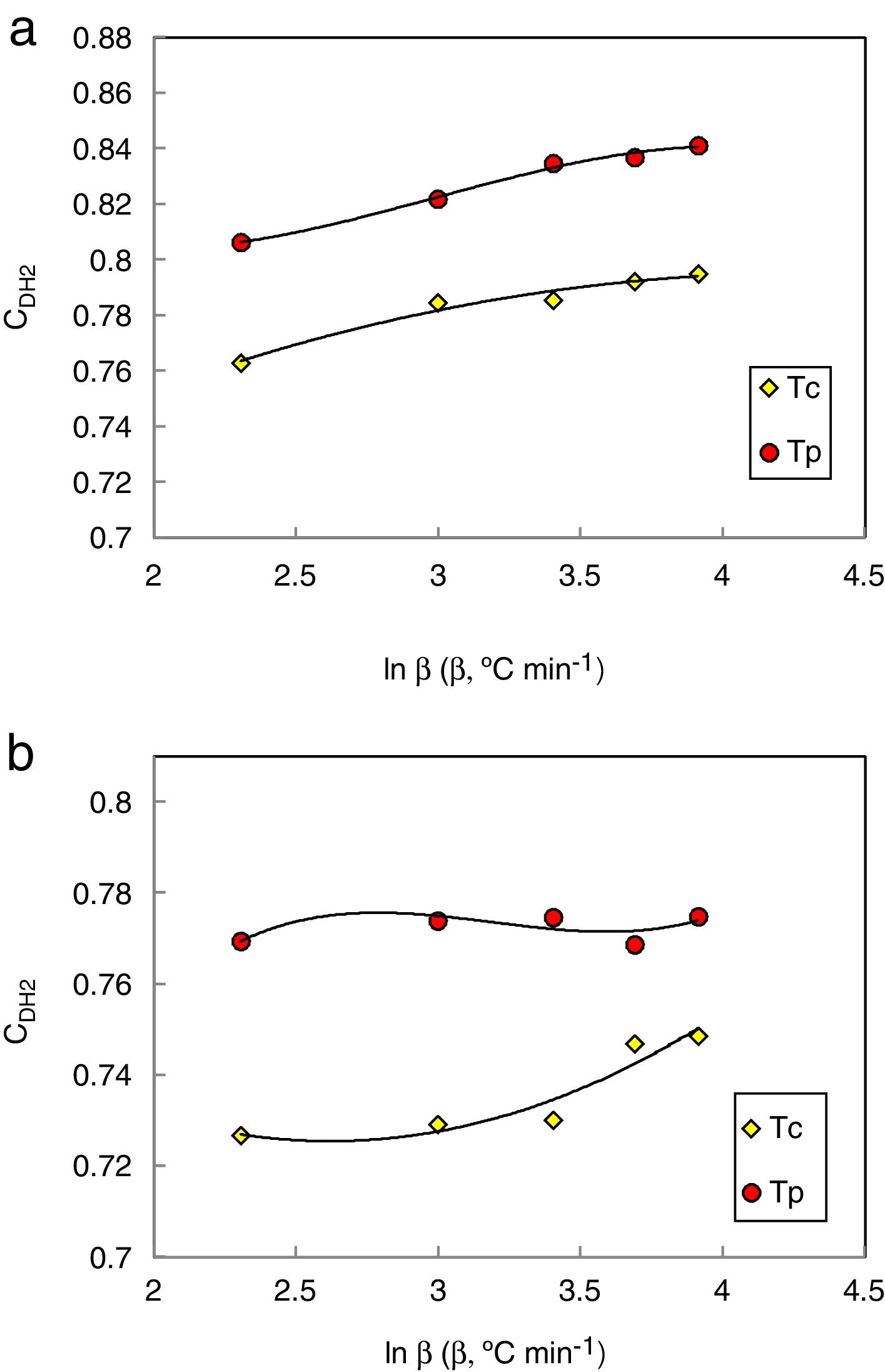
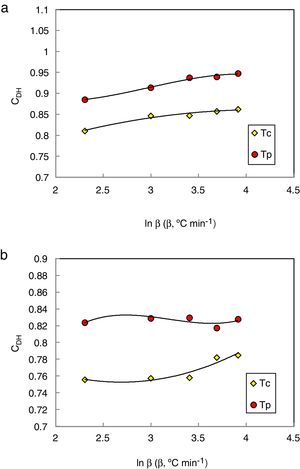
Du et al. modified the CDH criterion [31] and derived another new criterion as follows:
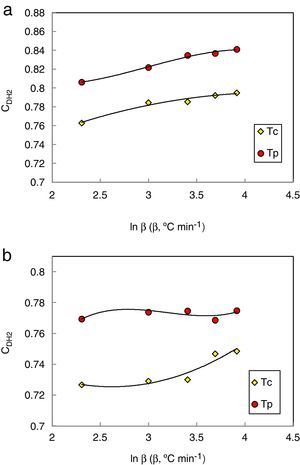
Here ΔTxg=Tx−Tg, which is considered as a good indicator of thermal stability, because the higher value of ΔTxg causes the delay in nucleation. Figs. 5–7 show the calculated values of CCS, CDH, and CDH2 criteria respectively calculated according to Tc and Tp as well for the investigated compositions.
Fan et al. [32] proposed a new dimensionless glass stability criterion based on a theoretical calculation using the fragility concept and nucleation theory for a model of glass formation system which indicates an excellent correlation with the critical cooling rate. This criterion can be expressed according to the following equation.
Here m is an exponent equal to 0.143 obtained by application of KF criterion to different glasses [32]. As shown in equation (7), the stability of glass can be quantified by the width of the super-cooled liquid region ΔTxg because of the rapid decrease in the viscosity above Tg. Therefore, the overestimation of GFA using Trg can be corrected by introducing ΔTxg in this criterion.
On the other hand, a new criterion CYL was proposed by Yuan et al. [33] as follows:
Yuan et al. reported that the CYL criterion is statistically better than currently used criteria such as CCS and Trg and also was considered more sensitive than CFC and CLL criteria.
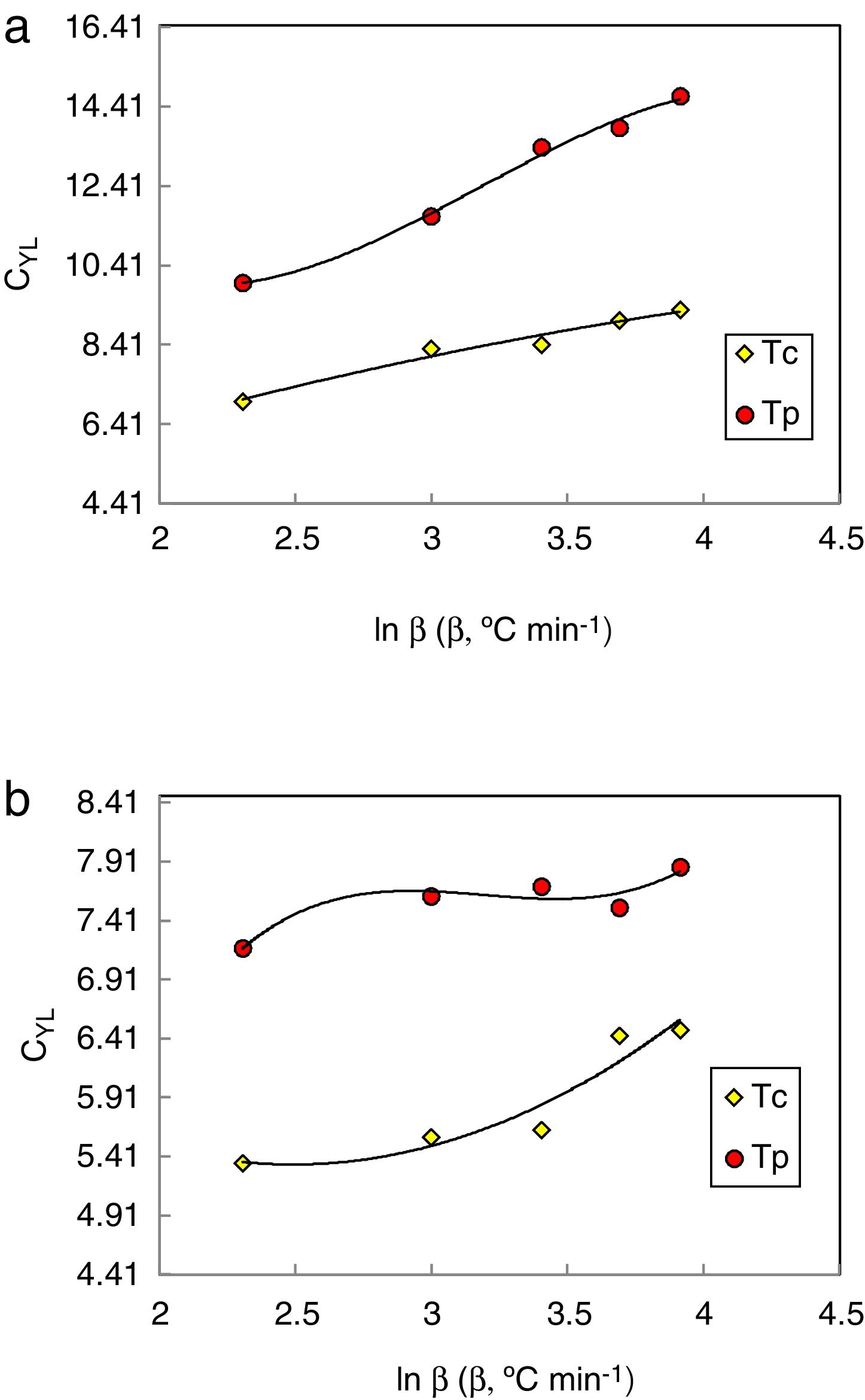
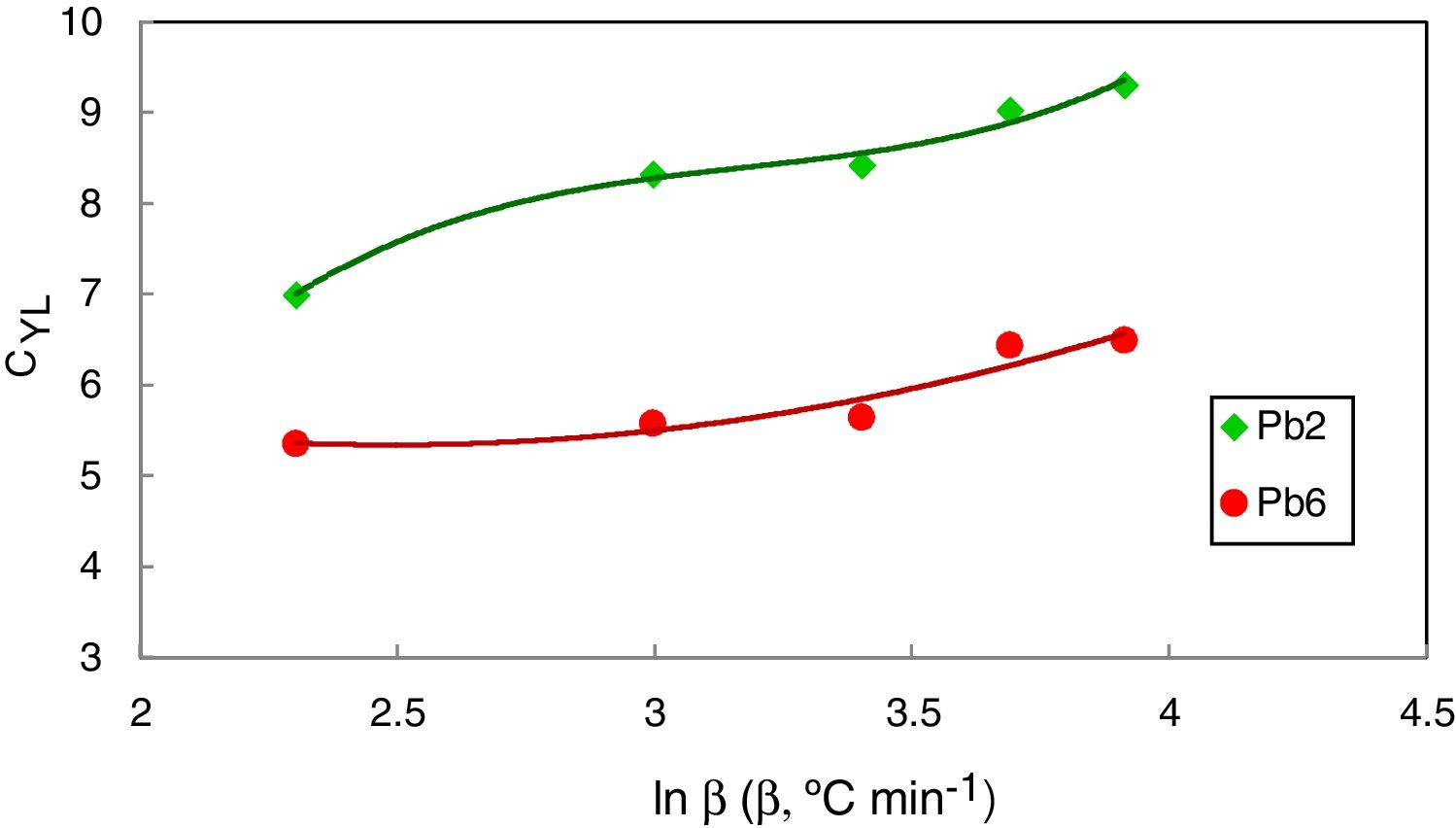
Figs. 8 and 9 represented the plots of CFC and CYL criteria versus ln β for amorphous Se90Te8Pb2 and Se90Te4Pb6 compositions respectively.
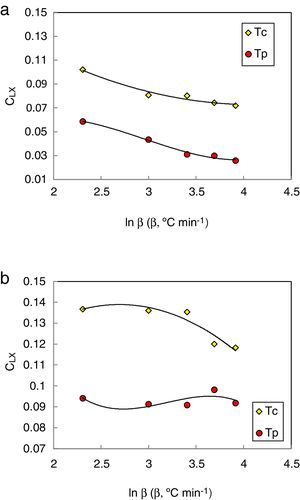
Based on the analysis of time-temperature transformation diagrams of glass formation using the fragility concept, a dimensionless glass stability criterion CLX can be written as:
This CLX criterion was proposed by Long et al. [34]. They reported that this criterion exhibits the strongest correlation with the critical cooling rate for glass formation among all currently available glass stability criteria.
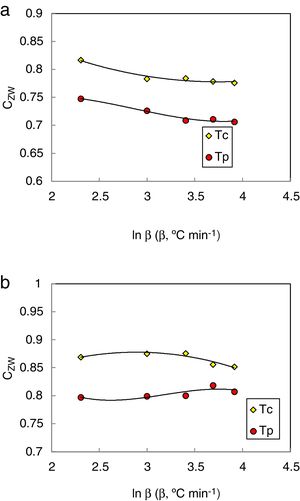
Another new criterion CZW has been proposed by Zhang et al. [35] to evaluate the glass stability for bulk glasses. This criterion can be expressed as follows:
Whereas, the glass stability of glasses could be in proportion to the (Tg/Tm) and (Tc+ΔTxg)/Tg) values which can be used to characterize the liquid phase stability and crystallization resistance respectively.
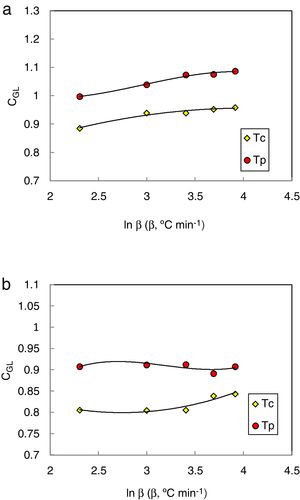
Since the glass stability is defined from the cooling of the glass-forming liquid, Guo and Liu et al. [36] derived a new glass stability criterion CGL as follows:
Considering the relationship between the cooling and heating process, this criterion correlates well to the critical cooling rate and agrees exceptionally with the physically acceptable boundary condition [36].
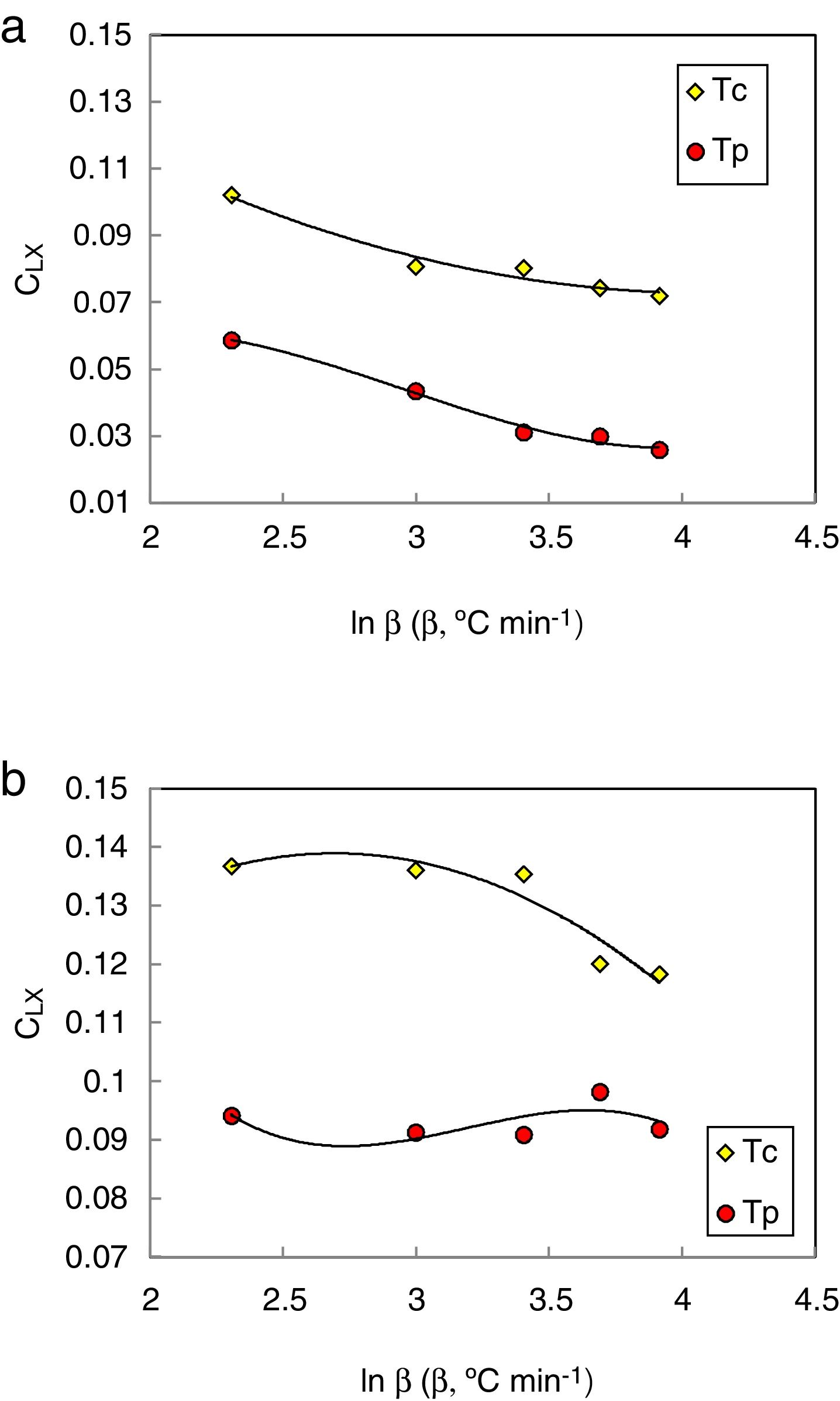
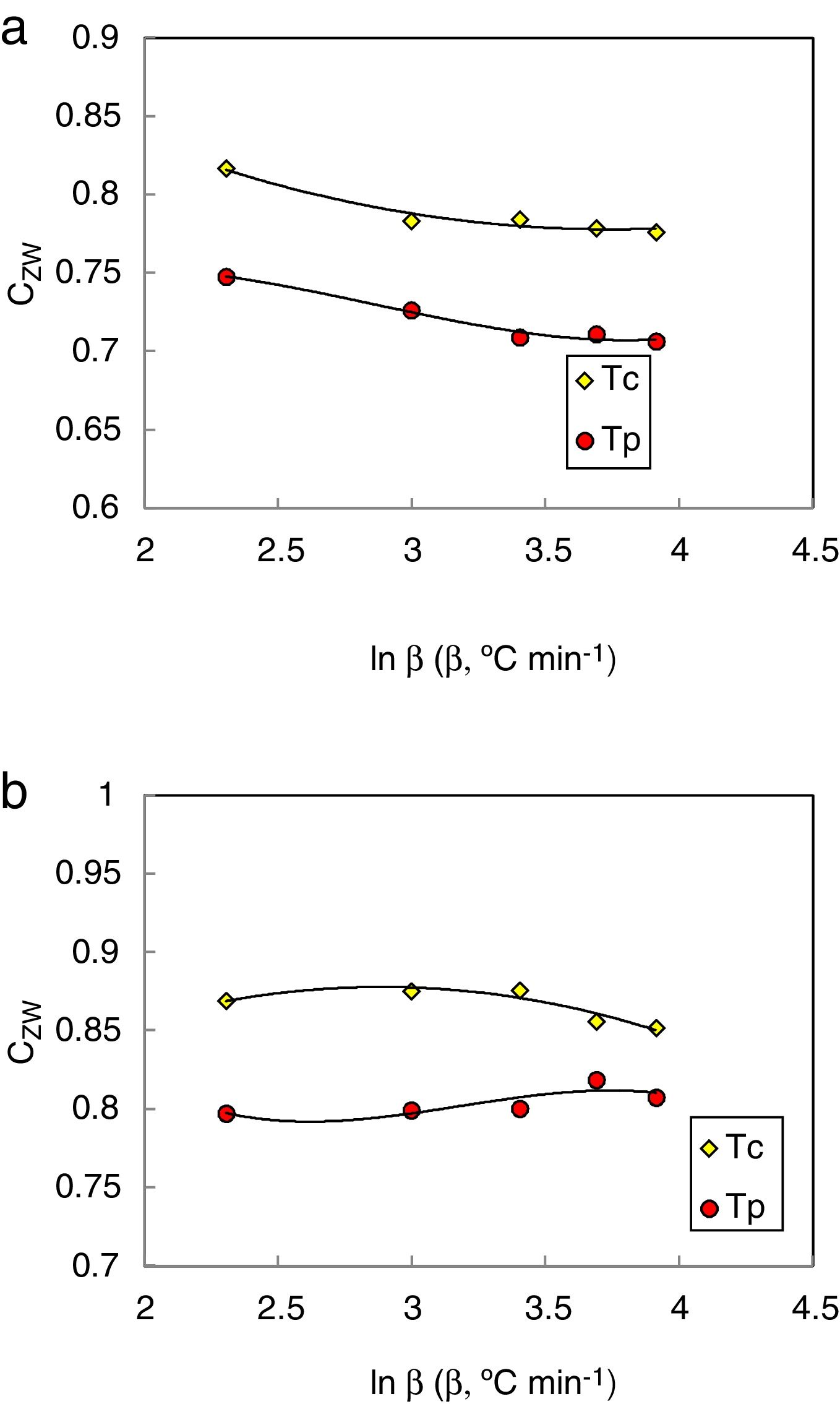
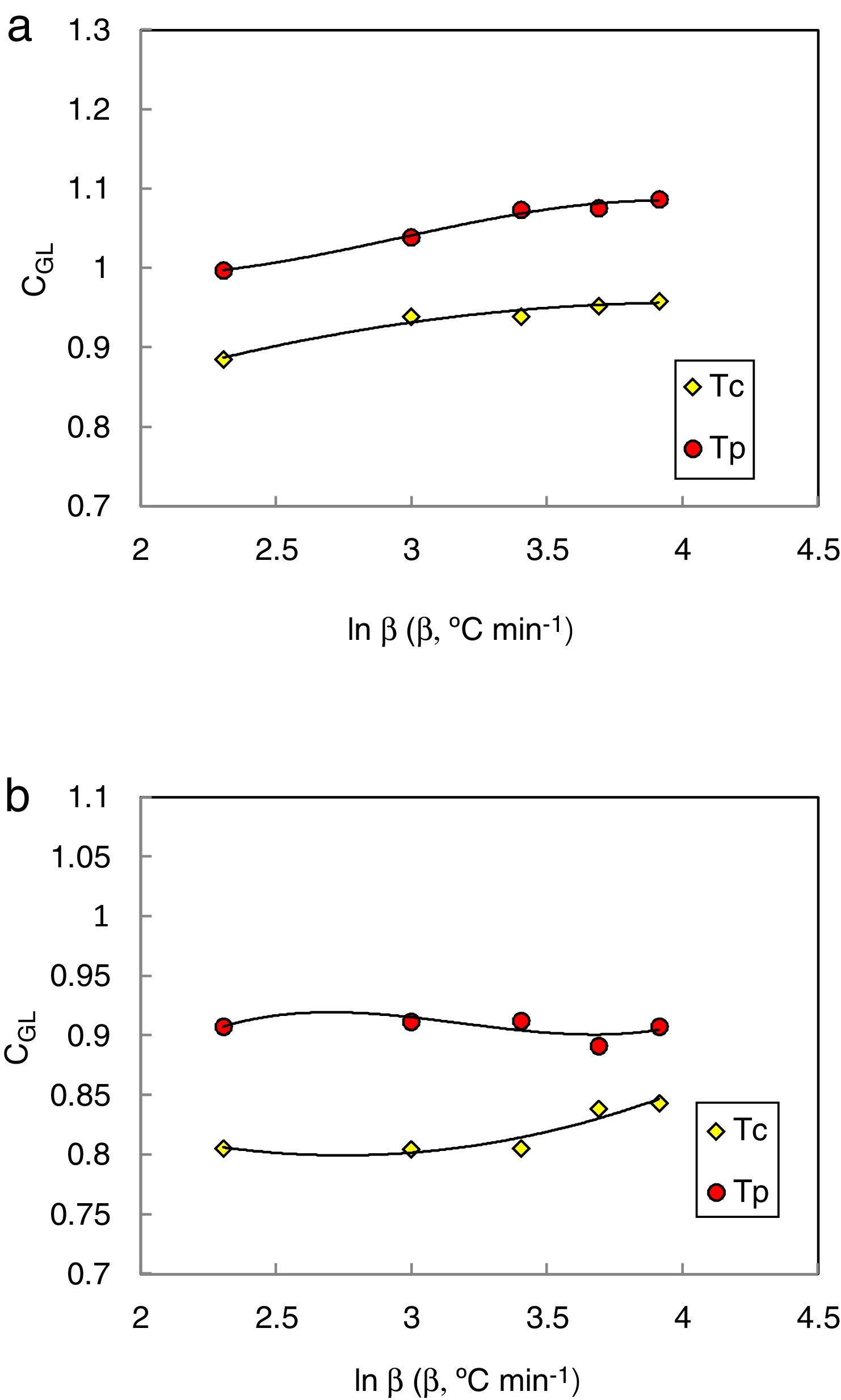
The heating rate dependence of the above criteria CLX, CZW, and CGL values according to equations 9, 10, and 11 (with Tc as well as Tp) is shown in Figs. 10–12 respectively.
Different comments can be concluded to describe the thermal stability aspects for the two studied compositions. Both of the two calculated glass stability criteria groups, based on the data of the corresponding maximum (peak) crystallization temperature Tp instead of the onset crystallization temperature Tc, have similar behavior as a function of composition as seen in Tables 1 and 2, whereas both of them have incompatible values for amorphous Se90Te8Pb2 and Se90Te4Pb6 compositions respectively. Furthermore, all of the glass stability criteria which calculated according to the peak crystallization temperature Tp have larger values than that calculated with the onset crystallization temperature Tc except two new criteria CLX and CZW which have opposite behavior for the two studied compositions as shown in Figs. 10 and 11.
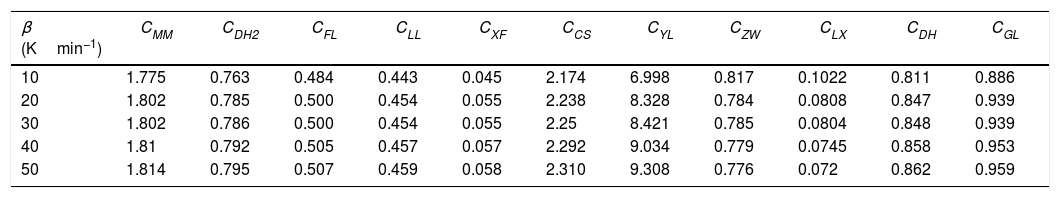
Glass stability criteria values for glassy Se90Te8Pb2 alloy.
| β (Kmin−1) | CMM | CDH2 | CFL | CLL | CXF | CCS | CYL | CZW | CLX | CDH | CGL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 1.775 | 0.763 | 0.484 | 0.443 | 0.045 | 2.174 | 6.998 | 0.817 | 0.1022 | 0.811 | 0.886 |
| 20 | 1.802 | 0.785 | 0.500 | 0.454 | 0.055 | 2.238 | 8.328 | 0.784 | 0.0808 | 0.847 | 0.939 |
| 30 | 1.802 | 0.786 | 0.500 | 0.454 | 0.055 | 2.25 | 8.421 | 0.785 | 0.0804 | 0.848 | 0.939 |
| 40 | 1.81 | 0.792 | 0.505 | 0.457 | 0.057 | 2.292 | 9.034 | 0.779 | 0.0745 | 0.858 | 0.953 |
| 50 | 1.814 | 0.795 | 0.507 | 0.459 | 0.058 | 2.310 | 9.308 | 0.776 | 0.072 | 0.862 | 0.959 |
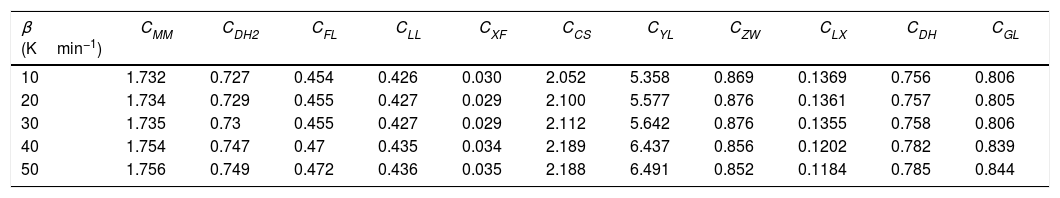
Glass stability criteria values for glassy Se90Te4Pb6 alloy.
| β (Kmin−1) | CMM | CDH2 | CFL | CLL | CXF | CCS | CYL | CZW | CLX | CDH | CGL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 1.732 | 0.727 | 0.454 | 0.426 | 0.030 | 2.052 | 5.358 | 0.869 | 0.1369 | 0.756 | 0.806 |
| 20 | 1.734 | 0.729 | 0.455 | 0.427 | 0.029 | 2.100 | 5.577 | 0.876 | 0.1361 | 0.757 | 0.805 |
| 30 | 1.735 | 0.73 | 0.455 | 0.427 | 0.029 | 2.112 | 5.642 | 0.876 | 0.1355 | 0.758 | 0.806 |
| 40 | 1.754 | 0.747 | 0.47 | 0.435 | 0.034 | 2.189 | 6.437 | 0.856 | 0.1202 | 0.782 | 0.839 |
| 50 | 1.756 | 0.749 | 0.472 | 0.436 | 0.035 | 2.188 | 6.491 | 0.852 | 0.1184 | 0.785 | 0.844 |
As seen, in general, all the glass stability criteria (with Tp and Tc) have the same behavior against the heating rate for Se90Te8Pb2 amorphous compositions. Since they were found to increase with increasing heating rate except for CLX and CZW criteria where their values were found to decrease with increasing heating rate in the Se90Te8Pb2 and Se90Te4Pb6 compositions.
According to the above results, one can conclude that the two new criteria CLX and CZW have been considered not suitable to evaluate and examine the glass stability for the two studied compositions.
The larger values of all glass stability criteria have been observed in the studied compositions with the lowest Lead content (Se90Te8Pb2 composition) than that of (Se90Te4Pb6 composition). This result concluded that the decrease of the glass stability of the SeTePb system with increasing Pb content may be attributed to the decrease of the bond energy with increasing Pb content. The addition of Pb atoms in Se–Te system reduces the effective bond energies of Se–Se bonds (79.5kcalmol−1) and Se–Te bonds (64kcalmol−1) by forming the lower bond energies as Se–Pb bonds (72.4kcalmol−1) and Te–Pb bonds (60kcalmol−1) [37]. This reveals that the studied Se90Te8Pb2 is more stable glass.
Estimation of the most suitable and sensitive glass stability criterion and the less one for the studied compositionsAll the criteria have been displayed and discussed for the investigation compositions. But their values have been considered not enough to evaluate glass stability for the studied compositions.
Another more important parameter called a relative change parameter P must be determined. This parameter requires estimating the largest relative changes of the glass stability criterion concerning the relative change parameters of the other glass stability criteria. The parameter PRC which determined for different criteria can be expressed as follows [38]:
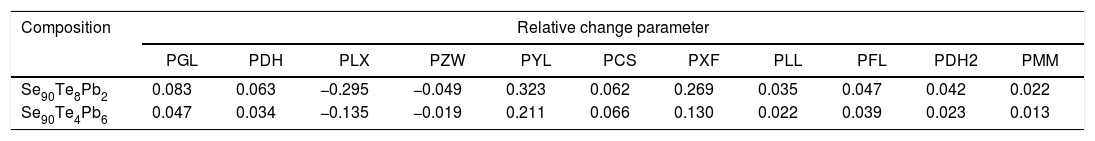
Here both Cmax and Cnim are the certain criterion values estimated at maximum and minimum heating rate values respectively. The values of the PRC parameter for the different proposed glass stability criteria have been given in Tables 3 and 4 for glassy Se90Te8Pb2 and Se90Te4Pb6 compositions respectively. The different PRC values corresponding to different glass stability criteria can be explained according to the change in the different values of (Tg∼Tc) and (Tm∼Tg) or between (Tg∼Tp) and (Tg∼Tm) at different heating rates. The composition dependence of PRC parameter values for different studied glass stability criteria shows that. The PRC parameter values for Se90Te8Pb2 composition are higher than their values for Se90Te4Pb6 composition.
Values of relative change parameters of different glass stability criteria (calculating with the onset crystallization temperature Tc) for glassy Se90Te8Pb2 and Se90Te4Pb6 alloys.
| Composition | Relative change parameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PGL | PDH | PLX | PZW | PYL | PCS | PXF | PLL | PFL | PDH2 | PMM | |
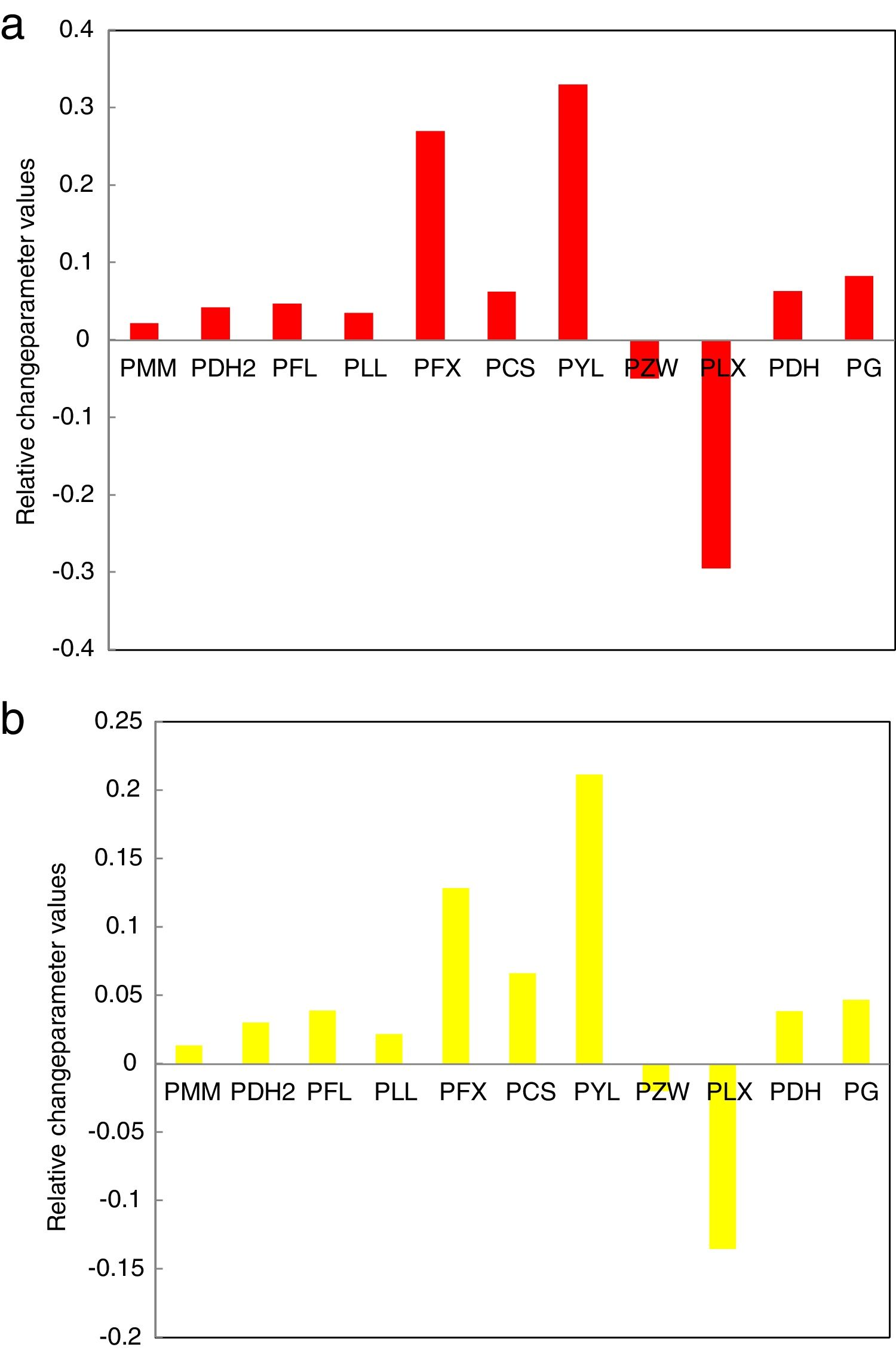
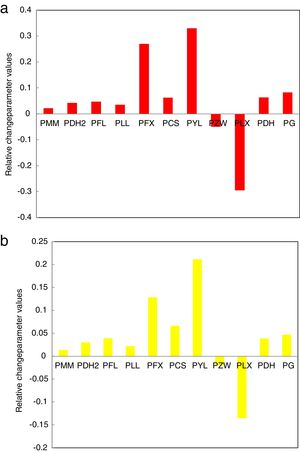
| Se90Te8Pb2 | 0.083 | 0.063 | −0.295 | −0.049 | 0.323 | 0.062 | 0.269 | 0.035 | 0.047 | 0.042 | 0.022 |
| Se90Te4Pb6 | 0.047 | 0.034 | −0.135 | −0.019 | 0.211 | 0.066 | 0.130 | 0.022 | 0.039 | 0.023 | 0.013 |
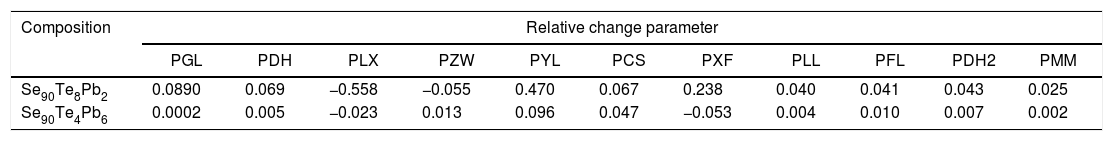
Values of relative change parameters of different glass stability criteria (calculating with the maximum (peak) crystallization temperature Tp) for glassy Se90Te8Pb2 and Se90Te4Pb6 alloys.
| Composition | Relative change parameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PGL | PDH | PLX | PZW | PYL | PCS | PXF | PLL | PFL | PDH2 | PMM | |
| Se90Te8Pb2 | 0.0890 | 0.069 | −0.558 | −0.055 | 0.470 | 0.067 | 0.238 | 0.040 | 0.041 | 0.043 | 0.025 |
| Se90Te4Pb6 | 0.0002 | 0.005 | −0.023 | 0.013 | 0.096 | 0.047 | −0.053 | 0.004 | 0.010 | 0.007 | 0.002 |
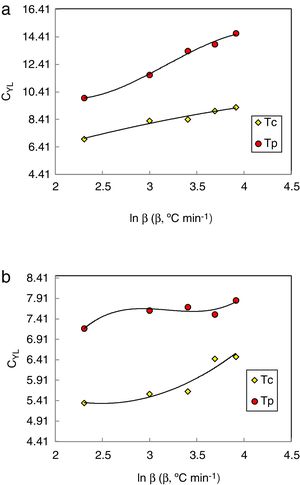
The data was extracted from Tables 3 and 4 can be plotted in Fig. 13 to show the values of relative change parameter P for different studied criteria. As seen from Tables 3 and 4 and Fig. 13 as well, the most sensitive and suitable criterion is Yuan et al. CYL, which have the greatest relative change parameter value for two studied compositions than other criteria. Also, the criteria itself have the greatest value as comparing with anther studied criteria. The variation of the more suitable and more sensitive criterion CYL with the heating rate is shown in Fig. 14 for both samples. Meanwhile, the less sensitive criterion is the Mondal and Murty criterion CMM for glassy Se90Te8Pb2 and Se90Te4Pb6 compositions.
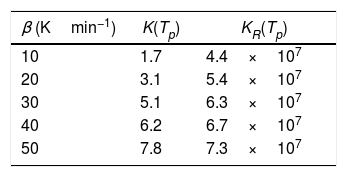
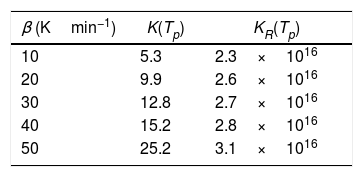
To evaluate the stability in glasses, some stability criterions based on the rate of crystallization were also developed. Hu et al. [39] and Vazquez et al. [40] proposed the following K(Tp) and KR(Tp) criteria respectively:
Here HR is Hruby glass-forming ability parameter which is itself a stability factor and can be expressed as:
The values of K(Tp) and KR(Tp) criteria are tabulated in Tables 5 and 6 for the present samples at all heating rates. From these tables, it is clear that the rate of crystallization is fast for Se90Te4Pb6 alloy as compared to Se90Te8Pb2 because of the value of K(Tp) and KR(Tp) are higher for Se90Te4Pb6 alloy as compared to Se90Te8Pb2 alloy. Thus, the results of K(Tp) and KR(Tp) criteria are consistent with all stability criteria except CLX and CZW criteria.
Different characteristic temperatures (the glass transition peak Tg, the onset temperature of crystallization Tc, the peak temperature of crystallization Tp, the melting temperature Tm) have been determined from Differential Thermal Analysis (DTA) measurements under the non-isothermal conditions at different heating rates (10, 20, 30, 40, and 50Kmin−1). Based on the interrelationship between these characteristic temperatures, the more recently various glass stability criteria (CCS, CXF, CLL, CMM, CDH, etc.) have been estimated and studied as a function of heating rate and composition as well. They have been found to increase with increasing heating rate except for CLX and CZW criteria where their values were found decrease with increasing heating rate in the Se90Te8Pb2 and Se90Te4Pb6 alloys. The two new criteria CLX and CZW have been considered not suitable to evaluate and examine the glass stability for the two studied compositions. The stability criteria of Hu et al and Vazquez et al based on Arrhenius dependence of rate constant K are also analyzed and they have shown excellent agreement with all other stability criteria except CLX and CZW criteria.
The comparative analysis shows that the larger values of all glass stability criteria have been observed for the sample having the lowest Lead content of atomic weight percentage 2. The most sensitive and suitable criterion is Yuan et al. CYL, which have the greatest relative change parameter value for two studied compositions than other criteria and the less one is the Mondal and Murty criterion CMM for glassy Se90Te8Pb2 and Se90Te4Pb6 alloys. No direct link has been observed between the structure of the two glasses with the process of glass-formation and criteria applicability at the present stage. We are hopeful that the future experiments in this direction could provide the qualitative information to reveal some connection between the structural properties, the process of glass-formation, and criteria applicability.
NM is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India for providing financial assistance under a major Project (Scheme no. 03(1453)/19/EMR-II).