HCV has been suspected to potentially cause degenerations in the central nervous system. Parkinson's disease is the second most common neurodegenerative disorder. Our aim was to assess the prevalence of Parkinson's disease among patients with HCV infection.
Material and methodsFor this study, we used Medicare database from 2005-2010. Medicare database contains information on enrollment, coverage, diagnosis recorded with International Classification of Disease, Ninth Revision (ICD-9). From combined inpatient and outpatient files, Parkinson's disease was identified as the first diagnosis by ICD-9 code 332.0. Other study variables were; age, gender, race (White and No White), and Medicare eligibility status. Simple distribution comparison by HCV status examined with t-test for numerical variables and χ2 test for categorical variables in the main analytical cohort as well as in the propensity score matched cohort.
ResultsA total of 1,236,734 patients (median age 76 years, 41% male, and 85% White) was identified among over 47 million claims. Of these, 6040 patients (0.5%) were infected with HCV. Overall, 0.8% (N = 49) of the HCV group and 1.3% (N = 16,004) of the Non-HCV group had Parkinson's disease (P < 0.001). When the study groups matched for age, gender and race, the prevalence of Parkinson's disease was similar between HCV and Non-HCV groups (P > 0.05).
DiscussionThis study revealed that, among Medicare population, HCV was not associated with Parkinson disease.
Chronic infection with Hepatitis C virus (HCV) is a major cause of chronic liver disease, affecting approximately 185 million patients around the world and nearly 4 million in the United States (US).1 HCV is the leading cause of cirrhosis, hepatocellular carcinoma and liver transplantation in the US.2-5 In addition to the clinical outcomes, HCV infection is associated with significant resource utilization due to complications, lost years of life, impaired quality of life, and decreased work productivi-ty.6,7 Furthermore, HCV is associated not only with liver disease, but also with various extrahepatic manifestations, including mixed type 2 cryoglobinemia, autoimmune or lymphoproliferative disorders, rheumatic diseases, cardiovascular, renal, metabolic and central nervous system (CNS) diseases.8,9 Recently, there have been reports suggesting a possible link between HCV infection and Parkinson's disease, raising the suspicion that patients with chronic HCV infection have an increased risk of developing Parkinson's disease.10,11 These studies originated from Asian countries and the association has not been established for Western countries. Parkinson's disease is a progressive neurodegenerative disease characterized by loss of dopaminergic neurons in the substantia nigra. It is the second most common neurodegenerative disorder, after Alzheimer disease.12 Some neurotropic viruses have been associated with acute and chronic Parkinsonism, including influenza, coxsackie, herpes and human immunodeficiency virus.13 As HCV is a neurotropic virus and can replicate in the CNS, it may induce dopaminergic neuron death and may play a role in the development of Parkinson's disease.
In this study, our aim was to assess the prevalence of Parkinson's disease among patients with chronic HCV infection in a large, population-based US database.
Material and MethodsStudy design and populationThis study used 5% randomly drawn sample from the de-identified Medicare Denominator, Inpatient (Part A) and Outpatient (Part B) fee-for-service the U. S. beneficiaries during 2005 and 2010. The Medicare is a health insurance program that covers approximately 50 million beneficiaries who are 65 or older (about 80%), those who have permanently certain disability, end-stage renal disease (ESRD), or amyotrophic lateral sclerosis (ALS), regardless of their age. The Medicare contains information on enrollment, coverage, diagnosis recorded with International Classification of Disease, Ninth Revision (ICD-9). For inclusion in the cohort, we required the following criteria:
- •
Have a claim with the ICD-9 codes 070.41 as acute hepatitis C with hepatic coma, 070.44 as chronic hepatitis C with coma, 070.51 as acute hepatitis C without mention of hepatic coma, 070.54 as chronic hepatitis C without mention of hepatic coma, V02.62 as communicable disease of hepatitis C carrier, 070.2 as viral hepatitis B with hepatic coma, 070.22 as chronic viral hepatitis B with hepatic coma without hepatitis delta, 070.3 as viral hepatitis B without hepatic coma, V02.61 as communicable disease of hepatitis B carrier: at least once during the index date, which was defined as the first date visit during 2005 and 2006;
- •
Have at least 36 months of coverage for part A or Part B from the index date;
- •
Not to be diagnosed hepatitis B virus (HBV) after the index date;
- •
Not to be diagnosed HCV after the index date; and
- •
Not to be diagnosed HIV.
We also identified a control cohort in the absence of HCV and HBV.
The following patients were excluded from the final analytical cohort:
- •
Hepatitis B virus (HBV) or HCV diagnosed after 2006, n = 13,616.
- •
HIV, n = 4,314.
- •
Patients who did not have at least 3 years of follow-up or not hospitalized/outpatient service utilized in 2005/ 2006, n = 943,037.
A total of 2,197,701 patients (47,379,437 claims) were in the Inpatient and Outpatient Files, Medicare, from 2005 to 2010. After study exclusion criteria applied, a total of 1,236,734 patients were identified among over 47 million claims and 39% of them were utilized hospital and/or outpatient service at least once per year for 6 years continuously.
Outcome and study variablesThe outcome was incidence of Parkinson's disease. From combined inpatient and outpatient files, this was identified as the first diagnosis by the ICD-9 code 332.0 as Parkinson's disease with three to five years follow-up after the study index date. Other study variables were; age, gender, race (White and No White), and Medicare eligibility status (disabled and ESRD). Also, the following comor-bidities were identified:
- •
Dementia ICD-9 codes 290 as senile dementia, uncomplicated, 294.1 as dementia in conditions classified elsewhere, 331.2 as senile degeneration of brain;
- •
Diabetes without complications codes 250.0 as diabetes mellitus without mention of complication, 250.1 as diabetes with ketoacidosis, 250.2 as diabetes with hyperosmolarity, 250.3 as diabetes with other coma;
- •
Diabetes with complications codes 250.4 as diabetes with renal manifestations, 250.5 as diabetes with ophthalmic manifestations, 250.6 as diabetes with neurological manifestations, 250.8 as diabetes with other specified manifestations, 250.9 as diabetes with unspecified complication;
- •
Cerebrovascular disease codes 362.34 as transient retinal arterial occlusion, 430 as subarachnoid hemorrhage, 431 as intracerebral hemorrhage, 432 as other and unspecified intracranial hemorrhage, 433 as occlusion and stenosis of precerebral arteries, 434 as occlusion of cerebral arteries, 435 as transient cerebral ischemia, 436 as acute (but ill-defined) cerebrovascular disease, 437 as other and ill-defined cerebrovascular disease, 438 as late effects of cerebrovascular disease;
- •
Hypertension codes 401 as essential hypertension, 402 as hypertensive heart disease, 403 as hypertensive chronic kidney disease, 404 as hypertensive heart and chronic kidney disease, 405 as secondary hypertension; and
- •
Hyperlipidemia codes 272.0 as pure hypercholestero-lemia, 272.1 as pure hyperglyceridemia, 272.2 as nixed hyperlipidemia, 272.4 as other and unspecified hyperli-pidemia, 272.5 as lipoprotein deficiencies, 272.9 as unspecified disorder of lipoid metabolism.
Simple distribution comparison by HCV status examined with t-test for numerical variables and chi-square test for categorical variables in the main analytical cohort as well as in the propensity score matched cohort (frequency 1:4 match ratio while adjusting age, race, gender, and Medicare eligibility status for each liver disease group control was matched, separately with replacement using a random seed). All analyses were conducted using SAS Statistical Package (SAS v. 9.3, SAS Institute Inc., Cary, NC). In the excluded patients who were diagnosed HCV/HBV after 2006 [13,616 where 1,352 (10%) HCV with HBV, 9,236 (68%) HCV only, and 3,028 (22%) HBV only], the prevalence of PD was as following: 1.3% in HCV with HBV, 0.83% in HCV only, and 1.7% in HBV only.
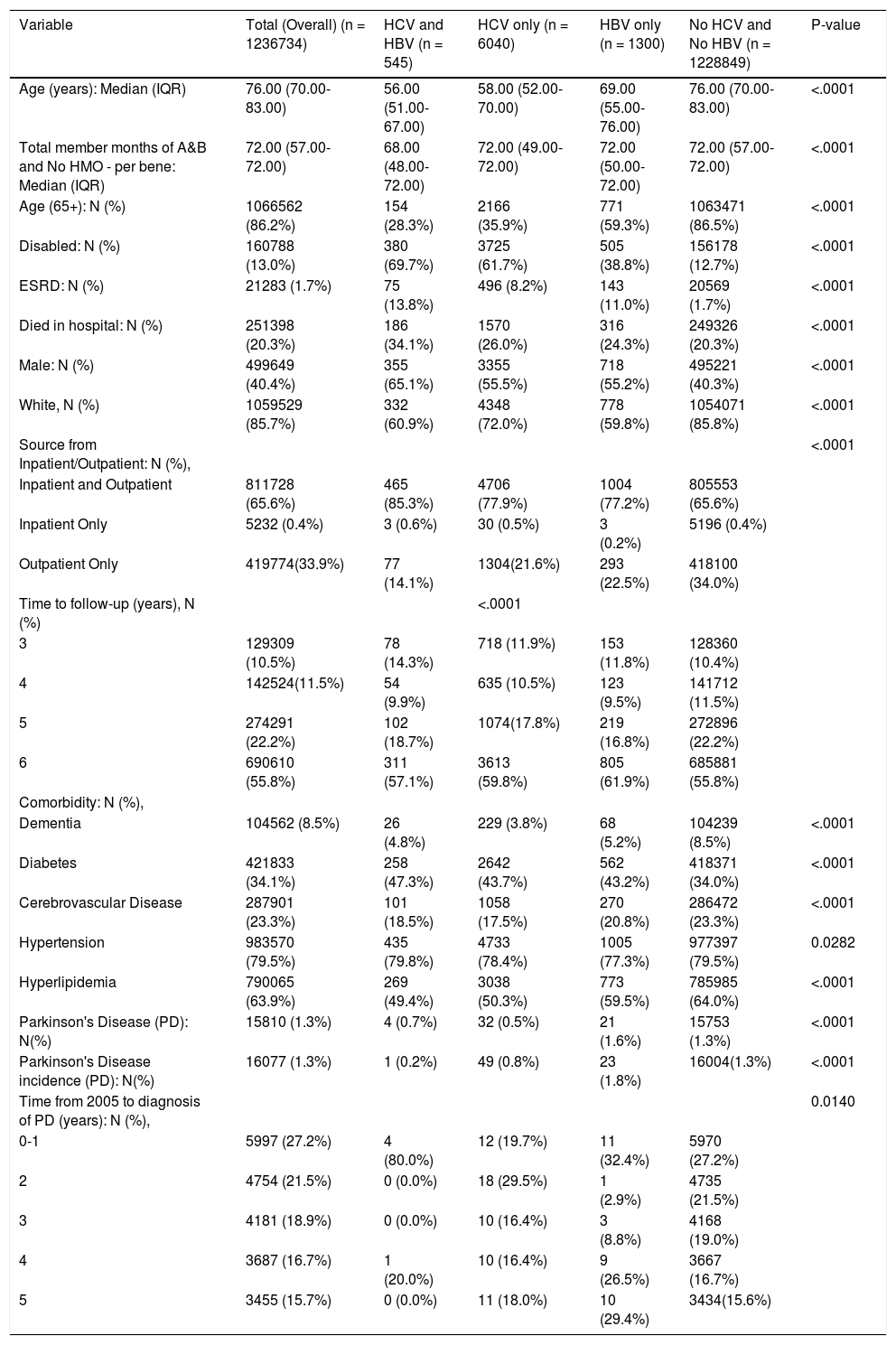
ResultsGeneral characteristics of the study populationMedian age of the study population was 76 years, 41% were male, and 85% were White (Table 1). In the main cohort, 545 (0.04%) patients were with co-infected HCV and HBV; 6,040 (0.49%) patients were infected with HCV only; 1,300 (0.11%) patients were infected with HBV only; whereas the remaining 1,228,849 (99.36%) patients did not have HCV and HBV infection. The prevalence of Parkinson's disease were significantly lower in the HCV groups as compared to Non-HCV (0.7% in HCV with HBV, 0.5% in HCV only; 1.6% in HBV only, and 1.3% in No HCV and No HBV, P < 0.00001). The proportion of patients older than 65 years of age was significantly higher in Non-HCV group (P < 0.0001). The prevalence of dementia were significantly lower in HCV/HBV than control (5% in HCV with HBV, 4% in HCV only; 5% in HBV only, and 9% in No HCV and No HBV, P < 0.00001). The proportion of diabetes was also higher in HCV/HBV than control (47% in HCV with HBV, 44% in HCV only; 43% in HBV only, and 34% in No HCV and No HBV, P < 0.00001).
Study characteristics, inpatient and outpatient, medicare, main cohort.
| Variable | Total (Overall) (n = 1236734) | HCV and HBV (n = 545) | HCV only (n = 6040) | HBV only (n = 1300) | No HCV and No HBV (n = 1228849) | P-value |
|---|---|---|---|---|---|---|
| Age (years): Median (IQR) | 76.00 (70.00-83.00) | 56.00 (51.00-67.00) | 58.00 (52.00-70.00) | 69.00 (55.00-76.00) | 76.00 (70.00-83.00) | <.0001 |
| Total member months of A&B and No HMO - per bene: Median (IQR) | 72.00 (57.00-72.00) | 68.00 (48.00-72.00) | 72.00 (49.00-72.00) | 72.00 (50.00-72.00) | 72.00 (57.00-72.00) | <.0001 |
| Age (65+): N (%) | 1066562 (86.2%) | 154 (28.3%) | 2166 (35.9%) | 771 (59.3%) | 1063471 (86.5%) | <.0001 |
| Disabled: N (%) | 160788 (13.0%) | 380 (69.7%) | 3725 (61.7%) | 505 (38.8%) | 156178 (12.7%) | <.0001 |
| ESRD: N (%) | 21283 (1.7%) | 75 (13.8%) | 496 (8.2%) | 143 (11.0%) | 20569 (1.7%) | <.0001 |
| Died in hospital: N (%) | 251398 (20.3%) | 186 (34.1%) | 1570 (26.0%) | 316 (24.3%) | 249326 (20.3%) | <.0001 |
| Male: N (%) | 499649 (40.4%) | 355 (65.1%) | 3355 (55.5%) | 718 (55.2%) | 495221 (40.3%) | <.0001 |
| White, N (%) | 1059529 (85.7%) | 332 (60.9%) | 4348 (72.0%) | 778 (59.8%) | 1054071 (85.8%) | <.0001 |
| Source from Inpatient/Outpatient: N (%), | <.0001 | |||||
| Inpatient and Outpatient | 811728 (65.6%) | 465 (85.3%) | 4706 (77.9%) | 1004 (77.2%) | 805553 (65.6%) | |
| Inpatient Only | 5232 (0.4%) | 3 (0.6%) | 30 (0.5%) | 3 (0.2%) | 5196 (0.4%) | |
| Outpatient Only | 419774(33.9%) | 77 (14.1%) | 1304(21.6%) | 293 (22.5%) | 418100 (34.0%) | |
| Time to follow-up (years), N (%) | <.0001 | |||||
| 3 | 129309 (10.5%) | 78 (14.3%) | 718 (11.9%) | 153 (11.8%) | 128360 (10.4%) | |
| 4 | 142524(11.5%) | 54 (9.9%) | 635 (10.5%) | 123 (9.5%) | 141712 (11.5%) | |
| 5 | 274291 (22.2%) | 102 (18.7%) | 1074(17.8%) | 219 (16.8%) | 272896 (22.2%) | |
| 6 | 690610 (55.8%) | 311 (57.1%) | 3613 (59.8%) | 805 (61.9%) | 685881 (55.8%) | |
| Comorbidity: N (%), | ||||||
| Dementia | 104562 (8.5%) | 26 (4.8%) | 229 (3.8%) | 68 (5.2%) | 104239 (8.5%) | <.0001 |
| Diabetes | 421833 (34.1%) | 258 (47.3%) | 2642 (43.7%) | 562 (43.2%) | 418371 (34.0%) | <.0001 |
| Cerebrovascular Disease | 287901 (23.3%) | 101 (18.5%) | 1058 (17.5%) | 270 (20.8%) | 286472 (23.3%) | <.0001 |
| Hypertension | 983570 (79.5%) | 435 (79.8%) | 4733 (78.4%) | 1005 (77.3%) | 977397 (79.5%) | 0.0282 |
| Hyperlipidemia | 790065 (63.9%) | 269 (49.4%) | 3038 (50.3%) | 773 (59.5%) | 785985 (64.0%) | <.0001 |
| Parkinson's Disease (PD): N(%) | 15810 (1.3%) | 4 (0.7%) | 32 (0.5%) | 21 (1.6%) | 15753 (1.3%) | <.0001 |
| Parkinson's Disease incidence (PD): N(%) | 16077 (1.3%) | 1 (0.2%) | 49 (0.8%) | 23 (1.8%) | 16004(1.3%) | <.0001 |
| Time from 2005 to diagnosis of PD (years): N (%), | 0.0140 | |||||
| 0-1 | 5997 (27.2%) | 4 (80.0%) | 12 (19.7%) | 11 (32.4%) | 5970 (27.2%) | |
| 2 | 4754 (21.5%) | 0 (0.0%) | 18 (29.5%) | 1 (2.9%) | 4735 (21.5%) | |
| 3 | 4181 (18.9%) | 0 (0.0%) | 10 (16.4%) | 3 (8.8%) | 4168 (19.0%) | |
| 4 | 3687 (16.7%) | 1 (20.0%) | 10 (16.4%) | 9 (26.5%) | 3667 (16.7%) | |
| 5 | 3455 (15.7%) | 0 (0.0%) | 11 (18.0%) | 10 (29.4%) | 3434(15.6%) |
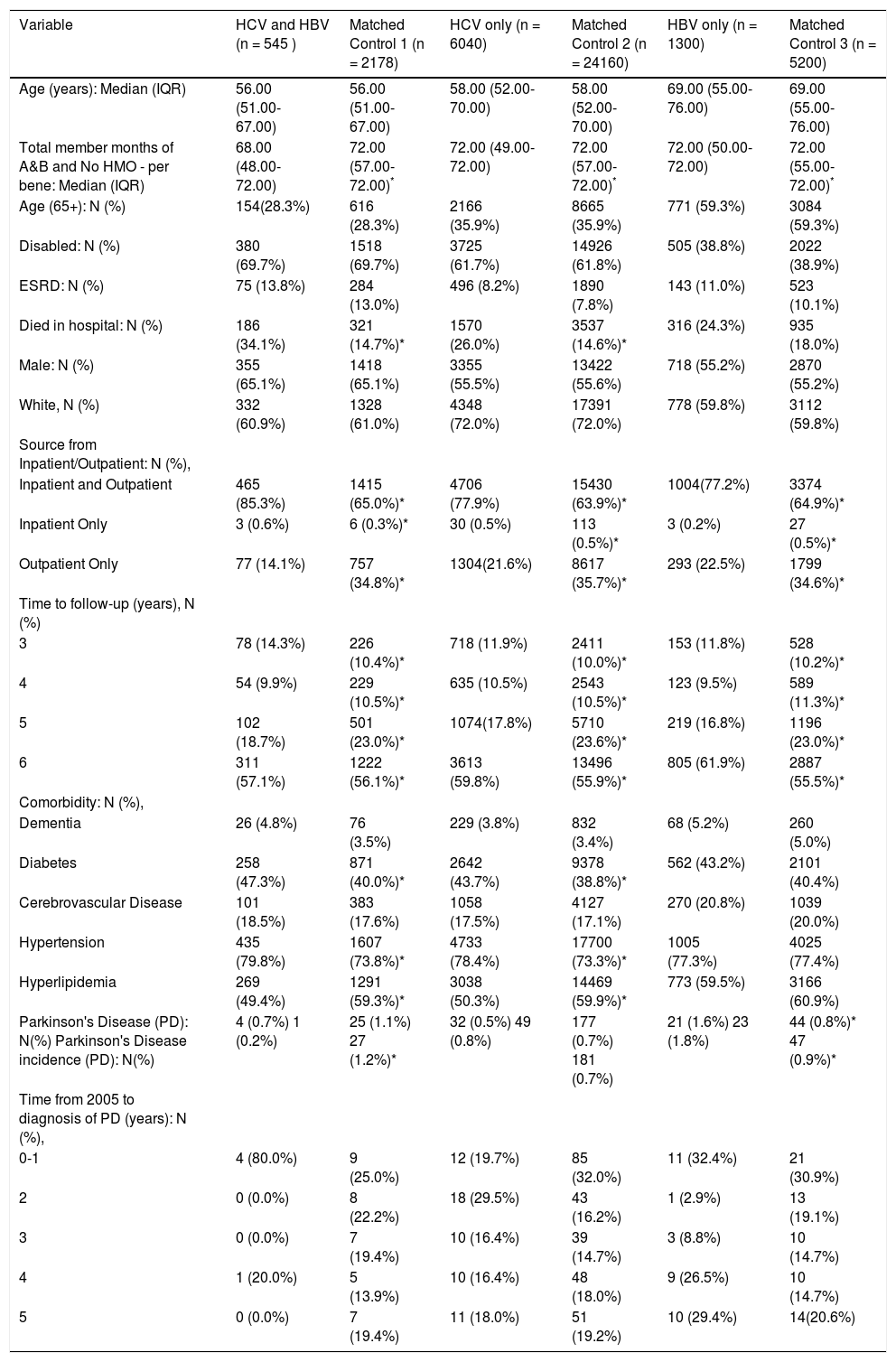
Table 2 is shown distributions of study characteristics after matching for age, race, gender, and Medicare eligibility status. In the each matched cohorts, for example, as compared with matched control, the prevalence of Parkinson's disease and the incidence of PD were higher in HBV (for prevalence of PD: 0.8% in matched control vs. 1.6% in HBV and for incidence of PD: 0.9% in matched control vs. 1.8% in HBV, All P < 0.05). The prevalence and incidence of PD were similar in HCV and matched control (for prevalence of PD 0.5% in HCV only vs. 0.7% in matched control and the incidence of PD 0.8% in HCV only vs. 0.7% in matched control, All P > 0.05). Again, the prevalence there was no difference in dementia prevalence between groups.
Study characteristics, inpatient and outpatient, medicare, matched cohort (based on age, race, gender, Medicare eligibility status, 1:4 ratio frequency match).
| Variable | HCV and HBV (n = 545 ) | Matched Control 1 (n = 2178) | HCV only (n = 6040) | Matched Control 2 (n = 24160) | HBV only (n = 1300) | Matched Control 3 (n = 5200) |
|---|---|---|---|---|---|---|
| Age (years): Median (IQR) | 56.00 (51.00-67.00) | 56.00 (51.00-67.00) | 58.00 (52.00-70.00) | 58.00 (52.00-70.00) | 69.00 (55.00-76.00) | 69.00 (55.00-76.00) |
| Total member months of A&B and No HMO - per bene: Median (IQR) | 68.00 (48.00-72.00) | 72.00 (57.00-72.00)* | 72.00 (49.00-72.00) | 72.00 (57.00-72.00)* | 72.00 (50.00-72.00) | 72.00 (55.00-72.00)* |
| Age (65+): N (%) | 154(28.3%) | 616 (28.3%) | 2166 (35.9%) | 8665 (35.9%) | 771 (59.3%) | 3084 (59.3%) |
| Disabled: N (%) | 380 (69.7%) | 1518 (69.7%) | 3725 (61.7%) | 14926 (61.8%) | 505 (38.8%) | 2022 (38.9%) |
| ESRD: N (%) | 75 (13.8%) | 284 (13.0%) | 496 (8.2%) | 1890 (7.8%) | 143 (11.0%) | 523 (10.1%) |
| Died in hospital: N (%) | 186 (34.1%) | 321 (14.7%)* | 1570 (26.0%) | 3537 (14.6%)* | 316 (24.3%) | 935 (18.0%) |
| Male: N (%) | 355 (65.1%) | 1418 (65.1%) | 3355 (55.5%) | 13422 (55.6%) | 718 (55.2%) | 2870 (55.2%) |
| White, N (%) | 332 (60.9%) | 1328 (61.0%) | 4348 (72.0%) | 17391 (72.0%) | 778 (59.8%) | 3112 (59.8%) |
| Source from Inpatient/Outpatient: N (%), | ||||||
| Inpatient and Outpatient | 465 (85.3%) | 1415 (65.0%)* | 4706 (77.9%) | 15430 (63.9%)* | 1004(77.2%) | 3374 (64.9%)* |
| Inpatient Only | 3 (0.6%) | 6 (0.3%)* | 30 (0.5%) | 113 (0.5%)* | 3 (0.2%) | 27 (0.5%)* |
| Outpatient Only | 77 (14.1%) | 757 (34.8%)* | 1304(21.6%) | 8617 (35.7%)* | 293 (22.5%) | 1799 (34.6%)* |
| Time to follow-up (years), N (%) | ||||||
| 3 | 78 (14.3%) | 226 (10.4%)* | 718 (11.9%) | 2411 (10.0%)* | 153 (11.8%) | 528 (10.2%)* |
| 4 | 54 (9.9%) | 229 (10.5%)* | 635 (10.5%) | 2543 (10.5%)* | 123 (9.5%) | 589 (11.3%)* |
| 5 | 102 (18.7%) | 501 (23.0%)* | 1074(17.8%) | 5710 (23.6%)* | 219 (16.8%) | 1196 (23.0%)* |
| 6 | 311 (57.1%) | 1222 (56.1%)* | 3613 (59.8%) | 13496 (55.9%)* | 805 (61.9%) | 2887 (55.5%)* |
| Comorbidity: N (%), | ||||||
| Dementia | 26 (4.8%) | 76 (3.5%) | 229 (3.8%) | 832 (3.4%) | 68 (5.2%) | 260 (5.0%) |
| Diabetes | 258 (47.3%) | 871 (40.0%)* | 2642 (43.7%) | 9378 (38.8%)* | 562 (43.2%) | 2101 (40.4%) |
| Cerebrovascular Disease | 101 (18.5%) | 383 (17.6%) | 1058 (17.5%) | 4127 (17.1%) | 270 (20.8%) | 1039 (20.0%) |
| Hypertension | 435 (79.8%) | 1607 (73.8%)* | 4733 (78.4%) | 17700 (73.3%)* | 1005 (77.3%) | 4025 (77.4%) |
| Hyperlipidemia | 269 (49.4%) | 1291 (59.3%)* | 3038 (50.3%) | 14469 (59.9%)* | 773 (59.5%) | 3166 (60.9%) |
| Parkinson's Disease (PD): N(%) Parkinson's Disease incidence (PD): N(%) | 4 (0.7%) 1 (0.2%) | 25 (1.1%) 27 (1.2%)* | 32 (0.5%) 49 (0.8%) | 177 (0.7%) 181 (0.7%) | 21 (1.6%) 23 (1.8%) | 44 (0.8%)* 47 (0.9%)* |
| Time from 2005 to diagnosis of PD (years): N (%), | ||||||
| 0-1 | 4 (80.0%) | 9 (25.0%) | 12 (19.7%) | 85 (32.0%) | 11 (32.4%) | 21 (30.9%) |
| 2 | 0 (0.0%) | 8 (22.2%) | 18 (29.5%) | 43 (16.2%) | 1 (2.9%) | 13 (19.1%) |
| 3 | 0 (0.0%) | 7 (19.4%) | 10 (16.4%) | 39 (14.7%) | 3 (8.8%) | 10 (14.7%) |
| 4 | 1 (20.0%) | 5 (13.9%) | 10 (16.4%) | 48 (18.0%) | 9 (26.5%) | 10 (14.7%) |
| 5 | 0 (0.0%) | 7 (19.4%) | 11 (18.0%) | 51 (19.2%) | 10 (29.4%) | 14(20.6%) |
This study of subjects enrolled in the Medicare database did not reveal any association between HCV and Parkinson's disease. This study is in contrast to previous reports that HCV may increase the risk of Parkinson disease.10,11
It is important to place this finding in the context of previously reported literature. HCV has been shown to replicate in the CNS.14,15 Additionally, patients with chronic HCV infection have higher prevalence of mental illness than the general population.16,17 Different types of mental disorders can be present in up to 50% of patients with chronic HCV infection, with depression and anxiety accounting for nearly 30% of them.18,19 One of the mechanisms that could be responsible for the psychiatric manifestations of HCV infection is defective dopaminergic neurotransmission in the CNS20-22 that could explain the potential linkage between HCV infection and Parkinson's disease. In a recent study, Wu, et al. assessed the association of HCV with Parkinson's disease among over 60,000 patients in Taiwan and found that patients with anti-HCV positivity had 1.9 times higher risk for developing Parkinson's disease.10 Additionally, the risk of developing Parkinson's disease was found to be even higher in another study. Tsai, et al. demonstrated an association between HCV infection and Parkinson's disease among nearly 50,000 Taiwanese people and stated that patients with HCV had 2.5 times higher risk of developing Parkinson's disease.11
Our results did not support such an association, as the prevalence of Parkinson's disease was similar between patients with and without HCV infection. There are a number of potential explanations for this discrepancy. First there is a substantial difference in the prevalence of HCV between two geographical areas. The prevalence of HCV among Taiwanese population has been estimated to be 5.5% and in fact, this rate is as high as 14.2% among elderly in the main island and even 26.4% in Penghu, an offshore island in Taiwan.23,24 On the other hand, the prevalence of HCV is between 1.1% and 1.9% in the general US population25,26 and higher among the Baby Boomer generation (3.2%-5.1%).5,27 In contrast, HCV rates were lower (0.5%) in the Medicare cohort.
Another possible explanation is the variability of the ex-trahepatic manifestation of HCV according to geographic regions. It is possible that HCV in Taiwan can have a different pathogenic profile in CNS than in the US. This brings up a very important point when comparing the two countries, the most common genotype responsible for HCV infection in these two different areas. Genotype 1 is the most common HCV genotype in the US, affecting approximately 70% of all HCV patients.28 Among these, more than half has genotype 1a. On the other hand, genotypes 1b and 2a are the two most common genotypes in Taiwan.29 Although there is no data on varying central nervous system virulence of different HCV genotypes, this might be a cause of the different findings between two areas, albeit unlikely.
Another finding of this study was that patients with HCV had significantly lower rates of comorbidities compared to patients without HCV, except for diabetes. Our results revealed that patients with HCV infection have significantly higher rates of diabetes. In fact, this finding is consistent with the previous publications.30,31 Mehta, et al. reported that patients older than 40 years of age with HCV infection had a threefold risk of developing diabetes compared to those without the infection.32 Although this relationship was attributed to insulin resistance in patients with HCV, more studies are warranted to better address this issue.
In summary, this analysis of the Medicare recipients in the United States does not support an association between HCV and of Parkinson's disease. Future studies assessing this association using other databases are warranted.
Abbreviations- •
ALS: Amyotrophic lateral sclerosis.
- •
CNS: Central nervous system.
- •
ESRD: End-stage renal disease.
- •
HBV: Hepatitis B virus.
- •
HCV: Hepatitis C virus.
- •
HIV: Human immunodeficiency virus.
- •
PD: Parkinson's disease
- •
US: United States.
The authors would like to thank Deena Hallaji and Brian Lam, PA/C for their great support during the formation of this study.
Conflict of InterestsThe authors have nothing to disclose. This study was internally funded.