Objective. To test the effects of peginterferon in an unrecoverable model of bile-duct ligation (BDL) induced liver fibrosis.
Material and methods.Thirty-seven Wistar rats were divided into five groups: group 1, BDL + peginterferon (n = 8); group 2, BDL (n = 8); group 3, sham + peginterferon (n = 7); group 4, sham (n = 7); and group 5, control group (n = 7). Peginterferon-alpha 2b (50 μgr/kg) or saline (1 mL/kg) was administered intraperitonealy every week for four weeks. Serum biochemical markers, liver tissue oxidative stress, collagen and transforming growth factor-β (TGF-β) levels were examined after four weeks. Liver slides were stained by hematoxylin and eosin and Masson trichrome/Gomory reticulum staining.
Results.The levels of tissue collagen, TGF-β, biochemical markers (AST, ALT, bilirubins, alkaline phosphates, gamma-glutamyl transpeptidase) and oxidative stress markers (Malondialdehyde, luminal, lucigenin) of the BDL group were higher than the sham operated and control groups (all-p < 0.001). Peginterferon improved malondialdehyde, luminal and glutathione levels in the BDL + peginterferon group (p < 0.05). Histopathological examination of the BDL groups showed stage-3 fibrosis, while all the control groups were normal. Peginterferon showed no improvement in fibrosis either histologically, or biochemically.
Conclusions. Peginterferon improved levels of malondialdehyde, glutathione and luminal in the rat model of BDL induced liver fibrosis. Peginterferon however, showed no anti-fibrotic effects in this model and therefore may not be a useful treatment for liver fibrosis.
Liver fibrosis is one of the main components of cirrhosis and is characterized by the accumulation of collagen and extra cellular matrix proteins in the Space of Disse. Hepatic stellate cells (HSCs) are the main component in producing these extra cellular matrix products which lead to fibrosis. There is still no effective treatment available for liver fibrosis but, recent experimental studies have reported partial success against liver fibrosis.1,2
Interferons not only have antiviral and immunomodulatory activities but also have effects on inhibition of cell growth and differentiation.3 Some studies have shown that interferons have some anti-fibrotic effects.4-8
Pegylated interferons (peginterferon) are superior to standard interferons in several ways. Peginterferons have longer half-lives with more constant serum levels, and are more tolerable due to weekly administration.9 These findings support the use of peginterferon as a potential agent for the prevention and management of hepatic fibrosis.
We aimed to test the effects of peginterferon-alpha 2b in an unrecoverable model of bile-duct ligation (BDL) induced liver fibrosis in rats as a potential therapeutic option. We preferred to give a higher dosage of the drug than in other similar studies to induce a higher therapeutic level for a possible anti-fibrotic effect.
Material and MethodsAnimals and study protocolThis experimental protocol had the full approval of the Ethical Committee on Animal Research, Marmara University School of Medicine, Turkey, and was performed according to the criteria of International Guidelines for animal research. Thirty-seven male Wistar rats, between 3.5-4 months old, weighing 190220 g, were obtained from Marmara University Animal Research Laboratory. Animals were kept at a constant temperature (22 ± 1 °C) with 12-hour light and dark cycles, in the same unit and allowed to acclimatize to their new conditions for one week before beginning the study. All animals received humane care in compliance with the National Institutes of Health criteria for laboratory animals. Rats had free access to standard rat chow and water.
Under general pentobarbital anesthesia, 37 male Wistar rats underwent BDL or a sham operation. Briefly, the common bile duct was exposed after laparotomy. Subsequently, two double knots were placed proximally and distally and the part of the bile duct between the two double knots was excised. In the sham-operated rats, the abdomen was closed without BDL. Sixteen of the rats underwent BDL and fourteen were sham operated. BDL rats were divided into two groups. The first group (BDL + Peginterferon; n = 8) received 50 μg/kg peginterferon-alpha 2b intraperitonealy per week. Sterile saline (1 mL/kg) was administered to the second group (BDL group; n = 8) intraperitonealy per week. The sham-operated rats were also divided into two groups and were given same dosages of either peginterferon (n = 7) or sterile saline (n = 7) intraperitonealy. An additional group of seven healthy rats were studied as the control group. All the animals survived the study period of four weeks. At the end of the study period, rats were weighed and their trunk blood collected after decapitation. The blood was centrifuged (3000 rpm, 10 min, 4 °C) and serum samples were obtained for biochemical analyses of AST, ALT, alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), total bilirubin, and direct bilirubin. The serum samples were stored at -80 °C and measured with automated standardized procedures (Roche Hitachi 917/747, Mannheim, Germany).
The left, middle, and right lobes of each liver were examined. Six different 5 × 5 × 5 mm slices were fixed in 10% buffered formalin, routinely processed, and blocked into paraffin for detection of collagen content by image analysis.2
The collagen content of the liver was assayed by the colorimetric method described by Lopez de Leon and Rojkind.10 The principle is the coloring of collagenous protein by Sirius red (36554-8, 2610-10-8; Aldrich Chemical, Deisenhofen, Germany) and noncollagenous proteins by fast green (14280; MERCK, Darmstadt, Germany). Fifteen micrometer-thick liver slices taken from each block were layered on glass slides and were assayed as originally described. Collagen content was calculated using the formula described by the authors as microgram collagen per milligram protein.10
Liver samples were weighed and homogenized in 0.15 M NaCl to determine reactive oxygen species by the following procedure. Homogenates were diluted up to 20% with 0.15 M NaCl. The homogenates were then sonicated two times for 30-second intervals at 4 °C. After sonication, the homogenates were centrifuged at 3000 rpm for 10 min and at 15,000 rpm for 15 min. Aliquots of the supernatants were used for studies.
Homogenized tissue supernatants were used for liver transforming growth factor-β (TGF-β) analyses. The level of TGF-β was quantified by an enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Zymed Laboratories, South San Francisco, CA).11 The minimal detectable level of TGF-β was 1.9 pg/mL, the intra-assay coefficient of variation for TGF-ß was 1%, and interassay coefficient of variation was 7.5%.
Oxidative stress parametersMeasurements of thiobarbituric acid reactive species (TBARS) were done according to Yagi.12 Liver tissues were homogenized in icy 10% trichloroacetic acid (TCA) solution and then centrifuged. The superficial liquid portion was mixed with equal volume of TBARS (0.67%) and heated at 90 °C for 15 min. TBARS were measured in nM/g tissue according to absorbance at 532 nm.
Reactive oxygen metabolites (ROM) were measured at room temperature via a chemiluminescent technique using a Mini Lumat LB 9506 Luminometer (EG&G, Berthold, Germany). Samples were placed in 2 mL of 0.02 M HEPES buffer (pH 7.4) containing 0.5 M phosphate-buffered saline. For measurement of ROM, 0.2 nM lucigenin (specific for superoxide radicals) or luminal (specific for HOCl-, H2O2, OH-) was used. Serial measurements at 15-second intervals for 5 min were done and the results were calculated as area under the curve and relative light unit (RLU); correction for fresh tissue weight was done (RLU per milligram of tissue area under the curve).13,14
Glutathione (GSH) levels were measured spectrophotometrically using Boyne and Ellman’s reagent and method.15 Results were calculated as pM GSH/g tissue.
Histopathological investigationsFive-micrometer liver sections were stained by hematoxylin and eosin and Masson trichrome/Gomory reticulum staining. The grading necroinflammatory activity and the staging fibrosis were set by Knodell’s criteria.16
Statistical evaluationData were expressed as mean values ± standard deviation or, in case of non-normal distribution, as median and range and were compared using the Kruskal-Wallis test. When significant, subsequent multiple comparison test was performed. P values below 0.05 were considered statistically significant. Comparisons between the groups were tested for significance by Mann-Whitney U and ϰ2 tests.
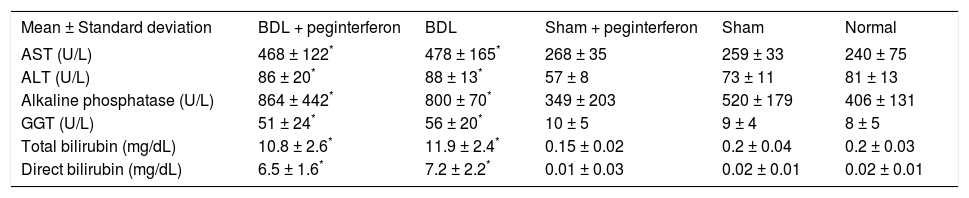
ResultsBiochemical findingsWeekly peginterferon therapy was performed in the study period of four weeks. All biochemical parameter levels, including AST, ALT, ALP, GGT, and total and direct bilirubin were significantly increased in both of the BDL groups in contrast to the control groups (all P > 0.001). No biochemical parameter difference was observed between the BDL subgroups. There was no significant difference in these biochemical parameters between the sham and the healthy control groups (Table 1).
Biochemical parameters measured in the current study.
| Mean ± Standard deviation | BDL + peginterferon | BDL | Sham + peginterferon | Sham | Normal |
|---|---|---|---|---|---|
| AST (U/L) | 468 ± 122* | 478 ± 165* | 268 ± 35 | 259 ± 33 | 240 ± 75 |
| ALT (U/L) | 86 ± 20* | 88 ± 13* | 57 ± 8 | 73 ± 11 | 81 ± 13 |
| Alkaline phosphatase (U/L) | 864 ± 442* | 800 ± 70* | 349 ± 203 | 520 ± 179 | 406 ± 131 |
| GGT (U/L) | 51 ± 24* | 56 ± 20* | 10 ± 5 | 9 ± 4 | 8 ± 5 |
| Total bilirubin (mg/dL) | 10.8 ± 2.6* | 11.9 ± 2.4* | 0.15 ± 0.02 | 0.2 ± 0.04 | 0.2 ± 0.03 |
| Direct bilirubin (mg/dL) | 6.5 ± 1.6* | 7.2 ± 2.2* | 0.01 ± 0.03 | 0.02 ± 0.01 | 0.02 ± 0.01 |
BDL: Bile duct ligation. GGT: γ-glutamyl transpeptidase. *P < 0.001 versus the sham + peginterferon,sham, and normal control groups.
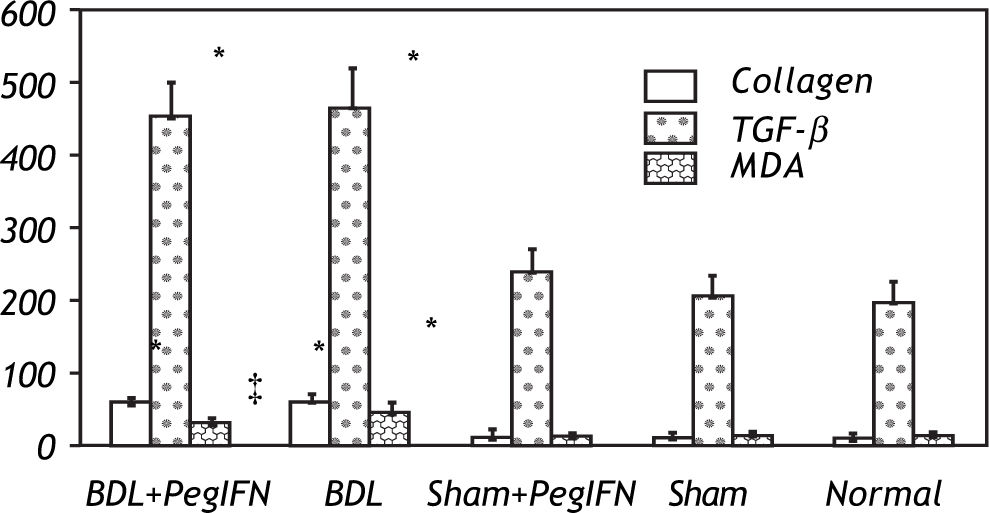
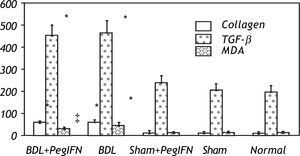
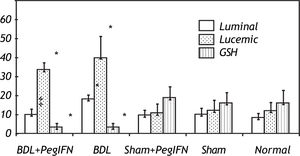
Hepatic collagen content in both BDL rat groups was significantly higher than the control groups (P < 0.001). However, the hepatic collagen levels were not found to be significantly affected by peginterferon administration. Similarly, no significant statistical difference was found between the control groups (P > 0.05; Figure 1).
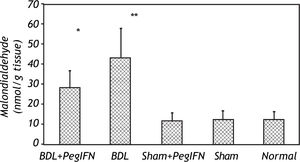
Tissue collagen (μg/mg protein), transforming growth factor-ß (TGF-ß; pg/g tissue) and malondialdehyde (MDA; nM/g tissue) levels. * P < 0.001 versus the sham + peginterferon (PegIFN), sham, and normal control groups. ‡ P = 0.036 versus bile duct ligation (BDL) group. Peginterferonal pha 2b (50 μg/kg) or saline (1 mL/kg) were administered intraperitoneally every week for four weeks.
Hepatic TGF-β levels in the both BDL rat groups were significantly higher than the control groups (P < 0.001). Peginterferon therapy did not change the tissue TGF-β levels in either the BDL and control groups or between them (P > 0.05; Figure 2).
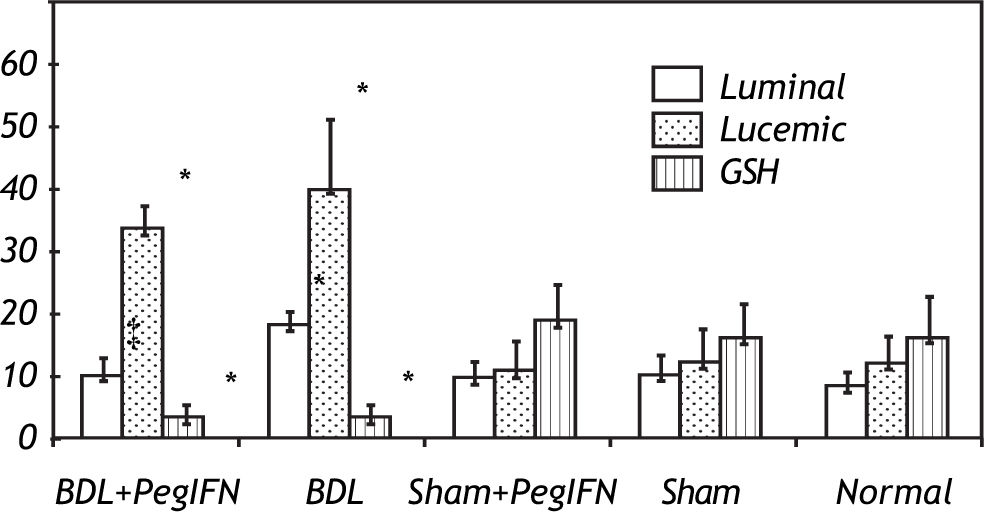
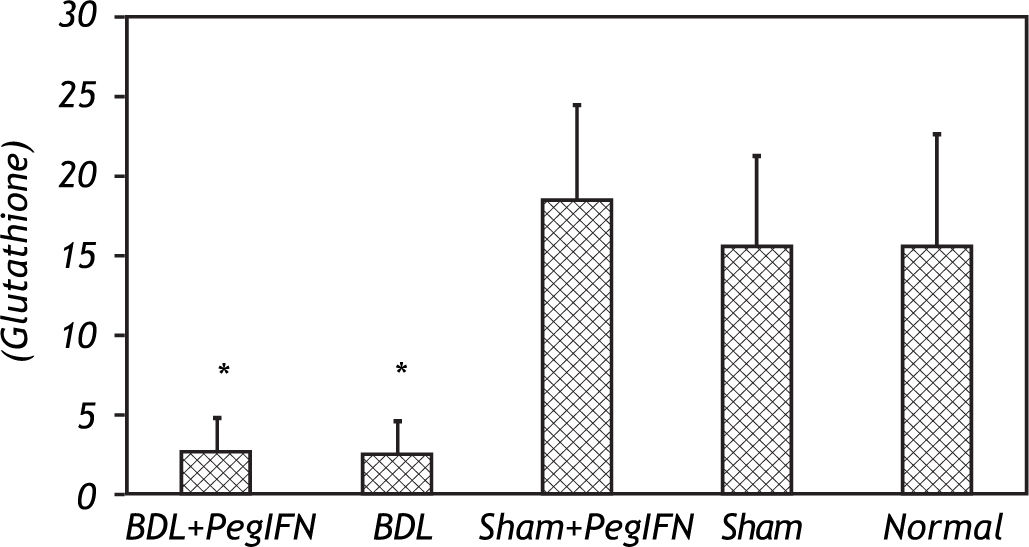
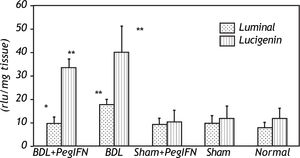
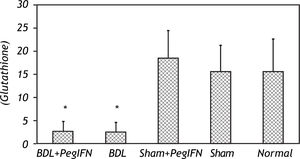
Tissue luminal (rlu/mg tissue), lucigenin (rlu/mg tissue) and glutathione (GSH; μM/g tissue) levels. ‡ P = 0.014 versus bile duct ligation (BDL) group. * P < 0.001 versus the sham + peginterferon (PegIFN), sham, and normal control groups. Peginterferon-alpha 2b (50 μjg/kg) or saline (1 mL/kg) were administered intraperitoneally every week for four weeks.
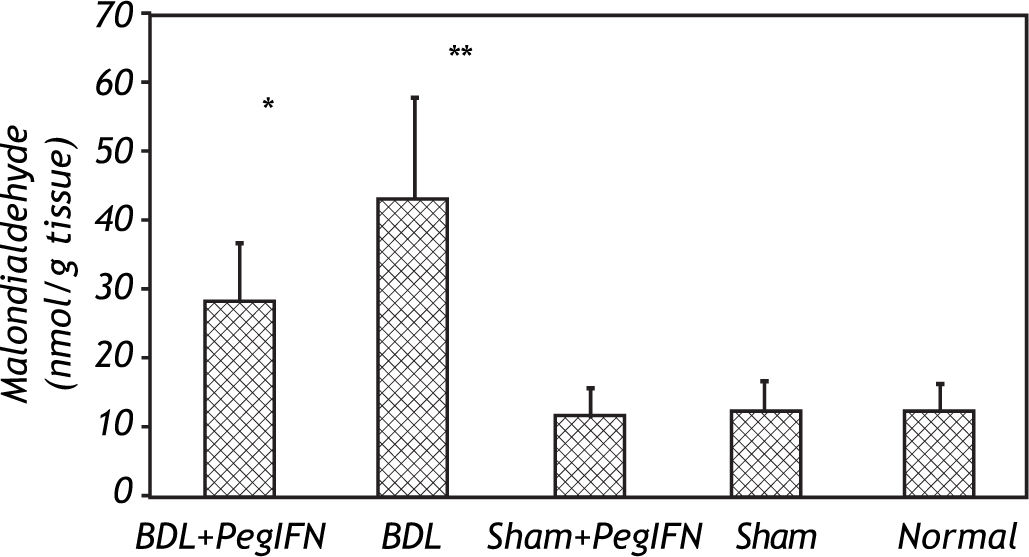
The mean liver malondialdehyde (MDA) levels of the BDL groups were significantly higher than those in the control groups (all P < 0.001). Peginterferon therapy improved the MDA level between the BDL subgroups (P = 0.036; Figure 3).
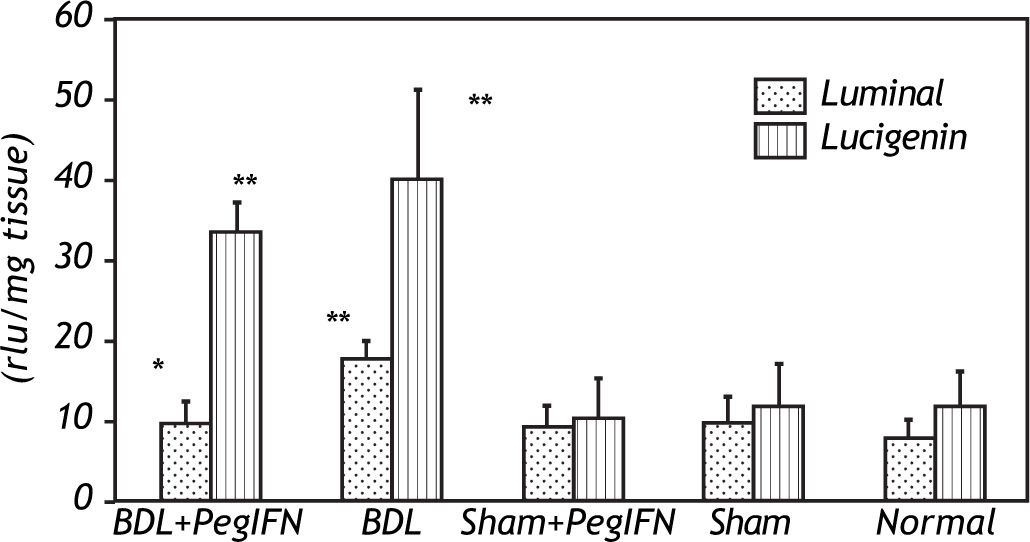
The tissue luminal level of the BDL + peginterferon group was significantly lower than the BDL group (P = 0.014). The tissue luminal and lucigenin levels in the BDL groups were significantly higher than the control groups (P < 0.001). Peginterferon-alpha 2b improved the luminal level of the BDL + peginterferon group, statistically similar to the control groups (Figure 4).
The tissue GSH levels of the BDL groups were significantly lower than the control groups (all P < 0.001). The tissue GSH levels between the BDL groups were not statistically different (Figure 5). No difference in the oxidative stress parameters was observed among the control groups.
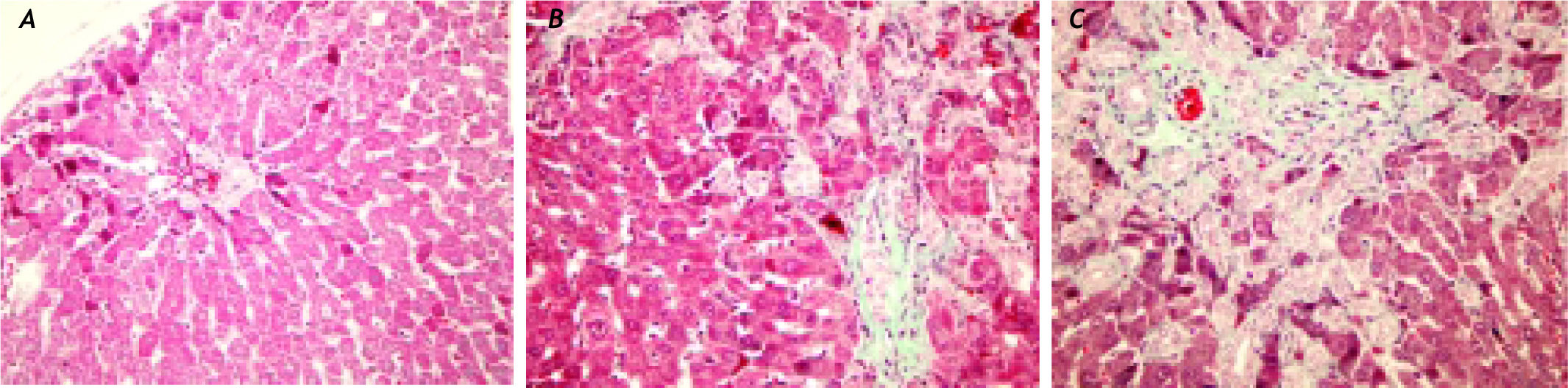
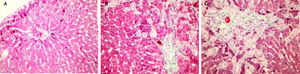
Histopathological findingsIn the histopathological examination of the liver sections, prominent bile duct proliferation and stage 3 fibrosis were demonstrated in all BDL groups. The histologic activity and fibrosis observed in the BDL groups by Knodell scoring did not change with peginterferon therapy (Figure 6).
A sham-operated group rat liver section with normal finding (HE, original magnification ×40). (B) A bile duct ligation (BDL) group liver slide with portal fibrosis and fibrous septa, (Trichrome-stained original magnification ×40). (C) A BDL+peginterferon group liver slide with cellular loss and portal-portal fibrous septa connecting portal areas to each others and lobule centers (Trichrome-stained original magnification ×40). Peginterferon-alpha 2b (50 μg/kg) or saline (1 mL/kg) were administered intraperitoneally every week for four weeks.
At present, no effective treatment of liver fibrosis is available for clinical use. A valuable therapeutic approach against the development of hepatic fibrosis is still needed.
Contradictory reports about the anti-fibrotic efficacy of interferon in some hepatic fibrosis models have been reported. Some of them have shown interferon as ineffective,2,17 while others found it effective as an anti-fibrotic agent.18,19 To the best of our knowledge this is the first study to investigate the effects of peginterferon in BDL induced liver fibrosis in rats.
Fort, etal.17 treated both CCl4 and BDL induced models with interferon-alpha 2a 100,000 UI/day in Sprague-Dawley rats. Inductions periods were eight weeks for the CCl4 and four weeks for the BDL models. The therapy drug was started with the initial inductions in the models and was given nine and four weeks to the CCl4 and BDL models, respectively. They studied liver fibrosis image analysis, liver hydroxyproline and mRNA (fibronectin, procollagen alpha) contents and serum hyalaronate. They reported that interferon-alpha 2a 100,000 UI/day was efficient in a CCl4-induced fibrosis model. However, they also noted that interferons were ineffective in the BDL model. Moreno and Muriel demonstrated that interferon-alpha (100,000 IU/rat /day, s.c.) was useful in reversing fibrosis and liver damage induced by BDL in rats. In their study, interferon-alpha reversed to normal the values of all collagen and biochemical markers measured and restored the normal architecture of the liver. Their study results on the model were restricted to 15 days only.20
Controversial results were also reported in chronic hepatitis C therapy with interferons. While some authors presented that the fibrosis score could decrease only in patients responding to interferon-alpha therapy,21-23 others noted that interferon decreased collagen synthesis in patients not responding to the therapy.24,25 There were also some studies that revealed that interferon-alpha improves necroinflammatory scores of the liver in sustained responders, although no effect was observed on the fibrosis.26,27
Tasci, etal.7 studied the effects of peginterferon-alpha 2b and ursodeoxycholic acid on CCI4 induced liver fibrosis in Sprague-Dawley rats. In their study, both of these agents improved the liver pathology. They observed significantly higher anti-fibrotic effects when using these agents in combination. Their therapeutic dose of peginterferon on rats was equivalent to human antiviral therapy dosage (1.5 pg/kg/week). In another study by the same group, peginterferon-alpha 2b and taurin showed similar anti-fibrotic effects on CCI4 induced liver fibrosis.8 They studied the effect of the drugs after 12 weeks of fibrosis induction by CCI4. Although we used much higher dosage of peginterferon-alpha 2b in Wistar rats; we could not find an anti-fibrotic effect of peginterferon in the BDL model.
CCI4 induced fibrosis resembles human liver fibrosis caused by alcohol consumption. Unlike human liver fibrosis, more significant cholangiolar cell hyperplasia is observed in the CCI4 induced model.28 The BDL model requires surgical skills and induces activation of hepatic stellate cells and corresponding fibrosis without any carcinogenic effect. In the BDL model, proliferation of biliary epithelial cells and oval cells are stimulated, resulting in proliferation of bile ductules with portal fibrosis.29 The BDL model causes pure fibrosis, whereas the CCI4 model also causes inflammation.17
Rat fibrosis models with CCI4 are disadvantageous, at least for interferon trials, because interferon directly prevents bioactivation of CCI4 by inhibiting cytochrome P450 2E1 enzyme activity.2,30-32 To beware of possible drug interaction in the CCI4 model, drug therapies are being given after the establishment of the model. The spontaneous recovery after cessation of toxic agent is another difficulty in the CCI4 model that requires a recovery group to compare the healing of the fibrosis in the groups. Ligation of the bile duct for four weeks caused advanced hepatic fibrosis in rats2,33,34 and this BDL model was recommended for assessing drug effects on liver fibrosis.35 In our study, we used peginterferon-alpha 2b as a long acting interferon molecule with higher doses (50 pg/kg/week) for four weeks in the BDL model. However, we did not find the outcomes of peginterferon-alpha 2b as efficient as observed in CCI4 models.7,8 The different outcomes can be attributed to the dosage and/or the model differences.
Our study results were correlated with the results of the study of Fort, etal.17 They showed that interferon was effective in the CCI4 model but was ineffective in the BDL model. We observed the same ineffectiveness with the new generation of the drug. The lack of effect in the BDL model may also be related to the inhibition of signal pathways used by interferons in cholestasis via hydrophobic biliary acids.36
Rat strain differences have been shown to explain fibrosis variation. Baba, etal. studied rat strain differences in the early development of porcine serum (PS)-induced hepatic fibrosis using Sprague Dawley and Wistar rats. They injected 0.5 mL PS i.p. twice a week. At four weeks, hepatic fibrosis with complementary fibrous septa developed in Sprague Dawley rats but not in Wistar rats.37
Current data indicate that oxidative stress is related with the activation of HSCs, which are the central mediators in the pathogenesis of liver fibrosis.38,39 One of the possible mechanisms of action of interferon, explaining its anti-fibrotic effect, is by decreasing oxidative stress through the reduction of reactive oxygen species from HSCs.40,41 Hydrophobic bile acids lead to oxidative stress via NADPH oxidase isoforms entering the pathway of immune activation.42 In our study we looked at the levels of oxidative stress markers as well as glutathione as an effect of peginterferon on BDL induced liver fibrosis. We believe that this mechanism may provide a potential pathway for peginterferon to treat liver fibrosis. Our study showed that peginterferon did improve levels of MDA, luminal and glutathione and that these changes could have a hepatoprotective effect but it showed no improvement in fibrosis using the BDL rat model.
In conclusion, weekly intraperitoneal peginterferon administration in the BDL model was found to have a beneficial effect on oxidative stress parameters (malondialdehyde, luminal and GSH). Our results on peginterferon in BDL model did not demonstrate the anti-fibrotic effects that have been reported previously with conventional interferon.
AcknowledgmentThe authors would like to thank Kenneth Dorko from the University of Pittsburgh for his suggestions and critical review of the manuscript.
Abbreviations- •
HSCs. Hepatic stellate cells
- •
BDL. Bile-duct ligation.
- •
ALP. Alkaline phosphatase.
- •
GGT. γ-glutamyl transpeptidase.
- •
TGF-β. Transforming growth factor-ß.
- •
TBARS. Thiobarbituric acid reactive species.
- •
MDA. Malondialdehyde.
- •
TCA. Trichloroacetic acid.
- •
RLU. Relative light unit.
- •
GSH. Glutathione.
- •
PS. Porcine serum.