Background/Objective. Inchin-ko-to (ICKT) is an herbal medicine used in Japan to treat jaundice and liver fibrosis. We investigated the effect of oral ICKT supplementation on endotoxin-induced cholestasis in the rat.
Material and methods.Lipopolysaccharide (LPS) injection (1 mg/kg body weight i.p.) was used as a model of sepsis-induced cholestasis. Bile flow, biliary bile salt secretion, biliary glutathione secretion and protein expression of the main hepatobiliary transporters Na(+)-taurocholate-cotransporting peptide (Ntcp), multidrug resistance protein 2 (Mrp2) and bile salt export pump (Bsep) were analyzed by conventional techniques in ICKT treated and non-treated animals.
Results.Injection of LPS induced a significant decrease of bile flow (-24%), biliary bile salts (-40%) and glutathione excretion (-70%) as well as a significant decrease in Ntcp (-90%) and Mrp2 (-80%) protein levels. ICKT supplementation partially prevented the effects of LPS determining a less intense reduction in bile flow (-10%), a normalization of glutathione excretion as well as a significant increase in Mrp2 protein levels to 60% of the levels observed in control animals. ICKT administration did not modify the effects of LPS on BS secretion or Ntcp protein levels.
Conclusion.Our data show that oral supplementation of ICKT partially prevents LPS-induced cholestasis by increasing Mrp2 protein levels and biliary glutathione excretion thus increasing bile salt-independent flow.
Cholestasis is a frequent clinical complication seen in patients with extrahepatic bacterial infection and sepsis.1-3
A cholestatic picture is associated to a wide variety of infections caused by several organisms including aerobic and anaerobic as well as gramnegative and gram-positive bacteria, with gram-negative bacteria being the cause of most cases.2,4 In the critical care setting, cholestasis frequently poses a clinical challenge both in diagnostic evaluation and in management as well as may influence the clinical prognosis of a given patient.5
Significant advances in the understanding of the pathogenesis of sepsis-associated cholestasis have been made in the recent years.6-9 Thus, now it is known that hepatocellular and ductal bile formation is critically impaired as a result of the effects of pro-inflammatory cytokines on specific transport proteins of both hepatocytes and cholangiocytes.1,2 Proinflammatory cytokines (mainly interleukin-1ß [IL-1ß], interleukin-6, and tumor necrosis factor a [TNFα]) are in turn generated after exposure of Kupfer cells to Lipopolysaccharide (LPS) which is derived from gram-negative bacteria wall.10 Data on the pathophysiology of sepsis-associated cholestasis has been mostly generated challenging the rodent liver with LPS.1,11-19 A single dose of LPS elicits a profound decrease of canalicular excretion of conjugated bile acids, bilirubin glucuronides, and glutathione conjugates.12,14,15,17 Moreover, the function and expression of the canalicular bile salt exporter BSEP (bile salt export pump, [BSEP, ABCB11]) is impaired and both protein and mRNA levels of the multispecific organic anion transporter Mrp2 (multidrug resistant-associated protein 2 [MRP2, ABCC2]) are significantly down-regulated after a single dose of LPS, providing a molecular mechanism for the diminished excretion of several bile and non-bile acid organic anions.1,20 In addition to profound alterations in canalicular transport systems, LPS injection also induces significant changes in sinusoidal transport function, markedly decreasing bile acid uptake.12,13,19 This correlates well with reduced protein and mRNA levels of the main bile acid importer of the hepatocyte Ntcp (Na+/taurocholate cotransporting polypeptide [NTCP, SLC10A1]) after hepatic exposure to endotoxin or effector cytokines such as TNF-α or IL-1ß.1,7,11
The herbal medicine Inchin-ko-to (ICKT) has long been used in Japan and China for the treatment of liver diseases,21,22 ICKT is an aqueous extract from three herbs; Artemisia capillaris spica (A. capillaris), Gardenia fructus (G. fructus), and Rhei rhizome (R. rhizome) with a weight ratio of 4:3:1 which is now manufactured under modern scientific quality controls.23 ICKT is considered a choleretic and hepatoprotective agent with relevant effects on bile formation,24 hepatic oxidative stress, hepatic fibrogenesis and stellate cells apoptosis.23-26
In this study, we tested the hypothesis that ICKT is able to influence the cholestasis development in the LPS-challenged rat liver through its known choleretic activity. Our results show that in fact, oral supplementation of ICKT partially prevents LPS-induced cholestasis through its influence on bile salt-independent flow particularly through an increase Mrp2 protein levels and biliary glutathione excretion.
Material and MethodsAnimalsMale Sprague-Dawley rats (170-190 g body weight) were housed in transparent polycarbonate cages, with wood chip bedding at a 12 h light/ darkness cycle, at a temperature of 21 °C and a relative humidity of 50% throughout the accommodation (at least one week) period and permitted ad libitum consumption of water and food. Animal experiments were approved by the Local Ethics Review Committee on Animal Experiments.
ReagentsLipopolysaccharide from S. typhimurium, sodium pentobarbital, 3-alpha-hydroxysteroid dehydrogenase and most reagents and organic chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All inorganic chemicals and organic solvents were obtained from E. Merck (Darmstadt, Germany). ICKT extract powder was from Tsumura and Co. (Tokyo, Japan).
ICKT and LPS treatmentsAnimals were randomized in four groups: two of them were fed ICKT [2% w/w] mixed with control diet for four weeks (ICKT groups). The remnant groups were fed a control diet. At the end of the feeding period, a single i.p. injection of LPS (1 mg/kg body weight, dissolved in saline) was given to rats belonging to one of the ICKT groups (ICKT+LPS group) and to one group of rats assigned to be fed a control diet (LPS group). LPS was administered 16 hours before carrying out the experiments. After treatment, rats were anesthetized with a single dose of sodium pentobarbital (50 mg/kg body wt, i.p.) and bile collection was carried out, in two 10 min-period, after cannulation of the proximal common bile duct with a PE-10 polyethylene tube (Clay Adams, Division of Becton, Dickinson & Co., Parsippany, NJ, USA) just proximal to the bifurcation. The second tube containing 5% 5-sulfosalicylic acid was used to determine GSH bile excretion. Arterial blood samples were taken at the end of bile collection period and the livers were removed, snap-frozen in liquid nitrogen and stored at -70 °C until analyzed.
Analytical ProceduresBile flow was measured gravimetrically and total biliary bile acids were quantified by the 3-alpha-hydroxysteroid dehydrogenase method.27 Glutathione in bile was determined as described by Anderson ME.28
Expression of hepatic transporter proteinsThe protein mass of hepatic transporters in liver tissue from control and treated rats was measured as previously described29 using membrane-rich microsomal fractions to assess the protein expression of Bsep, Ntcp and Mrp2. Western analysis was performed with standard techniques using the Renaissance Western blotting kit (New England Nuclear, Boston, MA). Membranes were probed with: a polyclonal anti-rat Bsep antibody against a C-terminus 13-amino acid sequence,30 anti-Ntcp fusion protein IgG and monoclonal anti-rat Mrp2.31 Immunoreactive bands were quantified by laser densitometry.
Statistical AnalysisAll results are expressed as mean ± SD of experiments from four to six animals per group. The differences between groups were compared with Kruskal-Wallis test and the Dunn test for pairwise comparisons. Values were considered significantly different when the p value was equal to or less than 0.05.
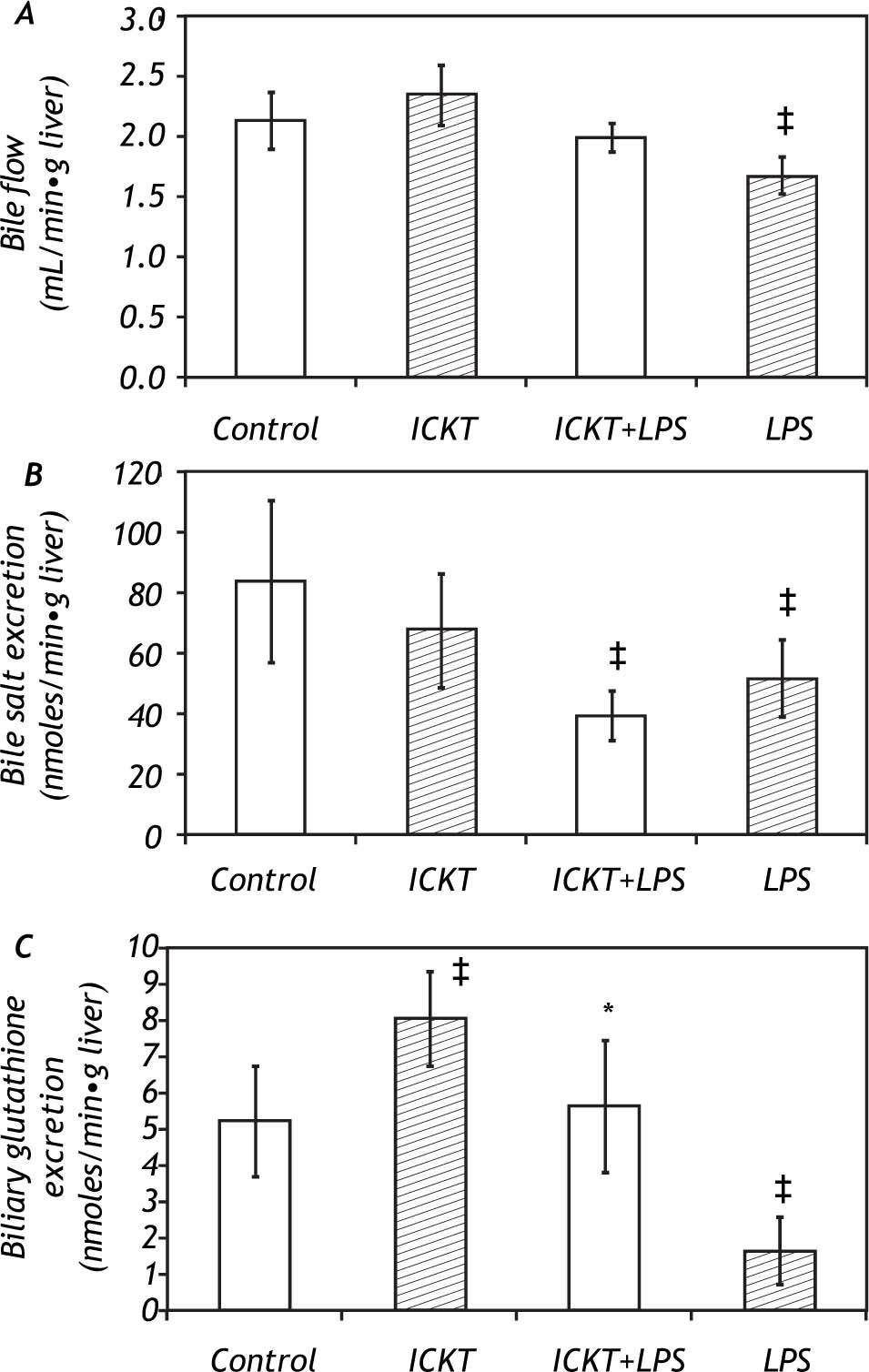
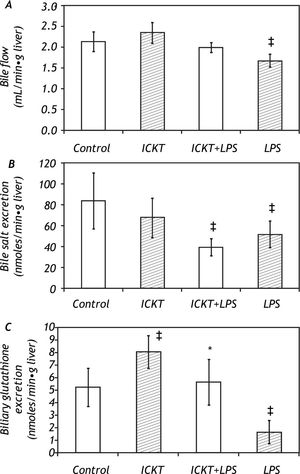
ResultsEffect of ICKT on Bile Flow and Biliary Secretion in control and LPS-challenged ratsICKT-supplementation determined a non-significant increase in basal bile flow (2.35 ± 0.25 vs. 2.13 ± 0.23 μl/g liver wet weight/min in ICKT-supplemented and control animals respectively). On the other hand, LPS injection determined a significant reduction of bile flow (1.67 ± 0.15 μl/g liver wet weight/min, 78% of the control value) which was partially prevented by ICKT administration. In fact, the observed reduction in bile flow was smaller than that observed in control animals and did not reach statistical significance (1.99 ± 0.12 μl/g to liver wet weight/min, 93% of the control value) (Figure 1).
Effect of Inchin-ko-to (ICKT) administration on bile flow (A), biliary bile salt secretion (B) and biliary glutathione secretion (C) in control and lipopolysaccharide (LPS)-treated rats [n = 5-6 animals per group, ‡ p < 0,05 compared with control group, * p < 0.05 compared with LPS group).
The observed biliary bile salt excretion rate in the control group was of 84.1 ± 26.8 nmoles/g of liver per minute. LPS administration induced a significant reduction in this variable to 51.7 ± 12.8 nmoles/g of liver per minute (61% of the control value, P > 0.05). ICKT supplementation did not affect the biliary bile salt excretion rate in LPS challenged animals (Figure 1).
LPS administration also induced a marked and significant reduction (-69%) in the biliary excretion of reduced glutathione (GSH) (1.6 ± 0.889 nmoles/ g of liver per minute in the LPS group vs. 5.16 ± 1.5 nmoles/g of liver per minute in control group). This was prevented by ICKT administration. In fact, rats belonging to the ICKT-LPS group exhibited biliary glutathione excretion rates similar to that seen in control animals (5.6 ± 1.8 nmoles/g of liver per minute) (Figure 1). Of note, oral ICKT administration significantly increased the biliary Excretion GSH (8.0 ± 1.28 nmoles/g of liver per minute, 1.5-fold higher than the value observed in control animals).
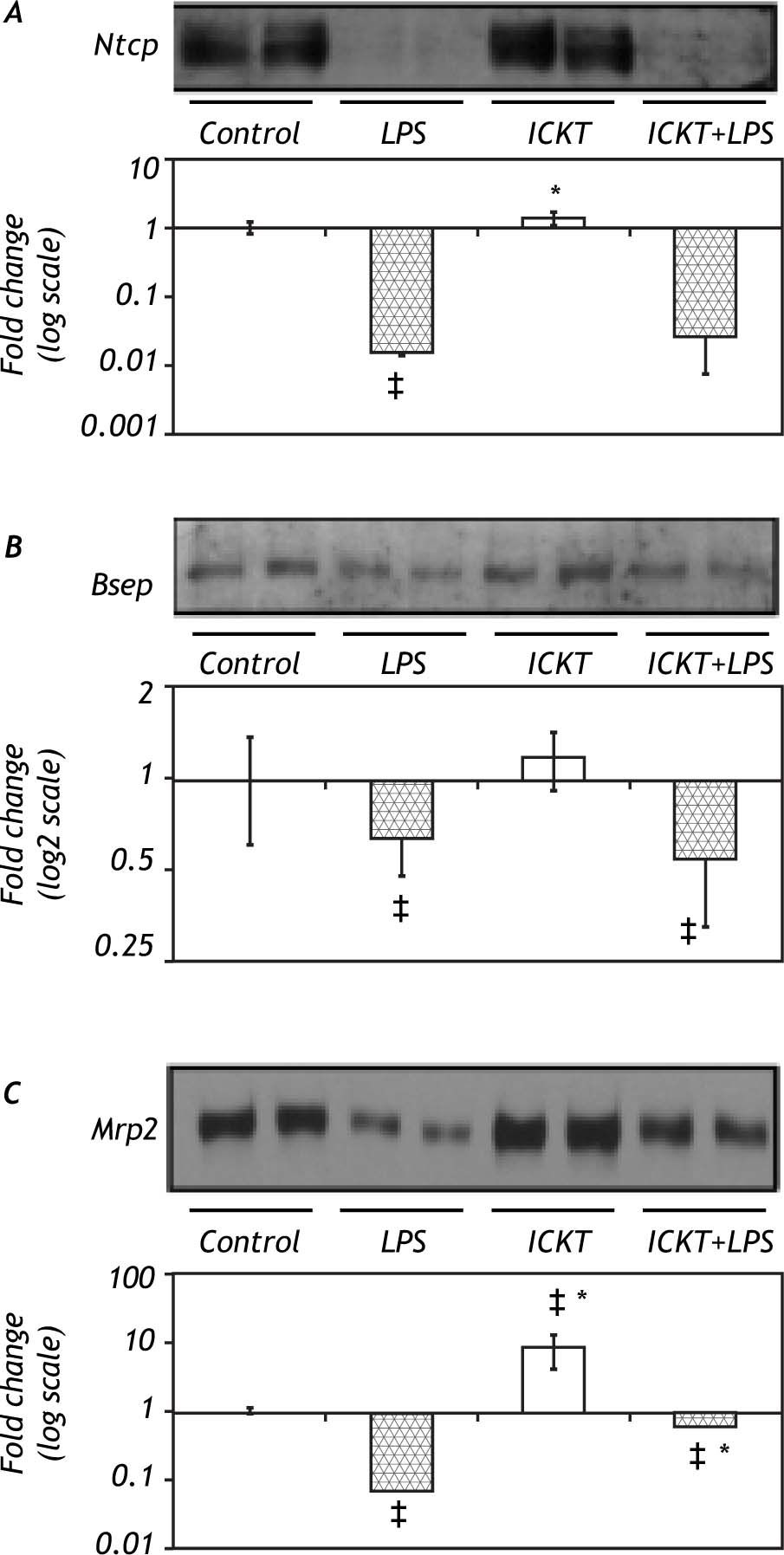
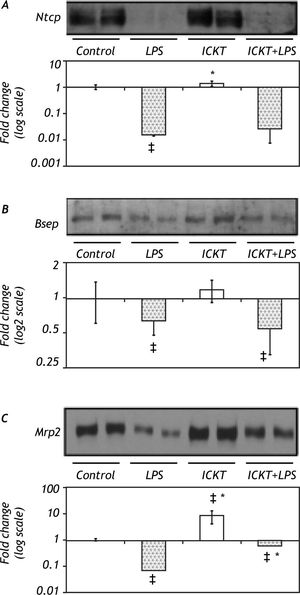
Immunoblot Analysis of hepatobiliary transporter proteinsIn order to clarify the molecular basis of the protective effect of ICKT on LPS-induced cholestasis the protein expression of critical hepatobiliary transporters Ntcp, Bsep and Mrp2 was assessed in rat liver extracts. Results of these experiments are shown in figure 2. As expected, Ntcp protein expression diminished significantly after LPS administration. ICKT administration did not affect Ntcp reduction after LPS injection. On the other hand, LPS injection reduced hepatic protein expression of Bsep which was not modified by previous administration of ICKT. Of note, neither Ntcp nor Bsep protein levels were influenced by ICKT administration. Finally, a significant decrease in Mrp2 protein levels was observed in LPS group (14.49-fold lower than control) which was partially reversed by ICKT supplementation (1.65-fold lower than control) (Figure 2C). Mrp2 protein expression levels were significantly increased in ICKT group compared to the control group (9.22-fold higher) and all other groups.
Representative Western blot analysis and quantitative densitometric analysis of hepatobiliary transport proteins. Protein levels of the Na(+)-taurocholate-cotransporting peptide (Ntcp, Panel A), the bile salt export pump (Bsep, Panel B) and the multidrug resistance protein 2 (Mrp2, Panel C) were measured. Values are expressed as fold change (log; mean ± SD) compared with control. ‡ p < 0.05 compared with control group, * p < 0.05 compared with LPS group).
The herbal medicine ICKT has been extensively used for the treatment of liver disease in Japan. Its use has been mainly based on traditional knowledge with no formal testing in clinical studies. However, in the last decade a number of experimental reports have shown that ICKT can positively influence several pathological processes relevant for liver diseases. In fact, ICKT seem to be able to induce hepatic stellate cell apoptosis and modulate liver fibrosis,25,26 to promote hepatic regeneration and exert anti-apoptotic effects in hepatocytes.32 Clinical experience using ICKT is limited to several case reports showing a decreasing effect on serum bilirubin levels after hepatectomy or an antifibrogenic action in patients with biliary atresia.33-35 Based on these communications, ICKT seems to be safe and devoid of adverse effects.
In the setting of clinical or experimental cholestasis, available information on the potential utility of ICKT administration is scarce. Several reports have shown that genipin, the active compound found in the gardenia fruit extract, has relevant effects on the hepatobiliary secretory function in rodents.23,24 However, no direct testing of the effects of ICKT in cholestatic models had been carried out. Considering that the potent choleretic effects of genipin is mainly mediated by enhancing Mrp2/MRP2-mediated secretory capacities through an increase in Mrp2 expression24 and the fact that Mrp2 down-regulation is a relevant phenomenon in LPS-induced cholestasis,20 we tested the effect of oral administration of ICKT on hepatobiliary secretory function and LPS-induced cholestasis. Our results showed that indeed ICKT was able to prevent the LPS-induced decrease of bile flow. We also confirmed the choleretic properties of ICKT as well as its ability to increase the Mrp2 protein levels in the liver. The latter was associated to a markedly increase in biliary GSH excretion and probably of the bile salt-independent bile flow. These effects are probably the underlying mechanisms responsible of the observed attenuation of the cholestatic effects of LPS administration in the rat liver. Also, a colangiocelullar effects might not be excluded. The partial response may be attributable to the lack of effects of ICKT on biliary bile salt excretion or the expression of main bile salt transporters Ntcp and Bsep which are also affected by LPS.17
In conclusion, the current study showed that oral administration of ICKT has beneficial effects in the LPS-induced model of cholestasis mainly in virtue of its choleretic effects and the ability to up-regulate the main hepatobiliary organic anion transporter Mrp2 and increase the bile salt-independent bile flow. This study provides support for further testing of ICKT in other cholestatic models or human subjects. Another relevant aspect to be explored is the potential synergistic effects of the administration of ICKT and other anticholestatic agents such as ur-sodeoxycholic acid, a known choleretic bile acid which also influence Mrp2 levels in the liver.36,37
AcknowledgementThis work was partially funded by Grants from the Fondo Nacional De Ciencia y Tecnologia de Chile (Fondecyt #1050780 and #1080170 to MA).
The authors thank to Tsumura and Co. (Tokyo, Japan) for donating the TJ-135 compound.