Diabetes developed as a complication of cirrhosis is known as «hepatogenous diabetes» (HD). Around 30% to 60% of cirrhotic patients suffer from this metabolic disorder. Insulin resistance in muscular, hepatic and adipose tissues as well as hyperinsulinemia, seem to be pathophysiologic bases for HD. An impaired response of the islet β-cells of the pancreas and the hepatic insulin resistance are also contributing factors. Diabetes develops when defective oxidative and nonoxidative muscle glucose metabolism develops. Non-alcoholic fatty liver disease (NAFLD), alcoholic cirrhosis, chronic hepatitis C (CHC), and hemochromatosis are more frequently associated with HD. HD in early cirrhosis stages may be sub clinical. Only insulin resistance and glucose intolerance may be observed. As liver disease advances, diabetes becomes clinically manifest, therefore HD may be considered as a marker for liver function deterioration. HD is clinically different from that of type 2 DM since it is less frequently associated with microangiopathy and patients suffer complications of cirrhosis more frequently as well as increased mortality. Insulin resistance and HD associate to a decrease in the sustained response to antiviral therapy and an increased progression of fibrosis in patients with CHC. Diabetes treatment is complex due to liver damage and hepatotoxicity of oral hypoglycemic drugs that are frequently prescribed to these patients. This paper will review current concepts in relation to the pathopysiolo-gy, the impact on the clinical outcome of cirrhosis, and the therapy of HD. Finally, the role of HD as a risk factor for the occurrence and exacerbation of hepatocel-lular carcinoma (HCC) will also be reviewed.
From 17% to 30% of patients who suffer cirrhosis may be clinically diabetic.1 Diabetes that develops as a complication of cirrhosis is known as “hepatogenous diabetes” (HD).2–3
The liver has an important role in the metabolism of carbohydrates because it is responsible for balancing blood glucose.4–8 In the presence of hepatic disease, the metabolic homeostasis of glucose is impaired because of insulin resistance and impaired sensitivity of islet β-cells in the pancreas.3,5,8,9 Insulin resistance occurs in muscular, hepatic and adipose tissues.10 Furthermore, the etiology of liver disease is important in the incidence of diabetes: the non-alcoholic fatty liver disease (NAFLD), alcohol, hepatitis C virus (HCV) and hemochromatosis are more frequently associated with diabetes.11–13
Diabetes mellitus (DM) in patients with compensated liver cirrhosis may be sub clinical. In these cases, an oral glucose tolerance test (OGTT) may detect glucose intol-erance.14 The natural history of HD is different from that of hereditary type 2 DM, since it is less frequently associated with microangiopathy. Patients with HD suffer complications of cirrhosis more frequently causing death.14,15
The treatment of HD is complex due to the presence of liver damage and the hepatotoxicity of oral hypoglyce-mic drugs frequently prescribed to these patients. There-fore, pharmacological therapy must be closely monitored for the risk of hypoglycemia.2,12,14
This paper will review the new concepts found in literature regarding: a) factors involved in the genesis of HD; b) the impact of HD on the clinical outcome of patients with liver disease, and c) the treatment of diabetes in cirrhotic patients. Similarly, it will review the role of HD as a risk factor for the occurrence and exacerbation of hepatocellular carcinoma (HCC).
EpidemiologyDepending on the etiology, the degree of liver damage and the diagnostic criteria, the incidence of glucose intolerance varies from 60-80%, and that of diabetes between 20% and 60%.2,12,14,16 In the early stages of chronic liver disease, insulin resistance and glucose intolerance may be found in most of these patients.17,18 Diabetes manifests clinically as the liver function deteriorates, thus the HD can be considered as an indicator of advanced liver disease.19
The etiology of chronic liver disease is crucial in the development of HD: alcohol, HCV, hemochromatosis and NASH.
a) NASHNASH is a severe manifestation of NAFLD. NASH associates with visceral obesity, hypertriglyceridemia, and virtually all patients suffer insulin resistance. Therefore, it is not surprising that DM is present in 30% to 45% of patients who suffer NASH.20
Obesity by itself is an independent risk factor for severe liver disease.21 Obesity is an expanded adipose tissue that is in a state of chronic inflammation resulting in an increased secretion of adipokines, which have systemic effects particularly on the liver. These adipokines impair several metabolic functions in muscle, liver and pancreas leading to insulin resistance, hyperglycemia, and hyperinsulinemia; these abnormalities disrupt metabolism of lipids in the liver.22 Cytokines, out of which TNF-α is the most studied member of this family, stimulate the liver stellate cells inducing hepatic fibrosis.23
b) Chronic hepatitis C (CHC) and HCVIn a study conducted by The National Health and Nutrition Examination Survey, a risk 3-fold higher for DM and CHC was identified in individuals over 40 years old, compared with those patients with non-C chronic hepatitis.24 Knobler et al observed prevalence of DM in 33% of non-cirrhotic patients with CHC, compared with 5.6% in a control group.25 In patients chronically infected with HCV, fatty liver was observed in 30% to 70% of the cases.26
Glucose intolerance and DM is present in more than 40% and 17% respectively in patients with CHC. Moreover, the insulin resistance in these patients is an inde-pendent risk factor for steatosis in relation to the severity of fibrosis.4,27–29
The mechanisms by which the HCV produces insulin resistance and DM are not clearly known. It has been observed that HCV induces insulin resistance regardless of the body mass index and the fibrosis stage. In a study conducted in a transgenic animal model, the HCV core protein was able to induce insulin resistance, steatosis, and DM. The TNF-α overproduction seemed be the primary mechanism. This cytokine phosphorylates the serine residues of insulin receptor (IRS-1 and IRS-2), and stimulates the overproduction of suppressor of cytokines (SOC3). The substance SOC-3 inhibits the phosphorylation of Akt and phosphatidylinositol 3-kinase (PI3K). All these disorders –related to intracellular signals of insulin– could block the transactivation of GLUT-4, which might result in the blocking of the glucose uptake at the cellular level. Indeed, in the transgenic mouse, TNF-α correlated with the hyperinsulinism and TNF-α block induced by the administration of anti-TNF-α drugs such as infleximab avoided the appearance of insulin resistance. Therefore, the mechanisms inducing insulin resistance by the HCV included the production of TNF-α, serine phosphorylation of IRS and over expression of SOCs. Furthermore, in patients with CHC, the overproduction of TNF-α correlated with a faster progression of fibrosis and a lower response to interferon.22
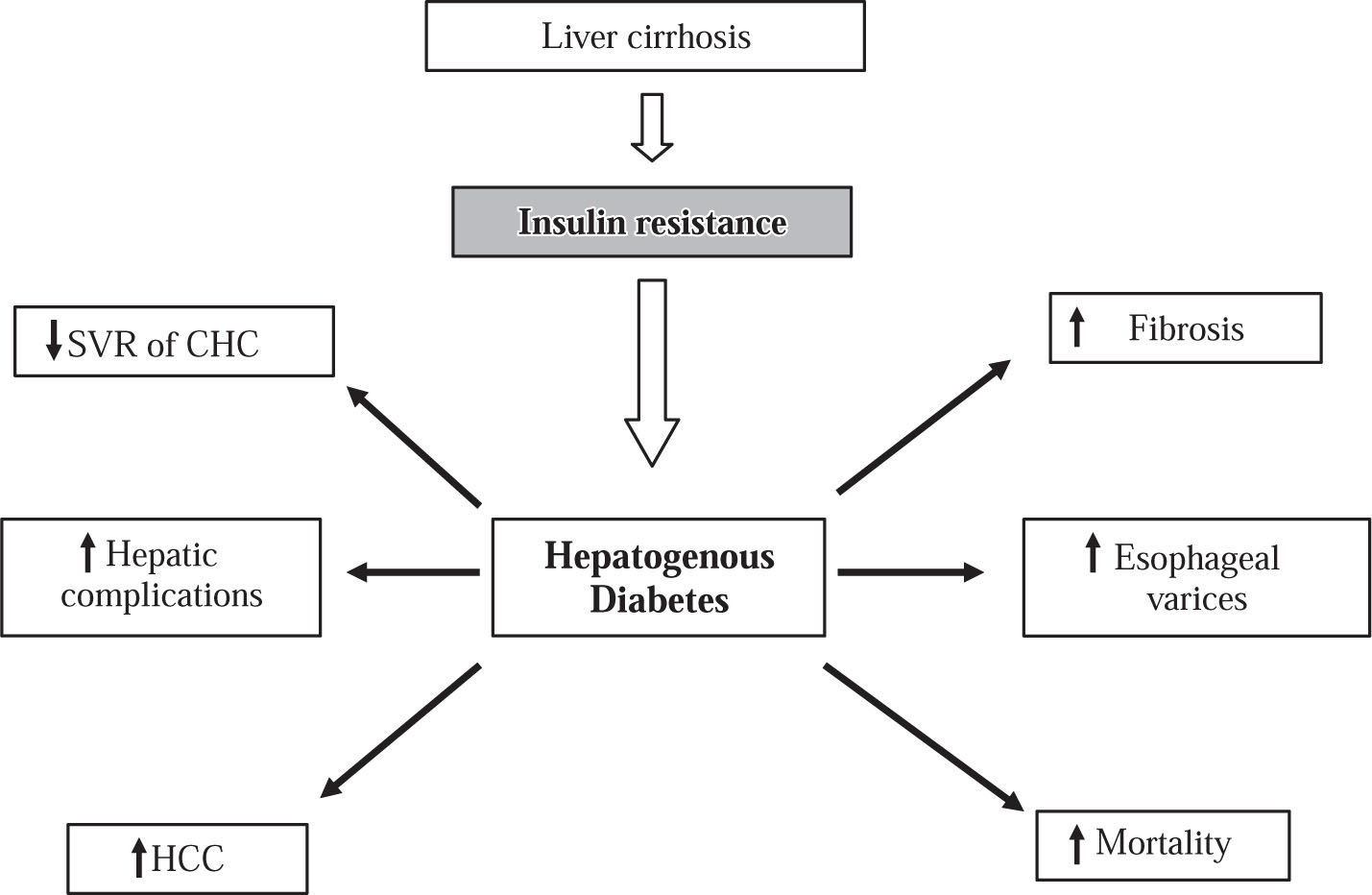
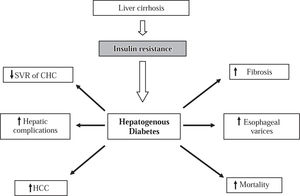
On the other hand, C virus some genotypes may be involved in the occurrence of glucose metabolic disorders, as genotypes 1 and 4 are significantly associated with in-sulin resistance more frequently than genotypes 2 and 3 (37% vs. 17%).28 It is well known that genotypes 1 and 4 are associated with lower viral sustained response to antiviral therapy than genotypes 2 and 3. Therefore, insulin resistance may be a cofactor that would increase the failure to antiviral treatment as it was recently reported in patients with CHC genotype 1: patients with HOMA > 2 (insulin resistance) had 2-fold lower sustained response to treatment compared with those patients with HOMA < 2 (32.8% vs. 60.5% respectively).30 In addition, in experiments carried out with Huh-7 cells infected with HCV RNA, viral replication was blocked by adding interferon to the system. However, the ability of interferon to block viral replication was abolished when insulin was added to interferon at a dose of 128 mcU/mL (a similar dose to that seen in the hyperinsulinemic states).31 These findings published in an abstract form have to be reproduced by other groups. Notwithstanding, it has been reported that patients with CHC and insulin resistance have a less sustained response to peginterferon plus ribavirin treatment compared with patients without insulin resis-tance22,30 (Figure 1).
The impact of insulin resistance and HD on the clinical outcome of patients with chronic liver disease. HD is associated with decrease of sustained viral response (SVR) and rapid progression of fibrosis in patients with chronic hepatitis C (CHC). HD is associated with an increased rate of complications of cirrhosis such as esophageal varicose veins and liver failure as well as increase of mortality. HD is a risk factor for occurrence and complications of hepatocellular carcinoma (HCC).
In patients with CHC, the eradication of HCV may be associated with a reduction in the incidence of diabetes. In a recent study 234 patients with biopsy-proven CHC and normal fasting glucose < 100 mg/dL were monitored for 3 years after finishing the antiviral therapy (interferon alpha-2b alone or with ribavirin for 6 or 12 months according to genotype). At the end of follow up 14 out of 96 (14.6%) patients with SVR and 47 out of 138 (34.1%) nonsustained responders developed glucose abnormalities (P < 0.05). Patients with SVR did not develop diabetes during monitoring, whereas nine cases of diabetes were detected in nonsustained responders (P < 0.05). After adjustment for the recognized predictors of type 2 diabetes, the hazard ratio for glucose abnormalities in patients with SVR was 0.48 (95% CI [0.24-0.98], P = 0.04).32 In the opposite side, others have suggested that the eradication of HVC did not reduce the risk of DM in patients with CHC and normal fasting blood glucose during a period of 8 years of monitoring after treatment. Patients with sustained response had a similar incidence of DM compared with those who did not respond to treatment (14.8% vs. 18.5% respectively).33 This controversy needs to be cleared in the near future.
c) AlcoholPatients with alcoholic liver disease have a high relative risk of suffering diabetes.34 This risk is directly related to the amount of ingested alcohol, as it rises 2-fold in the patients ingesting more than 270 grams of alcohol per week compared to those ingesting less than 120 grams per week.35 Acute alcohol ingestion produces a significant reduction of insulin mediated glucose uptake. On the other hand, patients with chronic alcoholism frequently have chronic pancreatic damage and injury of pancreatic islet β- cells resulting in DM.1
d) HemochromatosisHereditary hemochromatosis is a disease characterized by iron accumulation in several organs – particularly in the liver – because of a disorder of the metabolism of this metal. This abnormality is produced by a mutation of the HFE gene. In addition, the iron can infiltrate the pancreas and myocardium. In the pancreas, the concentration of iron is predominantly in the acinus of exocrine secretion. However, infiltration of Langerhans islets with damage to the insulin-producing β-cells can also be observed. That is the reason why DM can be observed in 50%-85% of patients with hereditary hemochromatosis in advanced stag-es.36 Additionally the glucose metabolic disorders resulting of the liver damage probably contribute to the high frequency of DM.2,18
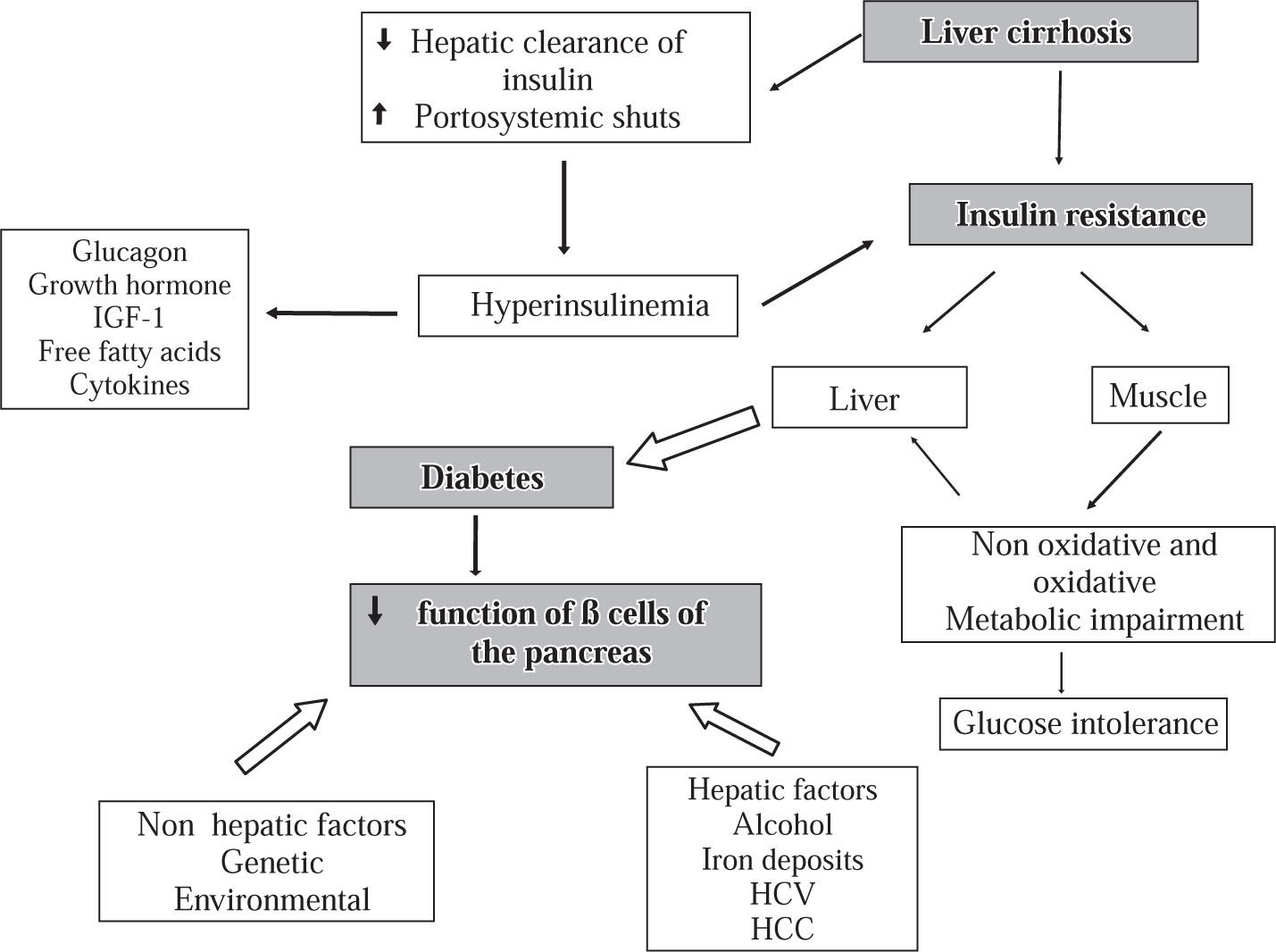
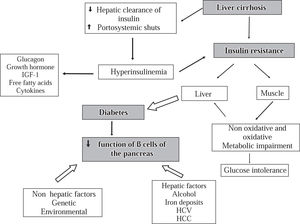
PathophysiologyThe pathophysiology of HD is complex and not precisely known. Insulin resistance in peripheral tissues (adipose and muscular tissue) plays a central role in the glucose metabolism disturbance.1,6,8,11,37–40 It was proposed that reduced insulin extraction by the damaged liver and portosystemic shunts result in hyperinsulinemia which is potentiated by high levels of contra-insulin hormones (glucagon, growth hormone, insulin-like growth factor, free fatty acids and cytokines).8,11,38–40 However, a recent study reported that in patients with Child B liver cirrhosis, the hyperinsulinism might be produced by an increase of the pancreatic β- cells sensitivity to glucose, whereas disturbance of hepatic insulin extraction did not seem to have a significant role.41 It has also been speculated that genetic and environmental factors and some etiologic agents of liver disease such as HCV, alcohol, and iron infiltration impair the insulin secretion activity of the β-cells of the pancreas.9 In conclusion it seems that glucose intolerance may result from two abnormalities that occur simultaneously: (a) insulin resistance of muscle and (b) an inadequate response to the β-cells to appropriately secrete insulin to overcome the defect in insulin action. Furthermore, diabetes mellitus develops as the result of progressive impairment in insulin secretion together with the development of hepatic insulin resistance leading to fasting hyperglycemia and a diabetic glucose tolerance profile37,38 (Figure 2).
Physiopathology of hepatogenous diabetes. One of the main abnormalities is insulin resistance in muscular cells and the hepatic tissue. Insulin resistance in muscle impairs non-oxidative and oxidative glucose metabolism. The reduction of insulin clearance by the damaged liver and the presence of portosystemic shunts in one hand and the desensitization of the beta cells of the pancreas produced by diverse factors on the other hand may produce hiperinsulinemia. With progression of the diabetes, there is a reduction of sensitivity of β-cell for production of insulin.
Discrimination between HD and type 2 DM may be difficult. In a recent study, comparing patients with HD vs. patients with type 2 DM the ratio of postprandial plasma glucose (PP2h) / Fasting plasma glucose (FPG) (2.27 vs. 1.69), fasting insulin (23.2 vs. 11.6 microIU/mL) and HOMA-Insulin Resistance index (8.38 vs. 3.52) were significantly higher in patients with HD. Therefore, insulin resistance in liver cirrhosis was higher than the type 2 DM, and impairment of hepatic insulin degradation might be an important mechanism of hiperinsulinemia in liver cirrhosis.42
The HD aggravates cirrhosis and hepatocellular carcinomaa) The HD increases morbidity and mortality of liver cirrhosis (Figure 1)The effect of HD in the clinical outcome of cirrhosis and HCC has been evaluated in only few studies.
Neither the Child-Pugh nor the Model for End-Stage Liver Disease (MELD) Scores (which are widely used as prognostic instruments of morbidity and mortality in the short and long term in cirrhotic patients) includes in their parameters the DM or glucose intolerance.43,44 However, interesting data were observed in some prospective studies involving cirrhotic patients in whom DM was studied as an independent prognostic factor. In a retrospective-prospective study 354 (98 with diabetes) of 382 eligible patients were followed during 6 years after inclusion into the study: 110 were alive at the end of follow-up. Prognostic factors identified by Kaplan-Meier analysis, followed by Cox’s stepwise regression demonstrated in sequence, albumin, ascites, age, encephalopathy, bilirubin, diabetes, and platelets as prognostic factors of mortality. The larger mortality rate in patients with diabetes was not due to complications of diabetes but to an increased risk of hepatocellular failure. Diabetes was no longer a risk factor as a covariate in a subgroup of 271 patients when varices were added but was again significant when patients who died of gastrointestinal bleeding were excluded.45
In another original study carried out in patients suffering from cirrhosis and refractory ascites on a waiting list for liver transplantation, the HCC and DM, but not the Child-Pugh score, were independent predictive factors of mortality. The patients suffering from refractory ascites and DM showed a 1 and 2-year probability of survival of 32% and 18%. By contrast 1 and 2-year survival rates of patients with refractory ascites without DM were of 62% and 58% respectively.46
Insulin resistance and diabetes mellitus may affect cognition in patients with hepatitis C cirrhosis. In a study, 128 cirrhotic patients were prospectively evaluated for the presence of hepatic encephalopathy (HE) according to the West-Haven criteria as well as by means of two psychometric tests and fasting plasma ammonium levels. Nutritional status, fasting plasma glucose and insulin resistance were also determined. Mul-tivariate analysis showed that the time needed to perform number connection test A was independently correlated to age, the Child-Pugh score, diabetes and malnutrition (P < 0.05 for all). Plasma ammonium levels were significantly related to insulin resistance and muscle mass. The above-mentioned data suggest that diabetes mellitus seemed to be related to HE in patients with liver cirrhosis and that insulin resistance might be implicated in the pathogenesis of HE.47 Certainly, more studies are needed in order to confirm these interesting findings.
Nishida et al studied a group of 56 patients with cirrhosis and normal fasting blood glucose. After OGTT 38% of the patients had DM, 23% glucose intolerance, and 39% were normal. After 5 years of follow-up, compared with normal patients, those with diabetes and glucose intolerance had significantly higher mortality (5%, 44% and 32% respectively). By a multiple regression analysis, only serum albumin and DM were independent negative predictive factors of survival.14
b) The DM increases the severity and mortality of HCCIn a recent case and control study that included 465 patients, the DM prevalence was more frequent in patients with HCC compared with controls (31.2 vs. 12.7%, OR 3.12 95% CI: 2.22-4.43). DM had been diagnosed prior to the occurrence of HCC in 84% of cases with an average duration of 181.4 months indicating that it was type 2 DM in most cases.48 The above data suggest that type 2 DM itself might be a risk factor for the occurrence of HCC. Nevertheless, others suggest that DM only in presence of other risk factors may be relevant in the genesis of HCC. In fact, some studies show that in diabetic patients with HCV, liver fibrosis and alcohol heavy consumption, the risk of having HCC increases. In the other hand, it was observed that patients suffering from chronic hepatitis C, DM and advanced fibrosis had a 3-fold greater risk than non-diabetic patients with mild to moderate fibrosis of developing HCC in 5 years of follow-up (13% vs. 5%).49
The HD together with the hepatitis B and C virus infection and alcoholic liver cirrhosis increases to 10-fold the risk of HCC.1,11
Finally, patients with HCC and DM have a higher mortality risk than those without DM. In another study involving 160 patients suffering from HCC, those who had DM had 1-year mortality rate higher than those patients without DM. Additionally they had more extensive disease.50
c) Pathophysiologic mechanismsThe mechanisms by which HD worsens the clinical course of liver cirrhosis have not been clearly established. Firstly, DM by accelerating liver fibrosis and in-flammation would give raise to severe liver failure. Secondly, DM may increase mortality by increasing the incidence of bacterial infections in the cirrhotic patient.51,52
In relation to the first mechanism, insulin resistance increases the adipokines production (cytokines secreted by adipose tissue), such as leptin and TNF-α, which acti-vates the inflammatory pathways that exacerbate liver damage.53 By contrast, another cytokine produced by adipose tissue (adiponectin) is a regulator of insulin sensitivity and tissue inflammation.54 The reduction in the adiponectin levels reflects peripheral and hepatic insulin re-sistance.55 There has been speculated that hypoadi-ponectinemia may play a role in the liver disease pro-gression.56,57 Notwithstanding, it has been recently observed that adiponectin plasma levels in cirrhosis are significantly elevated in patients with advanced stages of liver disease and altered hepatic hemodynamics. This paradoxal hyperadiponectinemia may be due to a reduced extraction by the liver.58
Regarding the second mechanism, DM may worsen immunosuppression of cirrhotic patients thus increasing the incidence of severe infections that may have deleterious effect on liver function. Cirrhotic patients with spontaneous bacterial peritonitis have an in-hospital high mortality rate due to sepsis, liver failure, and hepa-torenal syndrome. In the other hand patients with esoph-ageal variceal bleeding through a mechanism of intestinal translocation, have a high incidence of infections that increase their in-hospital mortality rate.59 Notwithstanding weather DM potentiates mortality rate in patients with other complications of cirrhosis is unknown.
In the future, the precise mechanisms by which DM may worsen liver function must be clarified, since their manipulation may be useful for reduction of complications.
Clinical implications of HD in liver cirrhosis.Clinical manifestations of HD in early stages of cirrhosis are virtually absent. In a recently published study involving compensated cirrhotic patients with normal fasting serum glucose and without a family history of type 2 DM up to 77% had either DM or glucose intolerance diagnosed by means of OGTT. In 38% of cases, DM was sub clinical.14 As liver function deteriorates, the incidence of diabetes increases so that it may be seen as a marker of liver failure.
Future research should clarify the impact of HD in the natural history of cirrhosis and the benefices of its early diagnosis and treatment for reduction of mortality.
TreatmentThe treatment of HD is complex since it has particular characteristics that make it different from type 2 DM: 1) About half of patients have malnutrition; 2) When diag-nosis of HD is performed, most of patients have end-stage liver disease; 3) Most of the oral hypoglycemic drugs are metabolized in the liver; 4) Patients often have episodes of hypoglycemia.12
Change of lifestyle. The initial treatment of patients suffering from mild to moderate hyperglycemia and compensated liver disease may be the lifestyle change, since at this stage the insulin resistance is a dominant factor. However, very restrictive diets may aggravate malnutrition of these patients and physical exercise, which improves insulin resistance, may not be advisable for patients with liver inflammation.38
Biguanides. Patients with HD and advanced stages of liver disease may require the use of oral hypoglycemic drugs. However, most of these drugs are metabolized in the liver; therefore, the blood glucose levels during treatment should be closely monitored in order to avoid hy-poglycemia.60 Biguanides, which reduce resistance to insulin, may be useful. Metformin is a biguanide that is relatively contraindicated in patients with advanced liver failure and in those who continue to ingest alcohol, due to the risk for lactic acidosis.61 Notwithstanding met-formin was effective to achieve biochemical response in patients with NAFLD who do not respond to lifestyle interventions and ursodeoxycoholic acid (UDCA). In a recent report 25 adult patients with NAFLD who did not achieve normalization of alanine transaminases (ALT) after 6 months of lifestyle interventions and UDCA were treated with metformin 500 mg daily for 6 months. In comparison to disease controls treated only with lifestyle, all patients treated with metformin had partial biochemical response (mean ALT 122.2 ± 26.8 vs. 74.3 ± 4.2 p < 0.05) and 14 (56%) of them achieved complete normalization of ALT.62
Insulin secretagogues. On the other hand, the insulin secretagogues despite the fact that are safe drugs in patients with liver disease probably are not useful, since they do not modify the insulin resistance and patients with alcoholic cirrhosis often have pancreatic islet β-cells damage.63 These patients have chronic compensatory hyperinsulinemia until the islet β- cells are exhausted.
Alpha-glycosidase inhibitors. These drugs can be useful in patients suffering from liver cirrhosis, since its action mechanism is by reducing the bowel-level carbohydrates absorption, thus reducing the risks for postprandial hyperglycemia. In a randomized double-blind controlled trial involving 100 patients with compensated liver cirrhosis and insulin-treated DM, the control of postprandial and fasting blood glucose levels improved significantly with the use of acarbose, an alpha-glycosi-dase.64 In another crossover placebo-controlled study involving patients with hepatic encephalopathy, the acar-bose produced a significant improvement of postprandial blood glucose level. Additionally, the patients had a reduction in plasma ammonia levels and an increase in the frequency of bowel movements.65 The reduction of ammonia levels was probably due to the decrease in the proliferation of intestinal proteolytic bacteria caused by bowel movements.65
Thiazolidines. These drugs may be particularly useful in cirrhotic patients with DM, since they increase the insulin sensitivity. However, troglitazone has been withdrawn from the market because of its potential hepato-toxic effects. Nevertheless, rosiglitazone and pioglita-zone are apparently safer drugs in patients with liver disease.66 Recently several studies reported beneficial effects of thiazolidines for the treatment of NASH. These drugs may normalize amino transaminases, reduce insulin resistance, and improve histological features.67,68
Insulin. Insulin requirements in the cirrhotic patient with diabetes may vary. In patients with decompensated cirrhosis requirements may be greater compared to patients with decompensate cirrhosis, since in the former predominate the insulin resistance while in the latter liver metabolism of insulin is highly deteriorated. Therefore, the therapy with insulin must be preferably performed in hospitalized patients accomplishing a close monitoring of blood glucose levels for the risk of hy-poglycemia.69
Liver transplantation. Finally, liver transplantation rapidly normalizes glucose tolerance and insulin sensitivity. It is thought that this effect is due to an improvement in the hepatic clearance and peripheral glucose disposal. The latter effect could be secondary to a correction of chronic hyperinsulinemia.39,70 Liver transplantation reduced insulin resistance and cured hepatogenous diabetes in 67% of cirrhotic-diabetic patients. In 33% of patients diabetes was not corrected because of persistence of a reduced β-cell function measured by means of an OGTT. This abnormality would make these patients eventually eligible for combined islet transplantation.71
Finally, other agents such as lipid-lowering drugs, an-tioxidants, bile salts, co-factors increasing the mitochon-drial transport of fatty acids, recently evaluated for the treatment of NASH,72 were not included in this review.
Future research should clarify if the treatment of HD may reduce the incidence and severity of complications of liver cirrhosis as well as the mechanisms by which HD increases morbidity and mortality of cirrhotic patient. Clearer guidelines for management of HD are necessary.