Special issue on hepatocellular carcinoma (HCC) and hepatitis B and C as its main causes worldwide
More infoDirect-acting antivirals (DAAs) revolutionized the treatment of chronic HCV-related disease achieving high rates of sustained virological response (SVR), even in advanced cirrhosis, with modest contraindications and a low rate of adverse events. However, the risk of hepatocellular carcinoma (HCC) persists due to the underlying chronic liver disease, both in patients with and without history of HCC. Although some initial studies reported a presumptive high risk of HCC development after DAA therapy, more recent observational studies denied this hypothesis. The residual risk for HCC occurrence after HCV eradication seems being progressively reduced with time after SVR. Data on recurrence of HCC after DAA exposure in patients with previously treated carcinoma initially reported conflicting results too, this being also due to methodological issues in analysis of retrospective multicenter studies. Anyway, current evidence support the use of DAAs in HCV-HCC treated patients, without any higher risk of tumor recurrence linked to antiviral therapy. Less effort has been made to evaluate the efficacy of DAA therapy in patients with untreated active HCC and it has been questioned whether a lower rate of SVR would be obtained among patients with active HCC. Studies conducted in this perspective concluded that HCC status does not influence the likelihood to obtain SVR with DAAs, making DAAs appropriate in HCC-active patients. As far as survival is concerned, recent studies conducted in cirrhotic HCV-related early-stage HCC found that DAAs improved overall survival, a benefit probably due to the reduction of hepatic decompensation.
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer and it represents the sixth most common neoplasm and the fourth most common cause of cancer-related death worldwide [1]. It is expected that incidence will increase in the future and more than 1 million patients will die from HCC in 2030, according to World Health Organization annual projections [2]. The development of HCC is closely related to the presence of chronic liver disease and it represents the leading cause of death in patients with compensated cirrhosis. The underlying chronic liver disease has a significant impact on the feasibility, the efficacy and the safety of treatments for HCC, although HCC rarely occurs in those who do not have advanced fibrosis. HCV infection represents an independent risk factor for the development of HCC, both in Western countries and in Japan,[3] and the prognosis of patients with HCV cirrhosis is driven by the progression toward hepatic decompensation and HCC, that represent competitive risks for death. In the past, HCV eradication obtained with interferon (IFN)-based therapies has been associated with a significant reduction in HCC risk in comparison with patients who did not obtain sustained virological response (SVR) [4]. More recently, direct-acting antiviral agents (DAAs) have decisively changed the outcomes of HCV-infected patients, with high SVR rates (> 90%) also in more advanced liver disease stages, that are associated with a stabilization or with an improvement of liver function,[5–7] and low rates of adverse events or contraindications [5]. However, patients with HCC or with more advanced liver disease were intentionally excluded from registrative randomized controlled trials (RCTs), therefore data about these patients come from post-marketing surveillance and from observational studies. In 2016, two alarm signals were released about an unexpected increased risk of early HCC recurrence after DAA therapy in patients with successfully treated HCC, and increased risk of HCC occurrence in patients without history of HCC [8–9] Since RCTs on this topic today would be unethical, unfeasible and untimely, observational studies and meta-analyses assessed the impact of DAA therapy on the risk of HCC. This brief review will focus on the available evidence on DAA treatment in HCV patients without history of HCC, in those with successfully treated HCC before DAA therapy and, in those with untreated active HCC.
2DAAs and HCC occurrenceHCC occurrence is defined as de novo appearance of HCC in patients without history or previous evidence of liver cancer. A positive impact of HCV eradication on the risk of HCC development has already observed in pre-DAA era in patients treated with IFN. In these patients SVR significantly reduces the risk for HCC, death and liver decompensation, regardless of the presence of esophageal varices [10]. In DAA treated patients, an Italian study initially reported an incidence of HCC after DAAs of 3.17% after 24 weeks of follow-up, raising a warning signal about a presumptive high risk of early HCC occurrence [9]. However, it must consider that these results have been obtained in the absence of a control group, have been expressed as a crude rate rather than actuarial rate and that IFN-treated patients are significantly different from DAA-treated patients, in terms of severity of underlying cirrhosis and portal hypertension. Particularly, it has been showed that DAA treatment is safe and effective even in presence of more advanced stages of cirrhosis and significant portal hypertension. Therefore, HCC occurrence after DAA therapy may be related to the more recent opportunity of treating patients at intrinsically higher risk of cancer, while IFN was attempted only in patients with well compensated cirrhosis, often without clinically significant portal hypertension, and with lower risk of HCC. Since this first alarm signal, several observational studies have definitively denied this presumptive risk. A large retrospective study conducted on 129 Veterans Affairs (VA) hospitals, including more than 22,000 DAA-treated patients, showed an overall annual HCC incidence was 1.18% (95% CI, 1.04–1.32), although cirrhosis was present only in less than 40% of patients [11]. Most importantly, the risk for HCC occurrence was significantly higher among patients who did not achieve SVR (3.45%; 95% CI, 2.73–4.18) compared with those who did (0.90%; 95% CI, 0.77–1.03), suggesting that a residual risk for HCC, although low, may persist also in patients who have obtained viral eradication by DAAs. Subsequently, a prospective study of 2,249 DAA-treated consecutive patients with HCV cirrhosis (about 10% with Child- Pugh class B) included in the HCV Sicily network (RESIST-HCV) showed a rate of HCC development of 2.1% in Child-Pugh class A patients with SVR and 6.6% of patients with no SVR, at 1 year after DAA treatment [12]. In Child-Pugh class B patients, 7.8% of patients who obtained SVR and 12.4% of patients without SVR (P < .001 by log-rank test) developed HCC after 1 year from DAA exposure. Low albumin values, low platelet count and No SVR were independently associated with higher risk for HCC. However, the results of these studies are limited by the lack of a control group of IFN-treated or untreated patients. An aggregate data meta-analysis indirectly compared the rates of HCC occurrence between IFN- and DAA-treated patients included in 26 studies (17 with IFN and 9 with DAAs), showing an incidence of 1.14/100 person-years (95% CI 0.86-1.52) in IFN and 2.96/100 person-years (95% CI 1.76-4.96) in DAA studies [13]. While considering the high heterogeneity and the limitations related to indirect comparisons, meta-regression did not show a significant association between DAA treatment and HCC occurrence (RR 0.68; 95% CI 0.18-2.55; p=0.55), after adjustment for age and follow-up. The issue of the control group was specifically addressed in a French study conducted on 1270 patients included in the ANRS CO12 CirVir cohort, that compared the rates of HCC occurrence between DAA-treated and IFN-treated HCV patients with cirrhosis [14]. Three-year cumulative incidences of HCC were 5.9% in the DAA group, 3.1% in the SVR-IFN group, and 12.7% in the non-SVR group. These differences could be explained by a higher prevalence of Child-Pugh class B, diabetes and portal hypertension in DAA-treated patients in comparison with IFN-induced SVR patients and using a time-dependent Cox model weighted by inverse probability of treatment to manage this selection bias, authors showed that DAAs were not significantly associated with an increase in the risk for HCC occurrence (HR 0.89; 95% CI 0.46-1.73; P = .73) nor with a more aggressive pattern of presentation. More recently, a retrospective study of VA health care assessed the impact of SVR on the survival in patients with advanced liver disease (defined as FIB-4 > 3.25), demonstrating a significant survival benefit after DAA treatment during a mean follow-up time of one year and half, stratifying the cohort according to the SVR status [15]. The reduction in mortality associated with SVR compared with No SVR was about 79% and multivariate analysis showed that SVR (HR 0.26, 95% CI 0-22-031, P<.001) was independently associated with a reduced risk for death, while the history of liver decompensation and low albumin values resulted independently associated with higher mortality (HR 1.57, 95% CI 1.34‐1.83; P < .001 and HR 2.7 per 1 g/dL decrease; 95% CI 2.38‐3.12; P < .001, respectively). Another recently addressed issue regards the potential relevance of the time association between DAA treatment and HCC occurrence. A prospective North Italian study based on the NAVIGATORE network showed that the residual risk for HCC occurrence after DAA therapy declines progressively with time after SVR, suggesting that early HCC occurrence after SVR could be related to the pre-existence of undetectable small tumors that may evolve in multinodular or infiltrative tumors after DAAs [16]. Not surprisingly, a No SVR status, diabetes and Child-Pugh class B together with HBV coinfection were independently associated with higher risk of HCC occurrence, by multivariate analysis. Finally, a multicenter retrospective study of 1,123 Spanish patients with centrally validated HCC diagnosis showed that HCC incidence was 3.73/100 person-years (95% CI 2.96-4.70), equivalent to 72 patients who developed HCC within a median of about 10 months after DAA start, suggesting a potential increase of short-term HCC risk after DAA treatment [17]. Interestingly, the presence of non-characterized nodules before DAAs resulted significantly associated with higher risk of HCC occurrence, although again the lack of a proper control group of untreated patients may have biased these results.
3DAAs and HCC recurrenceIf the definition of HCC occurrence is clear and simple, the same does not apply to HCC recurrence, for which there is no universally accepted definition. Recurrence could be defined as the reappearance of HCC in patients with a previous HCC radically treated by any technique; however, a wide heterogeneous definition exists in terms of temporal (early or late) and spatial (local or distant) features. From a pathogenic point of view, it is not clear if recurrence could be considered a de novo tumor in a patient with previous history of HCC or the reappearance of the same tumor. Heterogeneity and uncertainty in the definition contribute to the difficulty in the correct assessment of the real incidence of this outcome, as showed in an aggregate data meta-analysis of eleven studies including HCV patients who obtained a complete radiologic response after curative treatment and who did not receive antiviral treatment [18]. The pooled actuarial recurrence rate was 7.4% at 6 months, ranging from 0 to 12.5%, and 47% at 24 months, with a wider range from 32 to 100%. A similar degree of heterogeneity was not surprisingly observed also in survival rates, that were 80% at 3 years (65-95%) and 59% (47-78%). The assessment of the control rate (i.e., HCV-untreated patients) is essential for the assessment of the impact of DAAs on the risk for HCC recurrence and these results could be considered as a benchmark for this purpose. In the last few years, after a first alarming report estimating a HCC recurrence rate of 27.6% during a median follow-up time of 5.7 months,[8] several prospective studies did not show any association between DAAs and HCC recurrence (Table 1). Particularly, a prospective multicenter French study observed a recurrence rate of 2.2%,[19] while an English study including patients with decompensated cirrhosis treated with DAAs did not show significant differences in HCC recurrence between untreated and DAA-treated patients, neither in the first 6 months (4%) nor in 6-15 months (2.5%) after DAA treatment [20]. A prospective uncontrolled Italian study conducted on 143 patients included in the RESIST-HCV network showed that 6-, 12- and 18-month HCC recurrence rates were 12%, 26.6% and 29.1%, respectively. An infiltrative pattern was observed in 17% of recurrence and the main tumor size and the history of prior HCC recurrence resulted independently associated with higher risk of recurrence, by multivariate analysis [21]. Very recently Sapena et al[22], with a massive effort, lead an individual patient data meta-analysis of a world-wide cohort of 977 consecutive patients. Previous HCC recurrence before DAA therapy, alpha-fetoprotein level, multifocality and tumor burden of last HCC before DAA appeared to be significant predictive factors of recurrence in multivariate analysis. Moreover, a control matched population of DAA-unexposed patients and DAA-exposed patients with treated monofocal or within Milan criteria HCC showed to have a recurrence rate per 100 respectively of 23.2 and 14.7 (RR=0.64, 95% CI 0.37-1.1; p= 0.1). The OS rate per 100 was 3.4 in DAA exposed patients and 6.6 in DAA unexposed patients (RR 0.51, 95% CI 0.22 to 1.8; p=0.11), therefore clarifying that DAA therapy is not related with a higher recurrence rate of HCC.
Characteristics and results of studies that assessed hepatocellular carcinoma recurrence after treatment with direct-acting antiviral agents.
| Author - Year | Study location | HCC-DAAs exposed patients (n) | Type of HCC treatment | Median time from HCC treatment to DAAs exposure (months) | Median follow up period (months) | HCC recurrence in DAAs exposed patients (n; %) | HCC recurrence in DAAs exposed patients at 6 months and at 12 months (%) | HCC recurrence in No-DAAs exposed patients (n; %) | Survival rate at 12 months and at 36 months in DAAs exposed patients (%) |
| Conti[9]- 2016 | Italy | 59 | Resection, ablation, TACE | 12.4 | 5.5 | 17; 28 | NR | ———- | NR |
| ANRS collaborative study group[19]- 2016 | France | 189 | Resection, ablation, TACE | NR | 20.2 | 24; 12.7 | NR | 16; 20 | NR |
| Cheung[20]- 2016 | UK | 406 | NR | 9.5 | 15 | 10; 2.5 | 4; 2.5 | 11; 4 | NR |
| Cabibbo[21]- 2017 | Italy | 143 | Resection, ablation, TACE | 11 | 8.7 | 29; 20.3 | 12; 26.6 | ———- | NR |
| Cabibbo[24]-2019 | Italy | 102 | Resection, ablation | NR | NR | 28; 27.5 | 6; 15 | 38; 37.3 | 99; 90 |
| Singal[25]- 2019 | USA, Canada | 383 | Resection, ablation, TACE, TARE, SBRT | 7.7 | NR | 209; 54 | NR | 205; 49 | NR |
| Reig[43]- 2017 | Spain | 77 | Resection, ablation, TACE | 11.2 | 8.2 | 21; 27.3 | NR | ———- | NR |
| Ikeda[44] -2017 | Japan | 177 | Resection, ablation, TACE, | 10.7 | 20.7 | 61; 34.6 | NR; 18.1 | ———- | NR |
| Singal[45] - 2017 | USA | 207 | Resection, ablation, TACE | 7.2 | 22.7 | 95; 45.9 | NR | ———- | NR |
NR: Not Reported. TACE: Trans-Arterial Chemo Embolization. TARE: Trans-Arterial Radio Embolization. SBRT: Stereotactic Body Radiation Therapy.
Although the hypothetical link between DAAs therapy and HCC occurrence/recurrence has been extensively studied, less effort has been made to assess the impact of DAAs on overall survival. For this purpose, it is first necessary to investigate this outcome in a control arm, i.e. untreated HCV patients, and to identify which are the main drivers of death in this population. An observational study[23] conducted on 328 HCV cirrhotic patients with early HCC previously cured showed that the risk of death was significantly different depending on which first event occurred (early decompensation or early HCC recurrence). Using a time-dependent Cox model, patients who had an early hepatic decompensation event as a first event showed a significantly worse survival in comparison with patients having early HCC recurrence as a first event and these results were confirmed at multivariate analysis. Authors concluded that the survival of cirrhotic HCV-untreated patients with successfully treated early HCC is mainly driven by hepatic decompensation, suggesting that HCV eradication after DAAs could improve overall survival through a protective effect on liver function. A recent prospective multicenter study using observational data [24] investigated the role of DAAs in survival of patients with HCV-related compensated cirrhosis and a first diagnosis of HCC who had achieved a complete radiologic response after local-regional treatment. By emulating a randomized trial and matching a control group of DAA-unexposed patients using propensity score, the DAA group had a significantly higher survival rate than the No DAA group (HR 0.39, 95% CI 0.17-0.91, p=0.03). Moreover, a significantly lower number of patients in the DAAs group experienced hepatic decompensation (HR 0.32, 95% CI 0.13-0.84, p = 0.02). This result appears of great interest considering that, although DAAs did not change the risk of HCC recurrence, DAAs improved survival through a longer preservation of liver function.
Similar results were also obtained by Singal et al. [25]. They assessed overall survival between DAA-treated and untreated patients with prior HCC, distinguishing four causes of death: liver-related, HCC-related, non-liver/non-HCC related and unknown. As expected, liver-related deaths were significantly lower in DAAs-treated patients than untreated (16.3% vs 34%, p = 0.03), whereas HCC-related deaths were similar between the two groups (30.2% vs 29.1%, p = 0.89). These conclusions strongly support the potential benefit of DAA treatment to improve survival in HCV-related HCC by reducing progression of liver disease and slowing hepatic decompensation.
5Methodological issuesThe assessment of the impact of DAA treatment on overall survival and HCC occurrence/recurrence required the analysis of some methodological issues. When interpreting the results of HCC occurrence and recurrence studies, it should be considered the heterogeneity in their study design (retrospective and prospective) and in the inception points, the lack of a proper control group in most of them, and the impact of competitive risk (such as hepatic decompensation) on survival. At the same time, the studies on HCC recurrence are characterized by heterogeneity in baseline patients and tumor characteristics (also in terms of previous history of HCC recurrence), in the type of curative treatments and assessment of radiological response, in the definition of HCC recurrence (early or late, distant or local) and in the schedules of follow-up after HCC treatment [26]. Some of them assessed the risk for HCC recurrence only using crude rate, rather than actuarial methods, i.e. Kaplan-Meier method, which appears more appropriate to describe time-related events. Two aggregate data meta-analyses tried to summarize evidences on the risk of HCC occurrence and recurrence after DAA treatment but it included study with different designs and without parallel control groups of DAA-unexposed patients, therefore basing its conclusions on indirect comparisons with IFN-treated patients[13,27].
Most of the methodological uncertainties are related to the impossibility to assess the comparative effectiveness with RCTs given the fact that HCC patients and patients with advanced cirrhosis were excluded from registrative trials. As DAAs represented now the standard of care for patients with HCV infection, these RCTs would be unethical, untimely and finally unfeasible. In observational studies, the choice of treatment is often influenced by patients’ characteristics (i.e., more severe patients may be more or less likely to receive the intervention). Covariate adjustment is usually performed to adjust for baseline differences in observational studies, although these models might be overfitted when the number of covariates is disproportionate to the number of outcome events. To overcome the limitation of the confounding in observational studies, propensity score (PS) methods represent a valid alternative to conventional covariate adjustment[28] and it has been used to assess the impact of DAA treatment on overall survival[23,24] and the impact of SVR on the risk of liver-related and not liver-related complications[14].. Although there is no general agreement on the choice of PS method that is best suited to any particular scenario, PS matching or inverse probability to treatment weighting (IPTW) could be used according to the PS distribution, considering the differences between these two methods. PS matching usually provides excellent covariate balance and it is simple to present and interpret, but it leads to the loss of a variable proportion of unmatched patients. Conversely IPTW retains data from all study participants, providing a pseudopopulation with optimal covariate balance, but it is less accurate in presence of extreme weights. However, it should be considered that despite the use of PS methods, residual confounding from both measured and unmeasured cannot be simply ruled out.
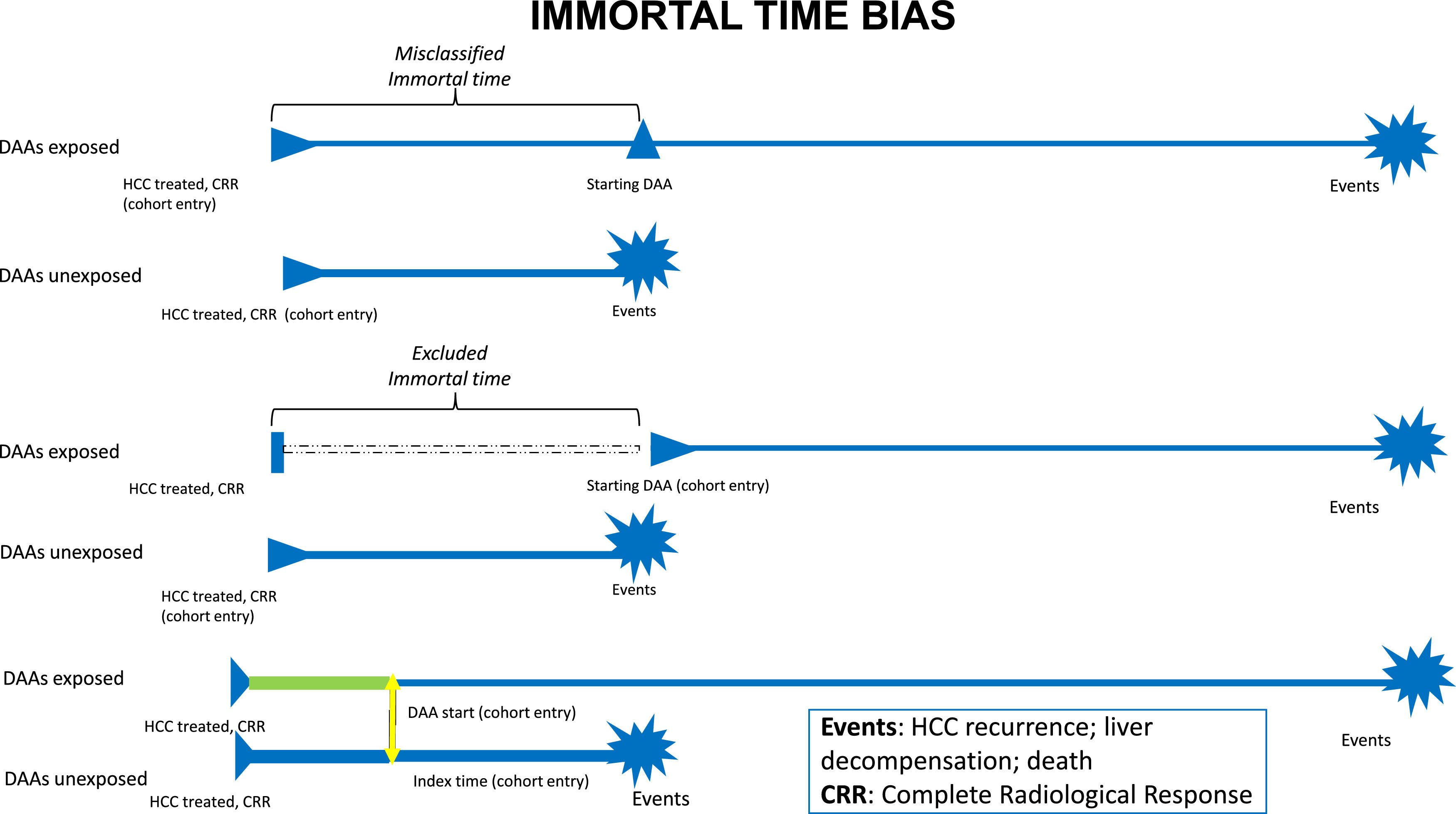
Observational studies suffer also from time-related bias[29,30]. Particularly in the studies that assessed overall survival in patients at risk for HCC recurrence, immortal-time bias is strongly related to the heterogeneity in the time of starting observation, such as the first complete radiological response to HCC treatment or the start of DAA treatment (Fig. 1). When starting follow-up from complete radiological response, misclassified immortal-time bias exists because the time between radiological response and DAA initiation cannot be attributed to DAA and it should be attributed to a "no DAA" state of treatment. To mitigate the effects of misclassified immortal-time bias in this scenario, DAA treatment should be evaluated as a time-dependent covariate. Furthermore, time-lag bias, that could be due to a change in disease stage between complete response and DAA start, should be excluded when the duration of this time-lag is long. Conversely, starting the observation from the beginning of DAA treatment is more appropriate in order to emulate a target trial[31,32], but it suffers from excluded immortal-time bias because of the exclusion of the patients who died or experienced an event during the time-lag between radiological response and DAA start. This approach makes complex to obtain a comparable starting point in the control arm, represented by HCV patients who did not receive DAAs. In this complex scenario, individual patients’ meta-analyses represent a great tool to compare data of different studies where all patients are individually analyzed and collected, differently from aggregate data meta-analyses[33].
6DAAs and untreated HCCGiven that SVR improves liver function, it may seem reasonable to provide DAA treatment to all patients with HCV-related cirrhosis, irrespective of the absence or presence of active hepatocellular carcinoma. Nevertheless, only few studies evaluated the efficacy of DAA therapy in patients with untreated active HCC and issues have been raised about the possibility of a lower rate of SVR among patients with active HCC. Beste et al. [34] conducted a retrospective study including 17487 HCV patients treated with DAAs, among whom 3.6% having HCC; some of them were transplanted and experienced DAAs therapy after liver transplantation, while others just received the cure for HCV-infection without being transplanted. Significantly lower SVR rates were found in HCC patients –regardless of tumor treatment received- compared to those without history of HCC (OR 0.38, 95% CI 0.29-0.48, p<0.001) suggesting that HCC itself could be causally linked to antiviral treatment failure. A retrospective cohort study based on a cohort of 421 cirrhotic patients treated for HCV with DAAs reached similar conclusions[35]. SVR was studied both on patients with active HCC and on those with a history of tumor; in effect, the group with active HCC showed a significant reduction in achievement of SVR, whereas patients who had their HCC treated and started antiviral therapy in the presence of an inactive tumor or after removal of tumor resulted in similar SVR rates to those without HCC.
Conversely, Chang and colleagues demonstrated different results [36]. They conducted a real-world experience with a HCV infected population, treated with DAAs. Overall SVR was highest among patients without HCC, cirrhosis, or liver transplant and it was lower among patients with HCC, decompensated cirrhosis or liver transplant. However, the difference of SVR rates among patients with or without HCC was not statistically significant, suggesting that HCC status does not influence the likelihood to obtain SVR with DAAs.
A recent meta-analysis[37] explored the effect of HCC on cure rates in DAA-treated patients with chronic hepatitis C. After pooling 49 articles and categorizing patients by several viral and host factors, it has been found that the overall SVR rate among patients with HCC was lower than in those without HCC, the former having a 4.8% reduction in SVR rate (95% CI 0.2-7.4), compared to the latter. Another point that was investigated concerns the comparison of pooled SVR rates for patients with HCC distinct from active vs inactive disease at the time of DAAs treatment. Interestingly, the presence of viable tumor was associated with lower SVR than having a history of HCC in complete response when starting antiviral therapy. Specifically, presence of active HCC implicated an 18.8% reduction in SVR rates (95% CI 7.3-31.8). SVR rates were also compared between patients with HCC who had undergone curative treatments (liver transplant, surgical resection, local ablation) or palliative treatments (such as chemo-radio embolization, systemic therapy), resulting in higher SVR rate in the first group. It was speculated that residual tumor might contribute to the lower SVR rates in patients with HCC, suggesting some biological mechanisms. Active tumor may function as a reservoir for HCV replication, or lead to distortion of liver architecture and alter liver inflammation, decreasing drug delivery of DAAs, or even lead to the development of resistant strains in HCC.
7DAAs and intermediate/advanced HCCData collected in the setting of patients listed for liver transplant (LT) provide indirect evidence about the impact of HCV eradication on the clinical outcome of patients with intermediate and advanced HCC. Pascasio et al [38] carried out a multicenter retrospective analysis evaluating patients positive for HCV, treated with an interferon-free regimen, while awaiting for LT; indication for transplantation was decompensated cirrhosis (with or without hepatocellular carcinoma) in 72% patients, and HCC in 28% patients. As expected, those who achieved higher response rates were compensated patients, with or without HCC, while among decompensated patients no differences were observed between the presence or not of HCC. Moreover, since patients in HCC group improved in liver function, scoring better in Child Pugh, they were frequently removed from transplantation waiting list, this meaning not only that DAAs treatment prior to LT is not associated with higher risk of HCC recurrence, but also that delisting could allow a better allocation of organs. Zanetto et al. [39] observed similar trends, even if their study population was not so large. They retrospectively compared two groups of patients with HCV-related cirrhosis and HCC, listed for LT; one group was treated with DAAs awaiting liver transplant, the other was not. All treated patients achieved SVR, and the 87% underwent downstaging with no significantly difference compared to patients with HCC not treated with DAAs. It must be stressed that HCC recurrence after LT was neither different between the two groups.
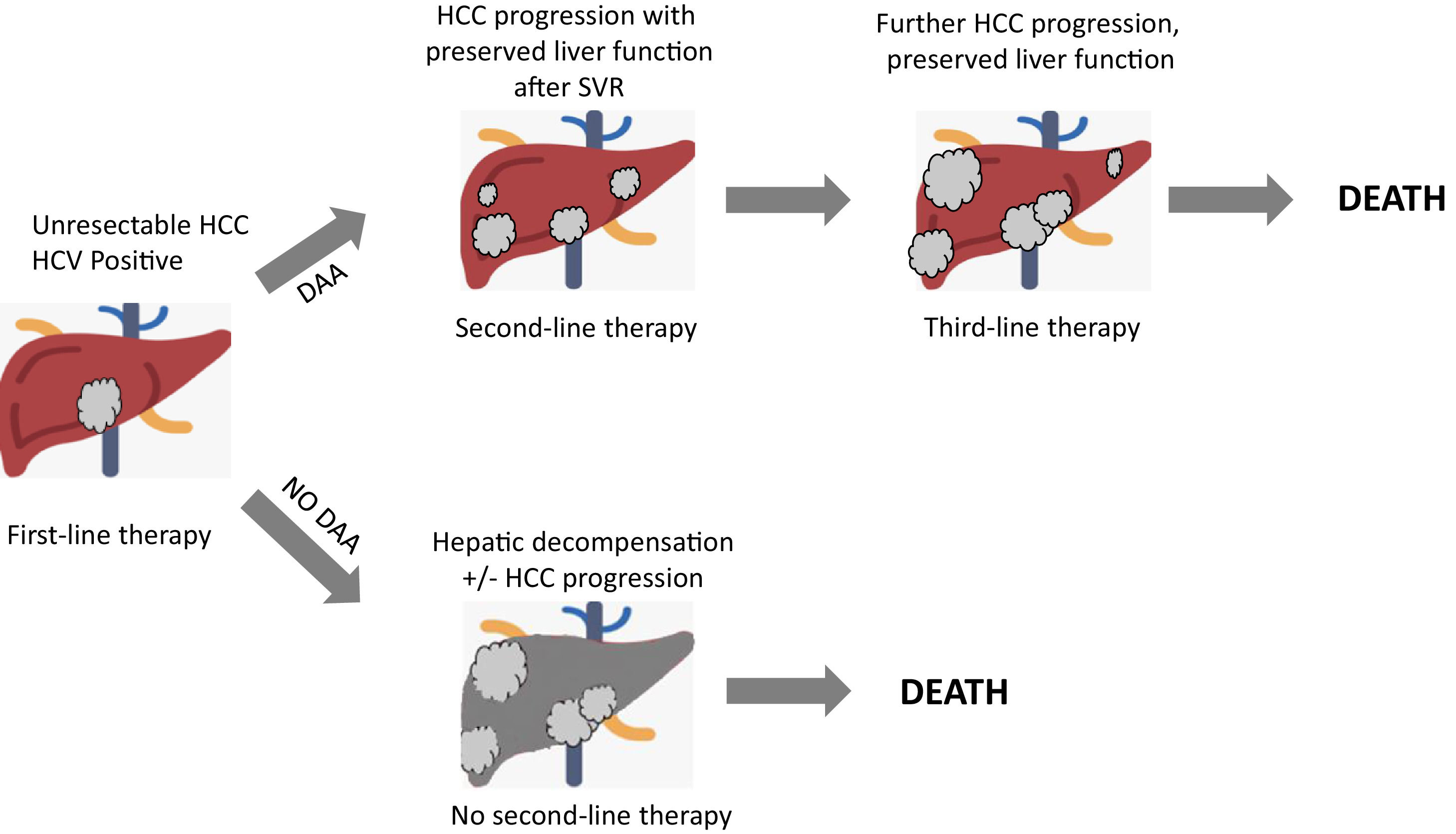
Recently, a retrospective cohort study[40] was performed to provide data about HCV eradication on patients with advanced HCC treated with systemic therapy. 27 SVR patients and 31 non-SVR patients were enrolled, all treated with Sorafenib. No differences in duration treatment, dose of administration, nor adverse events were registered. Response rate, disease control rate, median time to progression, time to treatment failure, post progression survival and overall survival were evaluated for the two groups. Interestingly, the SVR group exhibited a significantly better overall survival, time to treatment failure and post progression survival. This study further strengthens the necessity of HCV eradication in HCV-related HCC patients, in order to improve their prognosis by preventing deterioration of liver function especially during a systemic therapy and so even if HCC cannot be radically cured. More recently, in a multicenter multinational study[41], 1,676 HCV-related HCC patients were matched into two groups (HCV-untreated and DAA-treated patients with SVR), showing that SVR patients had a significantly higher 5-year survival compared to untreated patients, after time-varying adjustment for DAA treatment. Interestingly, the matched population of DAA-treated patients included about 30% of HCC treated with transarterial chemoembolization (TACE) or Sorafenib, suggesting that candidates for HCC therapy should also be considered for DAA therapy. Finally, it is fundamental to underline how crucial it is to properly evaluate residual liver function, HCC stage, potential risk of decompensation secondary to HCC treatment in order to assess in a patient-by-patients basis whether to use DAA treatment or not[42]. Fig. 2 depicts a competing risk model of patients with unresectable HCC undergoing systemic therapies, showing a critical role of DAAs treatment in order to prevent hepatic decompensation which allows the access to sequential systemic therapies.
8ConclusionsThe use of DAA therapy has deeply modified the management of HCV patients also in the setting of HCC. In these patients, DAAs resulted to be safe and effective, especially in reducing the risk for hepatic decompensation. This is particularly important during the coronavirus disease 19 pandemic where hepatic decompensation due to the direct effect of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection on cirrhotic patients with and without HCC could have a dramatic effect on the survival of these patients[46,47]. Also in this line, the positive effect on liver function could increase the feasibility of curative treatments for early HCC, finally resulting in a significant and well-demonstrated benefit in prolonging overall survival of patients with cured early HCC. Recent evidence showed that this benefit could be retained also in HCC patients listed for liver transplant and in patients with HCC not amenable to curative treatments and who will never obtain a complete response. Moreover, a very recent individual patient data meta-analysis [22] clarified that DAA therapy is not related with a higher recurrence rate of HCC, even though DAA-mediated HCV eradication does not eliminate the risk of HCC recurrence after a curative treatment. Basing on the lack of clear evidence of a harmful effect of DAAs in patients with HCC, both cured and active, the use of DAAs in these patients should be encouraged.
Author contributionsAll the authors equally contributed and approved the final version of the manuscript.
Authors did not receive any financial support for this work.