Background. To compare the survival of Chinese cirrhotic patients with hepatocellular carcinoma (HCC) ≤ 4 cm who underwent radiofrequency ablation (RFA) alone or a combination of RFA with percutaneous ethanol injection (PEI).
Material and methods. Retrospective analysis was performed for 681 cases with HCC ≤ 4 cm who were treated with RFA alone or RFA combined with PEI (RFA + PEI) between 2004 and 2011.
Results. As a result, 180 patients in each group were selected after propensity score matching (PSM). Higher overall survival (OS) and recurrence-free survival (RFS) rates were achieved by RFA + PEI compared with RFA alone (P = 0.019 and 0.009, respectively). The 1-, 3-, and 5-year cumulative OS rates were 78.0, 44.4, and 30.1% for patients in RFA group and 88.2, 58.0, and 41.1% for patients in RFA + PEI group, respectively. Besides, the 1-, 3-, and 5-year cumulative RFS rates were 77.0, 43.8, and 29.2% in RFA group, and 87.9, 57.6, and 38.4% in RFA + PEI group, respectively. The local recurrence, complete ablation and five-year mortality showed no distinct differences between RFA and RFA + PEI groups in three subgroups classified with tumor size. Moreover, Cox regression multivariate analysis results showed that sex and treatment approach were significantly related to OS, whereas sex, status of HBsAg, local recurrence, and number of tumor nodule were related to RFS.
Conclusion. Therefore, the combination of RFA and PEI yielded better OS and RFS rates than RFA alone for Chinese patients with HCC ≤ 4 cm.
Hepatocellular carcinoma (HCC) is the fifth most common tumor and ranks the third most common cause of cancer-related deaths worldwide.1,2 According to the World Health Organization, the as burden of HCC is expected to continue to increase until 2030, and this tumor has the second highest increase in overall death rate.3
Surgical treatments, including hepatic resection and liver transplantation, are considered as the most effective treatments for HCC. Unfortunately, because of the limited availability and inadequate function of the liver for resection or transplantation, less than 20-25% of HCC patients have been treated surgically.4 Minimally invasive percutaneous treatments are considered to be the best treatment alternatives for small HCC patients who are not eligible for surgical resection or transplantation.5,6 Percutaneous ethanol injection (PEI), a well-tolerated and inexpensive treatment schedule with few adverse effects, little complications, and a low risk of tumor seeding, has been shown to maintain the same overall survival (OS) and recurrence-free survival (RFS) as surgical resection.5,7 Furthermore, tumor ablation technologies, such as microwave and radiofrequency ablation (RFA), have been found to be effective for promoting thermally mediated coagulation necrosis for primary HCC.8 RFA has evolved from a palliative tool to a curative treatment modality.9,10 Several randomized trials have demonstrated the superior efficacy of RFA in treating patients with small HCC (especially < 4 cm) in terms of less operator variability, lower local, and overall recurrence, and higher RFS than PEL11,12
Recently, the combination therapy of RFA and PEI has been increasingly applied in the treatment of HCC. It is found that the combination of RFA and PEI is superior to RFA alone and to PEI alone, and can make significant improvement of therapeutic effects in RFA treatment for HCC with few sessions of treatment.8,13 Moreover, it has been reported that this combination therapy may obtain comparable outcomes in larger HCC (3.1-4 cm) and in high-risk locations.14 However, most of the studies only focused on the local patients with large or small HCC, while the long-term effect was limited by the short-term follow-up period. Therefore, the therapeutic effects of RFA and RFA + PEI remain to be further confirmed in a larger cohort of Chinese patients with HCC ≤ 4 cm with a long-term follow-up period by a precise matching.
In this study, we conducted a retrospective population-based study to assess the effects of RFA treatment and the combination treatment of RFA and PEI (under uncontrolled conditions and in usual clinical practice) on OS and RFS of Chinese patients with HCC ≤ 4 cm diagnosed and treated at the Tumor Hospital of Shandong Province from 2004 to 2011. Since the validity of treatment effects in observational studies may be affected by confounding factors and selection bias, we performed a propensity analysis.
Material and MethodsPatientsOur study was approved by the Ethics Committee of Weifang Medical College and conformed to the provisions of the Declaration of Helsinki. We obtained data from the Tumor Hospital Registry of Shandong Province from 1 January 2004 to 31 December 2011. In all cases, the diagnosis of cirrhosis was based on laboratory parameters and ultrasound or computed tomography features suggesting liver cirrhosis. The inclusive criteria for the patients were as follows:
- •
All patients were newly diagnosed with HCC and had cirrhosis (Child-Pugh class A or B).
- •
Patients with HCC nodules ≤ 3 and the largest being ≤ 4 cm in size.
- •
Patients received RFA treatment alone (termed as RFA group) or a combination of RFA and PEI (termed as RFA + PEI group).
To reduce occasional confounding, all patients undergoing surgery or any other type of treatment were excluded. The diagnosis of HCC was based on cytohistological evidence from liver biopsy specimens or, in the absence of biopsy results, on the diagnostic criteria of practice guidelines of the American Association for the Study of Liver Disease (AASLD).15 The demographics, clinical characteristics, initial HCC treatments, recurrence, and survival were recorded from medical records. In this study, the following variables were analyzed: age, sex, local recurrence, tumor family history, complete ablation, tumor size, nodule number, glutamyl transferase (GT) levels, alpha-fetoprotein (AFP) levels, Child-Pugh class, and status of hepatitis B surface antigen (HBsAg). Patients compensated and staged within the Milan criteria were excluded from surgical resection or liver transplantation because of several reasons:
- •
Special tumor locations (e.g. near the hepatic portal vein).
- •
Patients with poor condition or other diseases (diabetes mellitus or impaired renal function).
- •
The lack of the donors or the high cost of liver transplantation.
- •
Patients’ choices.
RFA was performed percutaneously under ultrasonographic guidance, with the patients under conscious sedation and local anesthesia. After skin disinfection, a 17-gauge straight needle (Cool-tip RF system, Valleylab, Boulder, CO, USA) type with a 2-3 cm active tip, which was connected to a 200 W radiofrequency (RF)-current (480 kHz) generator (CTRF-220, Valleylab, Boulder, CO, USA), was advanced until reaching the tumor. Then the tumor was ablated through multiple overlapping applications at a power of 60-120 W for 10-15 min. The end point of the session was complete ablation of the visible tumor and at least a 0.5-1.0 cm-wide margin of the normal liver parenchyma surrounding the tumor. Immediately or 1 day after RFA, a contrast-enhanced multiphase computed tomography (CT) scan was performed to evaluate the extent of the treated areas and possible complications, such as bleeding. Two weeks after the session, a second CT scan was performed to assess the degree of tumor necrosis and to define the residual viable tumor segments.16 All patients were followed up with contrast-enhanced CT every 3-6 months.
For the patients treated with the combination treatment of RFA and PEI, the RF electrode was positioned into the tumor first and a 22-gauge percutaneous transhepatic cholangiography needle (Hakko, Tokyo, Japan) was placed into the tumor under ultrasonographic guidance. Then ethanol (99%) injection sessions were given to induce necrosis in the viable tumor segments. The volume of the injected ethanol (V) was calculated according to the following formula: V = 4/3π (γ + 0.5)3
Where V is the volume of ethanol to be injected and γ is the radius of the tumor. The RFA program was performed immediately after PEI. The CT was also performed to assess the results of the treatment’s results and any residual enhancing tumor tissue. Tumor recurrences were treated with the best possible procedure according to tumor size, tumor spreading and liver function assessment. In both groups local recurrences were usually treated using a new course of the same ablation technique (RFA or RFA + PEI), but the decision to perform RFA or combination treatment was left to the operator’s discretion.
Assessment of therapeutic efficacyComplete ablation was termed as a low attenuated area in the liver after the treatment. Moreover, we defined the tumor nodules that appeared in the same hepatic segment in treated patients as local recurrences and the other tumor nodules as new lesions. Meanwhile, sonographically guided fine-needle biopsy was used when possible to obtain a pathologic diagnosis of recurrences.
Propensity score-matched (PSM) procedure
To reduce the confounding effects of covariates and selection bias, we performed propensity score (PS) to match patients treated with RFA alone or combination treatment of RFA and PEI. We computed the PS by using logistic regression with the independent variables, including age (< 40, 40-65 or > 65), gender (female or male), local recurrence (yes or no), complete ablation (yes or no), Child-Pugh class (A or B), status of HBsAg (positive or negative), family history (positive or negative), tumor size (< 2.0 cm, 2.0-2.9 cm or 3.0-4.0 cm), number of tumor nodule (one, two or three). Pairs of comparable cases were created using propensity-scoring algorithms (the matching method was 1:1 for the nearest neighbor, with a caliper of 0.2, and without replacement). To find matched patients from the two groups, we adopted a caliper matching approach that has the ability to avoid bad matches (large differences in PS). The matched sets with the same PS tended to have similar covariate distributions in the two groups. Besides, a subanalysis of recurrence and survival between the groups according to tumor size (< 2.0 cm, between 2.0 and 2.9 cm, and between 3.0 and 4.0 cm) was performed before and after PSM.
Follow-upPatients were followed up with contrast-enhanced CT every 3-6 months, and they were followed until the termination of this study (December 31, 2011) or until death. For each patient, tumor recurrence was calculated according to the time elapsed from ablation to recurrence diagnosis (local or distant). The prospective database was routinely updated with survival, symptoms, and recurrence data.
Statistical analysisThe χ2 test or Fisher’s exact test was used to analyze categorical data. The Wilcoxon rank-sum test was exerted to compare nonparametric continuous data, and the Student’s t-test was applied if the data were parametric. The Kaplan-Meier curve with log-rank test was used to compare survival in the two treatment-groups. PS-based analyses were performed for sensitivity analyses to ensure that previous results were not caused by shortage of covariate balance. The Cox proportional hazards model was applied to evaluate the therapeutic effect of RFA and RFA + PEI on overall death and recurrence before and after adjusting for significant covariates for baseline characteristics. The SAS statistical package (version 9.2; SAS Institute, Inc., Cary, NC, USA) and SPSS (version 13.0, SPSS Inc., Chicago, IL, USA) were utilized to analyze the data. All P values were derived from two-tailed tests and a level of P < 0.05 was accepted as statistically significant.
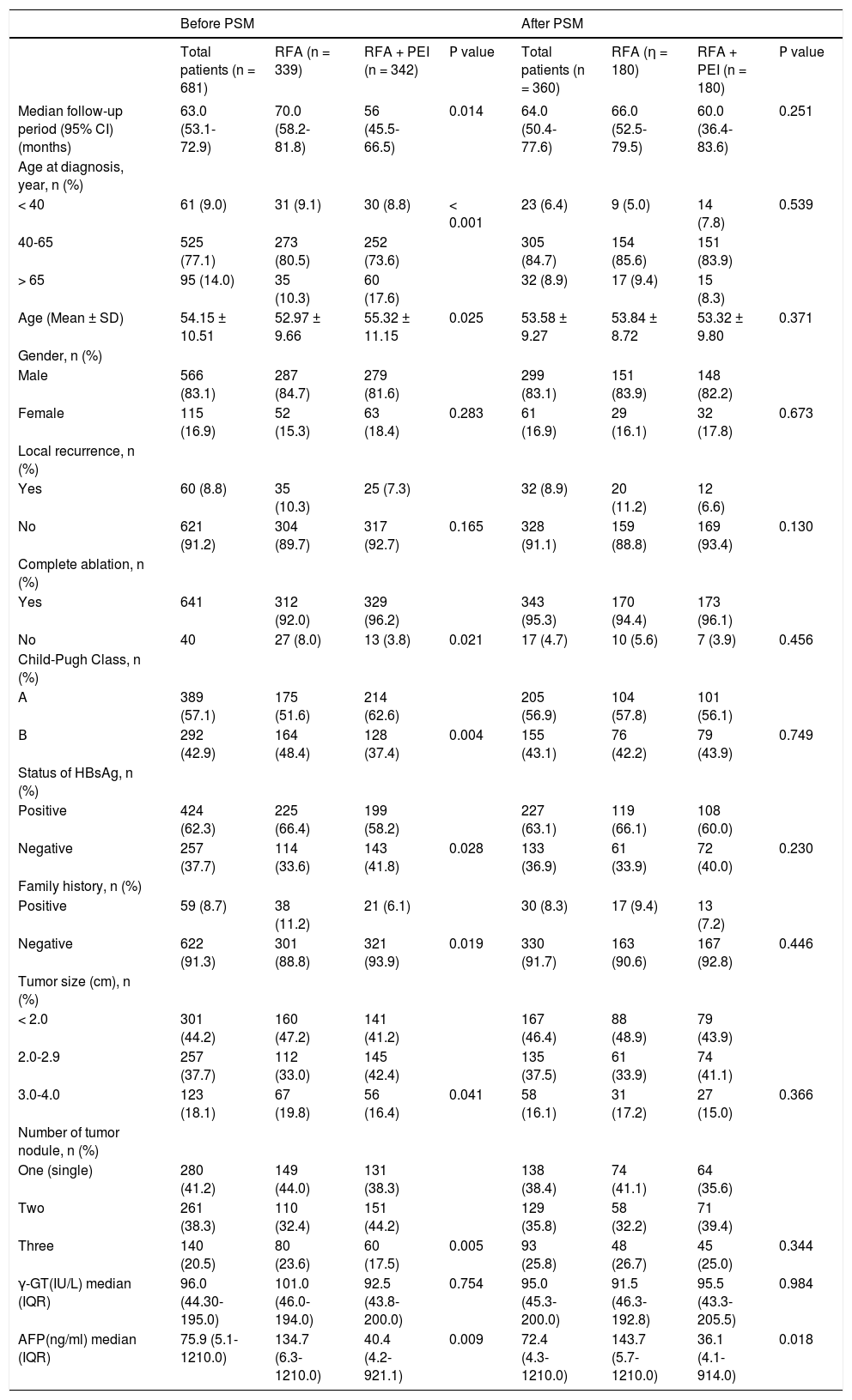
ResultsPatient characteristicsTable 1 summarized the descriptive statistics for the selected groups before and after PSM. We identified 681 Chinese patients with ≤ 4 cm HCC from the Tumor Hospital of Shandong Province between 2004 and 2011. Before PSM, 339 (49.7%) patients had undergone RFA treatment alone, and 342 (50.3%) were treated with RFA + PEI. The median follow-up periods were 70.0 (95% confidence interval (CI), 58.2-81.8) and 56.0 (95% CI, 45.5-66.5) months in the RFA and RFA + PEI groups, respectively. The mean patient age was 54.2 (SD, 10.5) and 53.0 (SD, 9.7) years in RFA and RFA + PEI groups, respectively. There were significant imbalances in clinical characteristics between the two groups, including complete ablation, Child-Pugh Class, status of HBsAg, tumor family history, tumor size, number of tumor nodule and AFP.
Clinical and epidemiological characteristics of patients treated with RFA alone or combined with PEI before and after PSM.
| Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Total patients (n = 681) | RFA (n = 339) | RFA + PEI (n = 342) | Ρ value | Total patients (n = 360) | RFA (η = 180) | RFA + PEI (n = 180) | Ρ value | |
| Median follow-up period (95% CI) (months) | 63.0 (53.1-72.9) | 70.0 (58.2-81.8) | 56 (45.5-66.5) | 0.014 | 64.0 (50.4-77.6) | 66.0 (52.5-79.5) | 60.0 (36.4-83.6) | 0.251 |
| Age at diagnosis, year, n (%) | ||||||||
| < 40 | 61 (9.0) | 31 (9.1) | 30 (8.8) | < 0.001 | 23 (6.4) | 9 (5.0) | 14 (7.8) | 0.539 |
| 40-65 | 525 (77.1) | 273 (80.5) | 252 (73.6) | 305 (84.7) | 154 (85.6) | 151 (83.9) | ||
| > 65 | 95 (14.0) | 35 (10.3) | 60 (17.6) | 32 (8.9) | 17 (9.4) | 15 (8.3) | ||
| Age (Mean ± SD) | 54.15 ± 10.51 | 52.97 ± 9.66 | 55.32 ± 11.15 | 0.025 | 53.58 ± 9.27 | 53.84 ± 8.72 | 53.32 ± 9.80 | 0.371 |
| Gender, n (%) | ||||||||
| Male | 566 (83.1) | 287 (84.7) | 279 (81.6) | 299 (83.1) | 151 (83.9) | 148 (82.2) | ||
| Female | 115 (16.9) | 52 (15.3) | 63 (18.4) | 0.283 | 61 (16.9) | 29 (16.1) | 32 (17.8) | 0.673 |
| Local recurrence, n (%) | ||||||||
| Yes | 60 (8.8) | 35 (10.3) | 25 (7.3) | 32 (8.9) | 20 (11.2) | 12 (6.6) | ||
| No | 621 (91.2) | 304 (89.7) | 317 (92.7) | 0.165 | 328 (91.1) | 159 (88.8) | 169 (93.4) | 0.130 |
| Complete ablation, n (%) | ||||||||
| Yes | 641 | 312 (92.0) | 329 (96.2) | 343 (95.3) | 170 (94.4) | 173 (96.1) | ||
| No | 40 | 27 (8.0) | 13 (3.8) | 0.021 | 17 (4.7) | 10 (5.6) | 7 (3.9) | 0.456 |
| Child-Pugh Class, n (%) | ||||||||
| A | 389 (57.1) | 175 (51.6) | 214 (62.6) | 205 (56.9) | 104 (57.8) | 101 (56.1) | ||
| Β | 292 (42.9) | 164 (48.4) | 128 (37.4) | 0.004 | 155 (43.1) | 76 (42.2) | 79 (43.9) | 0.749 |
| Status of HBsAg, n (%) | ||||||||
| Positive | 424 (62.3) | 225 (66.4) | 199 (58.2) | 227 (63.1) | 119 (66.1) | 108 (60.0) | ||
| Negative | 257 (37.7) | 114 (33.6) | 143 (41.8) | 0.028 | 133 (36.9) | 61 (33.9) | 72 (40.0) | 0.230 |
| Family history, n (%) | ||||||||
| Positive | 59 (8.7) | 38 (11.2) | 21 (6.1) | 30 (8.3) | 17 (9.4) | 13 (7.2) | ||
| Negative | 622 (91.3) | 301 (88.8) | 321 (93.9) | 0.019 | 330 (91.7) | 163 (90.6) | 167 (92.8) | 0.446 |
| Tumor size (cm), n (%) | ||||||||
| < 2.0 | 301 (44.2) | 160 (47.2) | 141 (41.2) | 167 (46.4) | 88 (48.9) | 79 (43.9) | ||
| 2.0-2.9 | 257 (37.7) | 112 (33.0) | 145 (42.4) | 135 (37.5) | 61 (33.9) | 74 (41.1) | ||
| 3.0-4.0 | 123 (18.1) | 67 (19.8) | 56 (16.4) | 0.041 | 58 (16.1) | 31 (17.2) | 27 (15.0) | 0.366 |
| Number of tumor nodule, n (%) | ||||||||
| One (single) | 280 (41.2) | 149 (44.0) | 131 (38.3) | 138 (38.4) | 74 (41.1) | 64 (35.6) | ||
| Two | 261 (38.3) | 110 (32.4) | 151 (44.2) | 129 (35.8) | 58 (32.2) | 71 (39.4) | ||
| Three | 140 (20.5) | 80 (23.6) | 60 (17.5) | 0.005 | 93 (25.8) | 48 (26.7) | 45 (25.0) | 0.344 |
| γ-GT(IU/L) median (IQR) | 96.0 (44.30-195.0) | 101.0 (46.0-194.0) | 92.5 (43.8-200.0) | 0.754 | 95.0 (45.3-200.0) | 91.5 (46.3-192.8) | 95.5 (43.3-205.5) | 0.984 |
| AFP(ng/ml) median (IQR) | 75.9 (5.1-1210.0) | 134.7 (6.3-1210.0) | 40.4 (4.2-921.1) | 0.009 | 72.4 (4.3-1210.0) | 143.7 (5.7-1210.0) | 36.1 (4.1-914.0) | 0.018 |
RFA: radiofrequency ablation, PEI: percutaneous ethanol injection, PSM: propensity score matching, HBsAg: hepatitis Β virus surface antigen, GT: glutamyltansferase, AFP: alpha-fetoprotein, IQR: interquartile range.
Based on the estimated PS, 180 patients who underwent RFA + PEI were successfully matched one-to-one with 180 RFA-treated patients. In total, 321 patients were excluded from the matched cohort because of a lack of appropriate matching. After PSM, the median follow-up periods were 66.0 (95% CI, 52.5-79.5) and 60.0 (95% CI, 36.4-83.6) months in the RFA and RFA + PEI groups, respectively. The mean age was 53.8 (SD, 8.7) and 53.3 (SD, 9.8) years for patients who underwent RFA alone and RFA + PEI, respectively. The clinical characteristics of patients after PS were also well balanced and did not show any significant difference between the two groups, including complete ablation, Child-Pugh Class, status of HBsAg, tumor family history, tumor size and number of tumor nodule (Table 1). Meanwhile, there were no significant differences in gender, local recurrence and γ-GT both before and after matching between two groups. Besides, the level of AFP showed significant difference between two groups after matching.
Survival analysisThe absolute numbers of patients died in five years (five-year mortality) in RFA and RFA + PEI groups after PSM were 24 and 30, respectively. The median follow-up periods before and after PSM were 63.0 (95% CI, 53.1-72.9) and 64.0 (95% CI, 50.4-77.6) months, respectively. AFP was included as a covariate in the survival analysis. In the 339 patients who were treated with RFA, the 1-, 3-, and 5-year cumulative OS rates were 82.6, 54.2, and 40.0%, respectively. The 1-, 3-, and 5-year cumulative RFS rates were 82.4, 54.0, and 35.1%, respectively. The median survival time was 42.0 (95% CI, 35.0-48.0) months. In the 342 patients who were treated with RFA + PEI, the 1-, 3-, and 5-year cumulative OS rates were 90.3, 59.3, and 43.1%, respectively. The 1-, 3-, and 5-year RFS rates were 90.0, 58.5, and 41.0%, respectively. The median survival time was 48 (95% CI, 41.0-59.0) months. There was no significant benefit in either OS rates or RFS rates in the RFA + PEI group. Twenty-five patients (7.3%) who underwent RFA + PEI treatment and 35 patients (10.3%) treated with RFA alone developed local tumor recurrence, with no significant difference between the two groups.
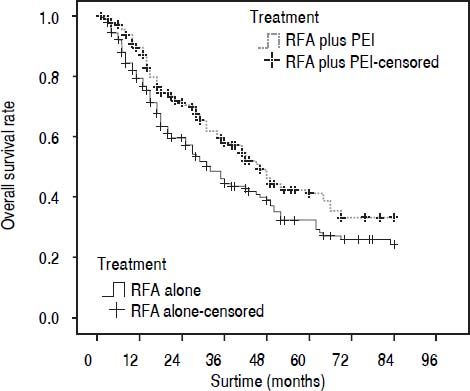
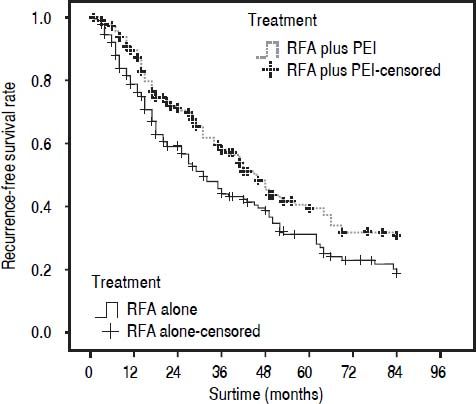
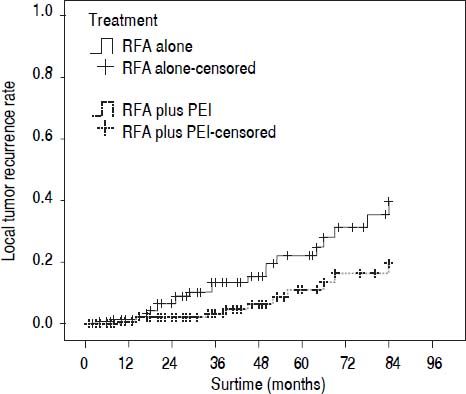
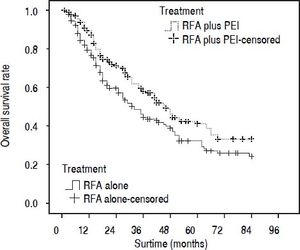
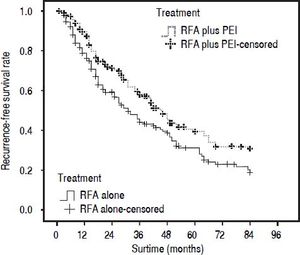
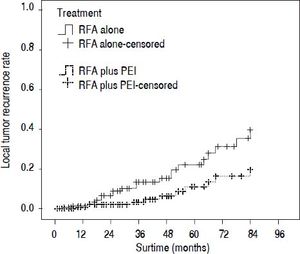
Our results after PSM showed that the 1-, 3-, and 5-year cumulative OS rates were 78.0, 44.4, and 30.1% for patients in the RFA group, and 88.2, 58.0, and 41.1% in the RFA + PEI group, respectively. The median survival time was 32.0 (95% CI, 25.0-43.0) and 46.0 (95% CI, 36.0-59.0) months in RFA group and RFA + PEI group, respectively. After adjusting of covariates, patients in the RFA + PEI group showed significantly better cumulative OS than patients in the RFA group (Figure 1). In this matched cohort, the 1-, 3-, and 5-year cumulative RFS rates were 77.0, 43.8, and 29.2% for patients in the RFA group, and 87.9, 57.6, and 38.4 for patients in the RFA + PEI group, respectively. Additionally, patients in RFA + PEI group showed better cumulative RFS than patients in the RFA group (Figure 2), and less (11, 6.1%) patients in the RFA + PEI group developed local tumor recurrence than those in the RFA group (21, 11.7%) (Figure 3).
Propensity score-matched Kaplan-Meier curves of patients treated with radiofrequency ablation (RFA) alone (n = 180) or RFA and percutaneous ethanol injection (PEI) (n = 180). The 1-, 3-, and 5-year cumulative overall survival (OS) rates were 78.0, 44.4, and 30.1% for patients in RFA group, and 88.2, 58.0, and 41.1% for patients in RFA + PEI group, respectively. There was a significant difference in OS between the two groups (P = 0.019).
Cumulative recurrence-free survival (RFS) curve of patients withi HCC ≤ 4 cm who underwent radiofrequency ablation (RFA) alone (n = 180) or RFA and percutaneous ethhanol injection (PEi) (n = 180) after propensity score matching. The 1-, 3-, and 5-year cumulative RFS rates were 77.0, 43.8, and 292% for patients in RFA group, and 87.9, 57.6, and 38.4% for patients in RFA + PEI group, respectively. There was a signifcant difference between the two groups (P = 0.009).
Comparison of the local tumor recurrence rate for main tumors in radiofrequency ablation (RFA)-treated and combination treatment of RFA and ethanol injection (PEI) after matching. The local tumor recurrence rate was significantly lower in the RFA + PEI group than in the RFA group (P = 0.011).
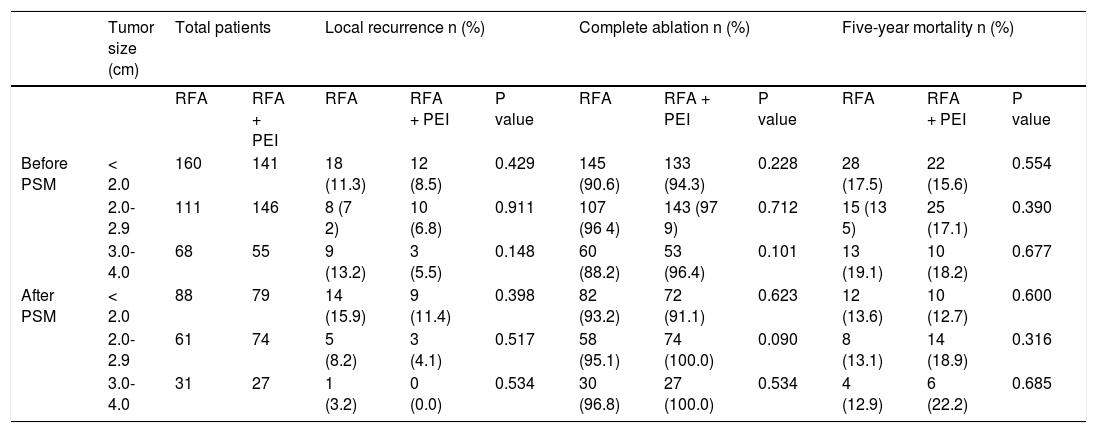
In addition, the subanalysis of patients with tumor size < 2.0 cm, between 2.0 and 2.9 cm, and between 3.0 and 4.0 cm was performed before and after PSM. As a result, local recurrence, complete ablation and five-year mortality showed no significant differences between patients treated with RFA and RFA + PEI before and after PSM (Table 2).
Analysis of patients with tumors smaller than 2 cm, between 2 and 3 cm, and between 3 and 4 cm treated with RFA alone or combined with PEI before and after psm.
| Tumor size (cm) | Total patients | Local recurrence n (%) | Complete ablation n (%) | Five-year mortality n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RFA | RFA + PEI | RFA | RFA + PEI | P value | RFA | RFA + PEI | P value | RFA | RFA + PEI | P value | ||
| Before PSM | < 2.0 | 160 | 141 | 18 (11.3) | 12 (8.5) | 0.429 | 145 (90.6) | 133 (94.3) | 0.228 | 28 (17.5) | 22 (15.6) | 0.554 |
| 2.0-2.9 | 111 | 146 | 8 (7 2) | 10 (6.8) | 0.911 | 107 (96 4) | 143 (97 9) | 0.712 | 15 (13 5) | 25 (17.1) | 0.390 | |
| 3.0-4.0 | 68 | 55 | 9 (13.2) | 3 (5.5) | 0.148 | 60 (88.2) | 53 (96.4) | 0.101 | 13 (19.1) | 10 (18.2) | 0.677 | |
| After PSM | < 2.0 | 88 | 79 | 14 (15.9) | 9 (11.4) | 0.398 | 82 (93.2) | 72 (91.1) | 0.623 | 12 (13.6) | 10 (12.7) | 0.600 |
| 2.0-2.9 | 61 | 74 | 5 (8.2) | 3 (4.1) | 0.517 | 58 (95.1) | 74 (100.0) | 0.090 | 8 (13.1) | 14 (18.9) | 0.316 | |
| 3.0-4.0 | 31 | 27 | 1 (3.2) | 0 (0.0) | 0.534 | 30 (96.8) | 27 (100.0) | 0.534 | 4 (12.9) | 6 (22.2) | 0.685 | |
RFA: radiofrequency ablation, PEI: percutaneous ethanol Injection, PSM: propensity score matching. Five-year mortality the absolute numbers of patients died In five years.
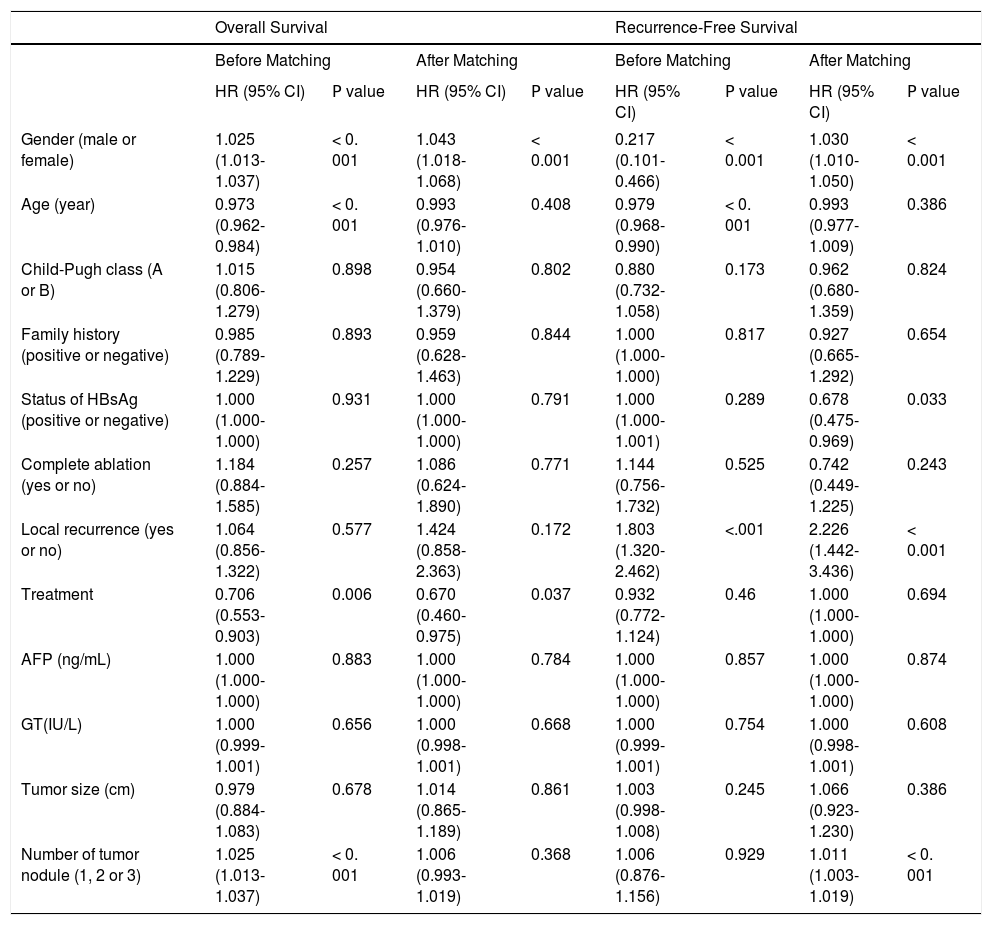
The logistic regression analysis results indicated that age, Child-Pugh class, status of HBsAg, family history, complete ablation, tumor size, number of tumor nodule, and AFP levels were significantly related to the treatment approach (P < 0.05, all variables). These variables were used to calculate PS indices. Before matching, multivariate Cox regression analysis results showed that sex, age, treatment, and number of tumor nodule were significantly related to the OS of HCC patients (P < 0.05) (Table 3). After matching, according to Cox regression analysis results, sex and treatment significantly influenced OS (P < 0.05) (Table 3). Besides, multivariate Cox regression analysis results also showed that sex, age, and local recurrence were significantly related to RFS (P < 0.05) before matching, while sex, status of HBsAg, local recurrence, and number of tumor nodule were related to RFS (P < 0.05) (Table 3) after matching.
Multivariate analysis of overall survival and recurrence-free survival by the Cox proportional hazards model before and after matching.
| Overall Survival | Recurrence-Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Before Matching | After Matching | Before Matching | After Matching | |||||
| HR (95% CI) | Ρ value | HR (95% CI) | Ρ value | HR (95% CI) | Ρ value | HR (95% CI) | Ρ value | |
| Gender (male or female) | 1.025 (1.013-1.037) | < 0. 001 | 1.043 (1.018-1.068) | < 0.001 | 0.217 (0.101-0.466) | < 0.001 | 1.030 (1.010-1.050) | < 0.001 |
| Age (year) | 0.973 (0.962-0.984) | < 0. 001 | 0.993 (0.976-1.010) | 0.408 | 0.979 (0.968-0.990) | < 0. 001 | 0.993 (0.977-1.009) | 0.386 |
| Child-Pugh class (A or B) | 1.015 (0.806-1.279) | 0.898 | 0.954 (0.660-1.379) | 0.802 | 0.880 (0.732-1.058) | 0.173 | 0.962 (0.680-1.359) | 0.824 |
| Family history (positive or negative) | 0.985 (0.789-1.229) | 0.893 | 0.959 (0.628-1.463) | 0.844 | 1.000 (1.000-1.000) | 0.817 | 0.927 (0.665-1.292) | 0.654 |
| Status of HBsAg (positive or negative) | 1.000 (1.000-1.000) | 0.931 | 1.000 (1.000-1.000) | 0.791 | 1.000 (1.000-1.001) | 0.289 | 0.678 (0.475-0.969) | 0.033 |
| Complete ablation (yes or no) | 1.184 (0.884-1.585) | 0.257 | 1.086 (0.624-1.890) | 0.771 | 1.144 (0.756-1.732) | 0.525 | 0.742 (0.449-1.225) | 0.243 |
| Local recurrence (yes or no) | 1.064 (0.856-1.322) | 0.577 | 1.424 (0.858-2.363) | 0.172 | 1.803 (1.320-2.462) | <.001 | 2.226 (1.442-3.436) | < 0.001 |
| Treatment | 0.706 (0.553-0.903) | 0.006 | 0.670 (0.460-0.975) | 0.037 | 0.932 (0.772-1.124) | 0.46 | 1.000 (1.000-1.000) | 0.694 |
| AFP (ng/mL) | 1.000 (1.000-1.000) | 0.883 | 1.000 (1.000-1.000) | 0.784 | 1.000 (1.000-1.000) | 0.857 | 1.000 (1.000-1.000) | 0.874 |
| GT(IU/L) | 1.000 (0.999-1.001) | 0.656 | 1.000 (0.998-1.001) | 0.668 | 1.000 (0.999-1.001) | 0.754 | 1.000 (0.998-1.001) | 0.608 |
| Tumor size (cm) | 0.979 (0.884-1.083) | 0.678 | 1.014 (0.865-1.189) | 0.861 | 1.003 (0.998-1.008) | 0.245 | 1.066 (0.923-1.230) | 0.386 |
| Number of tumor nodule (1, 2 or 3) | 1.025 (1.013-1.037) | < 0. 001 | 1.006 (0.993-1.019) | 0.368 | 1.006 (0.876-1.156) | 0.929 | 1.011 (1.003-1.019) | < 0. 001 |
HR: hazard ratios. HBsAg: hepatitis Β virus surface antigen. GT: giutamyitransferase. AFP: aipha-fetoprotein.
RFA and/or PEI were widely used as treatment strategies for patients with HCC.17 In the present study, we compared the long-term outcomes of Chinese patients with HCC ≤ 4 cm treated with RFA alone or combined with PEI by performing a PSM survival analysis. Our results indicated that the combination of RFA and PEI improved the OS and RFS rates and decreased the local tumor recurrence rate in Chinese patients with HCC ≤ 4 cm.
We firstly compared the long-term outcomes of total 681 Chinese patients with HCC ≤ 4 cm in RFA and RFA + PEI groups, and found that the risks of death and recurrence were similar in these two groups, as well as their 1-, 3- and 5-year survival rates. These findings are consistent with those of previous studies showing similar survival rates in HCC patients (especially those with tumors < 3 cm) undergoing RFA alone and a combination of RFA and PEI treatment.18,19 In consideration of the baseline differences between RFA-treated alone and a combination treatment of RFA and PEI, we performed PSM analysis to decrease the influence of selection bias and confounding variables in this study. Moreover, Cox proportional hazards regression model is the most common method for texting the effect of explanatory variables on time-to-event outcomes.20 Then the relative benefit of the combined therapy vs. RFA therapy alone was assessed using the Cox proportional regression model and the PS. Those results demonstrated that HCC patients treated with combined therapy presented a significantly better OS rate than RFA-treated alone. Our results were consistent with those of previous studies, which showed that the combination treatment of RFA and PEI was more effective than the use of RFA alone for patients with HCC.8,21,22 Tumor size is related to the therapeutic efficiency. In order to assess the effects of two treatment approaches in different tumor sizes, we also performed a subanalysis for patients with tumors < 2.0 cm, between 2.0 and 2.9 cm, and between 3.0 and 4.0 cm before and after PSM. As a result, local recurrence, complete ablation and five-year mortality showed no significant differences between RFA and RFA + PEI groups in three subgroups classified with tumor size, which suggested that treatment strategies had no direct relations with the tumor size. However, some studies have shown that combined treatment of RFA and PEI is effective for larger HCCs. For example, Lin, ei al.23 have found that combined treatment of RFA and PEI may achieve comparable long-term outcomes in larger HCC of 3.1-4.0 cm when compared with HCC < 3.1 cm. Nevertheless, only tumors less than 3.1 cm and 3.1-4.0 cm were performed comparisons in their research. In addition, since the PSM in this study was performed in the whole population but not in these subgroups, the clinical and epidemiological characteristics of patients in each subgroup might be inconsonant. Also, the subtle differences of treatment protocols in several independent researches may influence the results, such as ablation time, concentration and volume of ethanol. Even so, based on a large population, this study suggests that the combination treatment of RFA + PEI is more effective than RFA-treated alone in better survival and local disease control for Chinese patients with HCC ≤ 4 cm.
In this study, AFP level in RFA + PEI group was significantly lower than that in RFA group. Shimada, et al. have found that the positivity of AFP is an independent indicator of a poor prognosis in patients with HCC in terms of disease-free survival and patient survival.24 However, the prognostic significance of AFP level is still controversial. Schulze, et al.25 have shown that preoperative AFP mRNA is not significantly associated with higher incidence of HCC recurrence. Jeng, et al.26 have found that AFP is not a sensitive marker of HCC cell dissemination. Therefore, the level of AFP may be not correlated to the OS and RFS of patients with HCC in our study.
In addition, the Kaplan-Meier curve indicated that the RFS rates in the RFA group were 77.0, 43.8, and 29.2% at 1, 3 and 5 years after matching, respectively, which were in agreement with the results of Rossi, et al.27 The RFS rates of the combined therapy group after matching were 87.9, 57.6, and 38.4% at 1, 3 and 5 years, respectively. Those results were similar to those reported by Zhang, et al.,22 which indicated that the combination therapy improved the RFS for patients with HCC ≤ 4 cm. Meanwhile, the multivariate analysis results showed that the treatment strategy had no significant influence on RFS. This may be caused by other factors that were not given in our study, such as the level of protein induced by vitamin K absence or antagonists-II (PIVKA-II),28 state of HCV antibody and level of total bilirubin.14 For example, the multiplication of AFP and PIVKA-II is closely associated with differentiation and microscopic vascular invasion and is found to be an independent factor for survival (patient survival and RFS) and recurrence in patients with HCC.29 Although treatment strategy was not the independent influencing factor for RFS, our results suggested that the combined therapy might achieve better RFS in patients with HCC.
Furthermore, the local tumor recurrence rates in RFA + PEI group were less than these in RFA group. It is also showed a lower rate of local recurrence in both groups, which was in contrast to other previous clinical studies.30,31 These differences between studies may have been caused, at least in part, by our selection of patients with small-sized tumors (nearly 82% cases had a tumor < 3 cm). Additionally, survival time is defined as the interval between the first treatment and either death or the last follow-up visit.32 In our study, median survival time in both groups appeared to be higher (36 months in the RFA group and 41 months in the RFA + PEI group) compared with other studies.31,33 The reason for this finding may be that our study cohort mainly had small-sized tumors, leading to a comparatively better prognosis. Besides, our multivariate analysis showed that Child-Pugh class did not affect the OS between patients in RFA and RFA + PEI groups. This is probably because the patients with Child-Pugh class C were absent and patients with Child-Pugh class B were mild (most were B7 scored and a very small number of patients got mild ascites) in our study. Even so, our results implied that the combination treatment of RFA and PEI is superior to RFA-treated alone for Chinese patients with HCC, especially in patients with HCC ≤ 4 cm.
Although our results indicated that PSM is an efficient and useful method of creating a matched case-control study to assess the effects of combined therapy, our study has some limitations. Firstly, this study was not a randomized study and potential bias may have influenced the results (e.g., selection of patients for the groups of treatment and possible selective losses). Secondly, our data analysis originated from secondary data, and some further information, such as the Barcelona Clinic Liver Cancer stage, histological type, types of tumor growth, associated morbidities, and several commonly used parameters (serum transaminase level, platelet count, presence of varices or ascites) were not available. Thirdly, because the PSM was only performed in the whole population but not in each subgroup classified by tumor size, some confounding factors might exist when we analyzed the subgroups. Meanwhile, apart from HbsAg status no other information is available about the etiology of cirrhosis in the patients. This might also result in unknown bias. In addition, all patients in our study had tumors < 4 cm. Therefore, whether the combination of RFA with PEI still yields better OS and RFS than RFA alone for patients with larger-sized tumors requires further study.
In summary, the combination of RFA and PEI results in better OS and RFS rates than RFA-treated alone for Chinese patients with HCC 4 cm or less by analyzing a large cohort with a long-term follow-up. Also, treatment approach was selected by multivariate analysis as an independent factor for OS of patients with HCC ≤ 4 cm. The combination treatment might be an optimal method and appropriate for use in clinical practice.
Abbreviations- •
AASLD: American Association for the Study of Liver Disease.
- •
AFP: alpha-fetoprotein.
- •
CI: confidence interval.
- •
CT: computed tomography.
- •
GT: glutamyl transferase.
- •
HBsAg: hepatitis B surface antigen.
- •
HCC: hepatocellular carcinoma.
- •
OS: overall survival.
- •
PEI: percutaneous ethanol injection.
- •
PS: propensity score.
- •
RFA: radiofrequency ablation.
- •
RFS: recurrence-free survival.
The authors declare that they have no competing interests.
AcknowledgementThis study was supported by National High Technology Research and Development Program of China (2012AA02A617); National Nature Science Foundation of China (No.81473071) and Shandong Province Natural Science Foundation (ZR2013HM045).