Objective. To evaluate the efficacy of low carbohydrate diet (LCD) as compared with low fat diet (LFD) to decrease aminotransferase levels in obese women with nonalcoholic fatty liver disease.
Material and methods. A total of 59 women were randomly enrolled in a non-controlled clinical intervention study to receive either LCD or LFD during six months. Apparently healthy non-pregnant obese women aged 20 to 65 years were eligible to participate. Previous diagnosis of hepatic disease, serum creatinine level > 1.5 mg/dL, severe life-limiting medical illness, pregnancy, active participation in other dietary program, use of weight loss drugs, or alcohol consumption ≥ 30 g per day were exclusion criteria.
Results. A total of 31 obese women who received LCD were compared with 28 women allocated in the LFD group. There were 3 (LCD group) and 2 (LFD group) women with lost of follow-up. No differences in the proportion of type 2 diabetes, hypertension and hyperlipidemia were noted between women in the LCD and LFD groups. At end of follow-up, there were not significant statistical differences in the anthropometric and biochemical characteristics between women in both groups. The weight loss was 5.7 and 5.5% for women in the LCD LFD groups. Although the decrease of AST (31.7 and 22.4%) and ALT (41 and 33.3%) levels was more elevated in the women of LCD group, as compared with the LFD group, there were not significant statistical differences.
Conclusions. Our results show that weight loss, irrespective of the type of diet, reduces aminotransferase levels in obese women with NAFLD.
Obesity is an important risk factor for liver injury; on this regard, it has been reported that liver inflammation can be induced by the metabolically active intraabdominal fat1 and that the high body mass index (BMI) and large waist circumference are significantly associated with elevation of aspar-tate aminotransferase (AST) and alanine amino-transferase (ALT) levels.2,3
Bright liver at ultrasound and the increase ALT and AST levels are hallmarks of nonalcoholic fatty liver disease (NAFLD); their increase is a biochemical indicator of hepatic steatosis in obese populations.4,5 ALT also might declines as fibrosis worsens.6,7
The reduction of body weight can be achieved with combining diet and physical activity strategies; on this regard, it has been reported that low carbohydrate diet (LCD), as compared with low fat diet (LFD), promotes significant higher weight loss, improves triglycerides, HDL-cholesterol, and glucose levels, particularly in patients with hyperglycemia.8,9 Furthermore, several studies have demonstrated that weight loss also can reduce aminotransferase levels.10,11 These findings suggest that LCD could be an important tool for promoting weight loss and to reduce ALT levels in obese individuals with hepatic steatosis.12
Given that current therapeutic guidelines for obese patients with NAFLD include, as the first-line of treatment, strategies for weight loss13 and that stu-dies that compare the relative benefits of hypocalo-ric diets restricted in carbohydrate versus diets restricted in fat for the treatment of obese patients with NAFLD are scarce.
ObjectiveTo evaluate the efficacy of LCD as compared with LFD to decrease aminotransferase levels in obese women with NAFLD.
Materials and MethodsWith the protocol approval by the Mexican Social Security Institute Scientific Research Committee, and after obtaining the subject informed consent, 54 obese women, aged 20 to 65 years, inhabitants of Durango, city in northern Mexico, were enrolled in a randomized non-controlled clinical trial.
Apparently healthy non-pregnant obese women with diagnosis of NAFLD and similar social and economic background were invited to participate in the study.
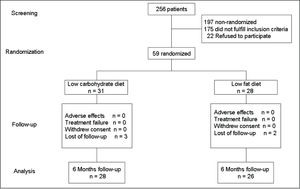
Obese women were randomly allocated to receive either LCD or LFD during 6 months. The primary trial end point was the decrease of AST and ALT levels. Previous diagnosis of hepatic disease, serum creatinine level ≥ 1.5 mg/dL, severe life-limiting medical illness, pregnancy, active participation in other dietary program, use of weight loss drugs, or alcohol consumption ≥ 30 g per day were exclusion criteria. The final distribution of the population in study is shown in figure 1.
The control visits for all participants were performed each month. Sessions were individually conducted by trained personnel of the Biomedical Research Unit. Record diaries of both, diet and exercise were completed by participants and reviewed in each visit. The recommendations for exercise were indicated according the physical status of each participant; these recommendations included physical activities such as walking, dancing, bicycling or swimming. The aim was to perform exercise at least one hour per day, 5 days for week.
Total caloric intake was calculated as we have previously reported.14 In brief, the LCD was based on the following percentage of total energy intake per nutrient: 27% protein, 28% fat and 45% carbohydrate; whereas the LFD was based on 21% of daily energy intake from fat, < 10% saturated fat, 25% protein, and 54% carbohydrate.
Adherence to diet and exercise was assessed every week by personal interview.
DefinitionsObesity was defined by body mass index (BMI) > 30 kg/m2.
Elevated ALT levels were defined by serum ALT levels > 29 U/L.15
Irrespective of ALT levels, diagnosis of NAFLD was established by the presence of hepatic ultraso-nographic findings such as different hepatorenal echo contrast, bright liver, deep attenuation, and blurred vessels.16
MeasurementsIn the standing position, weight and height were measured with the women in light clothing using a fixed scale with stadimeter (Tanita TBF-215, Tokyo, Japan). The precision of weight and height measurements was 0.1 kg and 0.01 m. Body Mass Index (BMI) was calculated as weight (kilograms) divided by height (meters) squared; total body fat (TBF) was measured by bioelectric impedance using a body composition analyzer (Tanita TBF-215, Tokyo, Japan).
Using Baumanometer (microlife AG, Heerbrugg, Switzerland) and stethoscope (3M Littman Classic II, Neuss, Germany), the technique for measurement of blood pressure was the recommended in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.17
AssaysA venous whole blood sample was collected after 8–10 h of fasting. Plasma glucose was assessed by glucose-oxidase method; the inter- and intra-assay variations were 2.5 and 1.8%. Total-cholesterol (inter- and intra-assay variations of 2.9 and 2.5%) and serum triglycerides (inter- and intra-assay variations of 3.7 and 3.0%) were determined by enzymatic methods. The AST and ALT levels were assessed by UV kinetic methods (Erlic, Tlalnepantla, Estado de Mexico, Mex.).
All measurements were performed in an Express 500 clinical chemistry autoanalyzer (Ciba Corning, Diagnostic Corp., Overling, Ohio).
Statistical analysisThe preplanned intention-to-treat analysis of the primary study end-point was done for all the randomly allocated participants who satisfactorily completed the follow-up (Figure 1).
Differences between the groups were assessed using unpaired Student t test (or Mann-Whitney U test for skewed data), and chi-square test (for qualitative variables). Differences between baseline and end of follow-up within the same group were assessed using paired Student t test.
The relationship between variables in study was estimated using the Pearson correlationship test.
ResultsA total of 28 obese women received LCD and 26 LFD (Figure 1). Average age for women in the LCD and LFD groups was 46.3 ± 9.1 and 45 ± 9.1 years, p = 0.80.
There were not statistical significant differences in the proportion of type 2 diabetes (21.4 and 7.6%, p = 0.25), hypertension (32 and 31%, p = 0.85), and hyperlipidemia (36 and 35%, p = 0.84), between women in the LCD and LFD groups, respectively.
Women in both groups received an average of 1,593 ± 82 kcal per day during the 6 months of treatment; adherence to diet and exercise was reached by > 90% of the women in both groups.
At baseline, glucose levels were higher in the women of LCD group as compared with women of LFD group; other anthropometric and biochemical characteristics showed not significant differences (Table 1).
Characteristics of the target population at baseline and end conditions.
| Baseline | p value* | End of follow-up (six months) | p value* | |||
|---|---|---|---|---|---|---|
| LCD (n= 28) | LFD (n= 26) | LCD (n = 28) | LFD (n= 26) | |||
| Age (years) | 46.3 ± 9.1 | 45 ± 9.1 | 0.80 | - | - | - |
| Weight (kg) | 96.3 ± 15.7 | 92.5 ± 17 | 0.35 | 90.8 ± 15.9 | 87.4 ± 18.0 | 0.47 |
| Body mass index (kg/m2) | 38.7 ± 6.1 | 36.9 ± 6.8 | 0.31 | 36.7 ± 6.8 | 34.8 ± 6.3 | 0.29 |
| Total body fat (%) | 47.3 ± 4.4 | 45.6 ± 5.2 | 0.18 | 44.8 ± 5.7 | 42.7 ± 5.3 | 0.16 |
| Sistolic blood pressure (mmHg) | 129.1 ± 15.1 | 122.7 ± 12.7 | 0.10 | 120.4 ± 9.1 | 117.1 ± 16.6 | 0.46 |
| Diastolic blood pressure (mmHg) | 83.5 ± 14.2 | 80.2 ± 10.2 | 0.32 | 77.1 ± 9.8 | 76.5 ± 13.6 | 0.86 |
| Glucose (mg/dL) | 121.1 ± 54.3 | 98.3 ± 15.6 | 0.03 | 120.4 ± 41.6 | 109.6 ± 52.7 | 0.41 |
| Cholesterol (mg/dL) | 202.3 ± 46 | 190.5 ± 49.6 | 0.37 | 189.1 ± 31.9 | 191.2 ± 39.9 | 0.83 |
| Triglycerides (mg/dL) | 200.3 ± 102.7 | 173.2 ± 82.7 | 0.29 | 173.5 ± 63.9 | 155 ± 53.8 | 0.25 |
| AST (U/L) | 48.1 ± 16.3 | 42 ± 14.8 | 0.15 | 32.9 ± 10.9 | 32.6 ± 8.8 | 0.91 |
| ALT (U/L) | 59.6 ± 27.4 | 53.6 ± 15.9 | 0.33 | 35.3 ± 11.6 | 35.8 ± 14.0 | 0.89 |
Data are mean ± standard deviation.
At the end of follow-up, participants in the LCD and LFD groups lost 5.7 and 5.5% of their initial weight. In both groups, systolic and diastolic blood pressure decreased in a parallel way to weight loss. At 6 months of follow-up weight, BMI, TBF, glucose, cholesterol and triglycerides levels decreased in a similar proportion in the women in both, LCD and LFD groups. Changes in TBF showed a positive relationship with changes in ALT (r = 0.203 and r = 0.258) and AST (r = 0.307 and r = 0.312) for the women in the LCD and LFD groups. In the same way, changes in weight also were positively related with changes in ALT (r = 0.258 and r = 0.242) and AST (r = 0.267 and r = 0.244) for the groups with LCD and LFD.
On the other hand, the intragrupal analysis showed a significant reduction, between baseline and the end of follow-up:
- •
In the body weight (p < 0.00001 and p < 0.00001).
- •
ALT (p = 0.0002 and p = 0.005).
- •
AST (p = 0.00002 and p = 0.0001) levels of the groups with LCD and LFD.
Stratified by age, at baseline there were not significant differences for:
- •
Body weight (99.4 ± 12.8 and 96.6 ± 16.3 kg, p = 0.92).
- •
TBF (49.0 ± 4.1 and 46.4 ± 5.0%, p = 0.20).
- •
AST (47.0 ± 18.3 and 37.3 ± 6.2 U/L, p = 0.09).
- •
ALT (58.9 ±34.4 and 54.6 ± 15.8 U/L, p = 0.69) for the women aged ≤ 43 y in the LCD (n = 13) and LFD (n = 10) groups.
At the end of follow-up, women in the LCD and LFD groups, in this age stratum, lost 5.5% and 5.1% of their initial weight without significant differences in the ALT (34.4 ± 9.4 and 39.5 ± 19.9 U/L, p = 0.47) and AST (31.4 ± 7.1 and 33.4 ± 11.7 U/L, p = 0.63) levels.
In the same way, there were not significant differences in:
- •
Body weight (93.3 ± 16.4 and 88.2 ± 15.5 kg, p = 0.29).
- •
TBF (46.1 ± 4.3 and 45.2 ± 5.4%, p = 0.61).
- •
AST (49.0 ± 44.8 U/L, p = 0.49).
- •
ALT (60.1 ± 20.8 and 53.0 ± 16.4 U/L, p = 0.30) levels, between the women aged > 43 years in the LCD (n = 15) and LFD (n= 16) groups.
At the end of follow-up, women in the LCD and LFD groups, in this age stratum, lost 5.9% and 6.0% of their initial weight without significant differences in the ALT (36.1 ± 13.6 and 33.5 ± 8.6 U/L, p = 0.53) and AST (34.2 ± 13.4 and 32.0 ± 6.8 U/L, p = 0.57) levels.
Furthermore, taking into account that glucose levels exert an adverse effect on ALT and AST, we analyzed changes in the ALT and AST levels at baseline and the end of follow-up between the women with diabetes in the LCD (n = 6) and LFD (n = 2) groups; there were not significant differences in the analyzed variables (Table 2).
Characteristics of the women with diabetes.
| Baseline | p value* | End of follow-up (six months) | p value* | |||
|---|---|---|---|---|---|---|
| LCD (n= 6) | LFD (n =2) | LCD (n = 6) | LFD (n = 2) | |||
| Weight (kg) | 89.9 (77.6–108.7) | 72.3 (69.9–74.7) | 0.07 | 87.1 (73.5–104.8) | 69.6 (69.2–70.1) | 0.70 |
| Total body fat (%) | 44.6 (42.5–50.9) | 40.0 (39.5–40-5) | 0.29 | 42.9 (40.9-50.8) | 38.2 (35.9–42.0) | 0.71 |
| Glucose (mg/dL) | 175.0 (129.7–238.2) | 125.5 (112–139) | 0.29 | 173.0 (143.0–216.2) | 281.5 (267.0–296.0) | 0.07 |
| AST (U/L) | 52.0 (42.5–66.2) | 31.0 (30.0–32.0) | 0.07 | 31.5 (24.5–41.0) | 33.0 (28.0–38.0) | 0.07 |
| ALT (U/L) | 54.0 (36.0–97.7) | 35.5 (31.0–40.0) | 0.29 | 32.5 (19.5–39.7) | 36.5 (35.0–38.0) | 0.64 |
Data are median (25-75 percentil).
Finally, in the overall group although decrease of AST (31.7 and 22.4%) and ALT (41 and 33.3%) levels was higher in the women of LCD group, as compared with the LFD group, there were not significant statistical differences between the groups (Figure 2).
DiscussionOur results show that, in the obese women with NAFLD, reduction of body weight decreases ALT and AST levels irrespective of the type of diet.
The elevated levels of ALT and AST, which prevalence increases progressively with the increase of BMI,18 are common in the subjects with obesity. The release, into the portal circulation, of free fatty acids from the increased depots of visceral adipose tissue, is among the mechanisms of hepatic injury.19 Thus, it is important to consider that in the obese patients, even in those without diagnosis of liver disease, increase of BMI and central obesity are major determinants for elevation of ALT levels and hepatic steatosis.12
Wong, et al.20 showed that weight reduction is associated with non-progression of hepatic disease and decrease of aminotransferase levels in patients with steatosis; finding that highlight the important role that interventions in life-style, focused in weight loss, plays in the strategies for improvement of health condition. It has been reported that LCD promotes favorable changes in cardiovascular disease risk factors and has greater beneficial effects in the induction of short term weight loss and the decrease of aminotransferase levels as compared with LFD 10,12,21–22
Our results showed that the weight loss as well as the decrease of AST, ALT, total cholesterol, and triglycerides levels were similar in the women who received LCD and LFD. These findings strongly suggest that, irrespective of type of diet, the metabolic changes depend of weight reduction per se.
Given that adherence to diet mainly depends of the aliment’s flavor, our results, showing that reduction of ALT and AST not depends of the type of diet, could be of importance in this field because dieticians may have alternatives to choose the proper diet for each individual in order to reduce the body weight with the concomitant reduction of ALT and AST levels.
Results of this study disagree with previous reports that show the advantages of LCD as compared with LFD,12,21,22 but agree with results showing that LFD is as effective as LCD for inducing weight loss, to decrease ALT levels, and to promote favorable changes in cardiovascular disease risk factors.23
Given that glucose levels have effect on ALT and AST levels and that there were an elevated proportion of women with diabetes in the LCD (n = 6) than in the LFD group (n = 2), we analyzed changes in ALT and AST in women stratified according the presence of diabetes. At baseline and the end of follow-up, glucose, ALT, and AST levels showed not significant differences between the groups in study. These finding suggest that, in the population in study, glucose levels are not related with changes in ALT and AST levels. However, because the number of diabetic women in study was small, further research is necessary to evaluate the relationship between glucose levels with ALT and AST levels among diabetic individuals who receive LCD or LFD.
Obesity, insulin resistance, elevated glucose and triglycerides levels are indicators of advanced stages of glucose metabolic disorders and liver fibrosis;24 on this regard, decrease of ALT, as consequence of weight loss, may be the result of decrease of insulin resistance induced by weight reduction.10 The statement that weight loss per se improves not only glucose metabolic disorders, but reduce AST and and ALT levels, requires further research.
Several limitations of this study deserve to be mentioned:
- •
Given that the primary trial end-point of the study was the change in ALT and AST levels in obese women with NAFLD, we did not repeat the hepatic ultrasound at the end of follow-up to verify changes in NAFLD. In addition, diagnosis of NAFLD was only established at baseline because it was an inclusion criterion, so, because changes in NAFLD are not related with the aim of study, absence of data about possible changes in the NAFLD do not affect our main conclusion.
- •
We did not perform hepatic biopsy; so, we have not hepatic histological data. Given that ALT could decline with worsening of hepatic fibrosis as NASH advances, we have not the certainty if the decline in ALT was secondary to weight loss or to the worsening of hepatic fibrosis. However, taking into account the design of the study that includes randomization of participants into the groups, it is expected that those women who had had worsening of hepatic fibrosis were similarly distributed in both groups, minimizing the analysis bias.
- •
The follow-up period of this study was only of six months; so, we have not the certainty if the changes in body weight as well as in the ALT and AST levels are sustained for long time; further research, with long follow-up period is required.
- •
The effect that LCD and LFD exert on the reduction of AST and ALT was evaluated only in women; so, our results can not be applied to men. Further research is needed in the field to verify our results and its possible application also in men.
The main conclusion of this study is that weight loss, irrespective of type of diet, reduces AST and ALT levels in obese women with NAFLD.