It has been 4 years since the first, long-term (> 3 years) prospective comparison of adult-to-adult living donor liver transplantation (A2ALLTx) to adult deceased donor liver transplantation (ADDLTx) was reported.1 In this follow up, prospective, IRB approved, 10-year comparison of A2ALLTx to ADDLTx we expand on our initial observations. This data includes: age, gender, ethnicity, primary liver disease, waiting time, pretrans-plant CTP/MELD score, cold ischemia time (CIT), perioperative mortality, acute and chronic rejection, graft and patient survival, charges and post-transplant complications.
In 10 years, 465 ADDLTx (81.37) and 107 A2ALLTx (18.7%) were performed at VCUHS. Hepatitis C virus (HCV) was the most common reason for transplantation in both groups (54.5% vs. 48.2%). Data regarding overall patient and graft survival and retransplantation rates were similar. Comparison of patient/graft survivals, retransplantation rates in patients with and without HCV were not statistically different. A2ALLTx patients had less acute rejection (9.6% vs. 21.7%) and more biliary complications (27.1% vs. 17.6%).
In conclusion, A2ALLTx is as durable a liver replacement technique as the ADDLTx. Patients with A2ALLTx were younger, had lower MELD scores, less acute rejection and similar histological HCV recurrence. Biliary complications were more common in A2ALLTx but were not associated with increased graft loss compared to ADDLTx.
Although liver transplantation (LTx) has undergone immense progress and is a curative treatment for most endstage liver diseases, shortage of donor organs continues to be the main obstacle in the treatment of this group of patients. With the increasing number of patients waiting for a liver transplant and the rising number of patients dying while waiting, many alternatives have been developed in the last decades with the intent to increase the liver donor pool. Some of these alternatives include: marginal donors, older donors (> 60 years), split LTx, HCV-positive donors and living donor LTx.
The use of live donors for LTx was initiated many years ago for children and small adults, among whom mortality was escalating as a direct result of the lack of deceased donors of appropriate size.2,3
In the last 10-15 years, adult-to-adult living donor LTx (A2ALLTx) has been developed on an international scale, multiplying the number of procedures performed and increasing the pool of liver donors.
Advantages and disadvantages of this technique are continuously under scrutiny. Some advantages include the increase of the pool of organs for transplantation, reduction of cold ischemia time (CIT) with an expected improvement in immediate post-transplant graft function, thorough donor and recipient evaluation and better organization of the timing for surgery. Disadvantages including donor risk, unclear standards regarding the donor, recipient selection and limits in the use of this technique, remain as difficult topics which still need clarification. Short-term outcomes after A2ALLTx seem to be comparable to deceased donor liver transplant based on experiences in Europe, Japan and the United States.4-6 However, the analyses of long-term outcomes and complications are still incomplete.
At our center the waiting list mortality from 1994 to 1997 averaged 6-15% per year. With the addition of a doubling recipient pool and regional sharing, the waiting list mortality doubled, reaching 21% per year in 1998, prompting our center to initiate an adult-to-adult living donor program in 1998. Since then, we have performed 107 such transplants in both emergent and more commonly elective circumstances. Donor safety has been our highest priority, followed closely by assurance of a functional graft and prevention of unnecessary recipient complications. This report describes the 10 years of follow-up in our A2ALLTx program. Data were collected prospectively, and the outcome of the A2ALLTx group is compared with the contemporaneous group of adult deceased donor LTx (ADDLTx) patients performed at VCU Health System, Medical College of Virginia Hospitals.
Materials and MethodsThe study group included 107 right lobe (RL) adult liver recipients, and the contemporaneous group of 465 ADDLTx performed at our institution from March 1998 to October 2009. A2ALLTx technique was offered only to patients eligible and listed on the UNOS liver transplant waiting list.
A2ALLTx donor selection and operationDonors were not solicited in any way and they had to express their intent to donate before discussing this option with any representative of the transplant team. Preoperative donor evaluation proceeded if ABO compatibility to the recipient was verified along with acceptable donor results from psychological and medical evaluations as previously published from our group.7 Intraoperative imaging included cholangiography and ultrasonography as the guide for transection; the middle hepatic vein was always left with the donor. A detailed technical description has already been described by our group.7 In brief, a bilateral, sub costal, with midline extension incision to the xiphoid was performed in all donors. The cystic duct was first identified with minimal dissection and an intraoperative cholangiogram was performed followed by a cholecystectomy. Intraoperative ultrasonography (IUS) was facilitated by mobilization of the RL. Simple division of the attachments to the diaphragm was adequate at this point. The left lobe and diaphragmatic attachments to the left of the middle hepatic vein from above, and to the left of the portal vein confluence below were never disturbed. IUS was used to define the course and relationship of the middle hepatic vein (MHV) to the right hepatic vein (RHV) as they drain into the vena cava, the presence and significance of accessory hepatic veins draining the RL, the least vascular plane of transection and the anatomy of the portal vein branches to segment 4 in relation to this intended plane. The superior surface of the liver was marked using the argon beam, approximately 0.5-1 cm to the right of the MHV, for later transection. A major ‘learning curve’ point was the moving to the left on the final third parenchymal transection anteriorly to the bile duct to prevent skeletonizing the anterior right hepatic duct after sharp transection of the bile duct at the bifurcation.8 After completing the parenchymal transection, vascular clamps were applied on the right hepatic artery at least one half centimeter to the right of the bifurcation followed by transection. Next, the portal vein was clamped at right angle (instead of parallel as originally described.7 one half centimeter to the right of the portal vein bifurcation and transected, with the left thumb placed over the transected right portal vein for hemostasis. Finally, accessory hepatic veins greater than 0.5 cm were clamped at their IVC vein junction and divided above the clamps. A Satinsky vascular clamp was placed on the RHV IVC junction and divided above the clamp. The RL was then passed off to the back table and the artery, veins and bile ducts were flushed with 1 L of ice cold UW solution.
A2ALLTx recipient selection and operationRecipients were not considered for A2ALLTx unless they had been listed for deceased donor transplantation according to UNOS criteria (available at: www.unos.org). A graft-to-recipient body weight ratio determined by MRI liver volume measurement of at least 0.8% was the minimum acceptable graft volume requirement. RLs were piggybacked to the native RHV orifice. The main portal vein was anastomosed to the donor right portal vein in end-to-end fashion. After reperfusion of the graft the arterial anastomoses was performed between the donor RHA and the recipients’ hepatic artery at the bifurcation of the right and left recipient artery stump. If this vessel appeared to be inadequate, a jump graft to the aorta was constructed with recipient saphenous vein. From June 1998 to August 2000, a Roux limb was used routinely for biliary reconstruction. As of September 2000, a duct-to-duct anastomoses has been the preferred biliary tract reconstruction technique used, unless recipient duct length and vascularity was felt technically inadequate. Bile duct stenting was used in all duct-to-duct anastomoses. Venous-venous bypass was used during IVC clamping in all cases.
Liver biopsies and follow-upAlmost all liver recipients (236/325 [73%]) ADDL-Tx and all surviving A2ALLTx recipients received protocol liver biopsies (LBx) at 6-, 12-, and 24-month post-transplantation. Furthermore, additional LBx were performed as needed to evaluate for abnormal liver chemistries (doubling of transamina-ses). Patients received follow-up with weekly visits the first month, every 15 days until 3 months, monthly until 6 months and then every 3 or 6 months for 2 years, followed by annual visits thereafter. HCV recurrence was defined as a Knodell necroinfla-mmatory score of ≥ 3 on liver biopsy.9
Data collection and statistical analysisThis is a prospective, IRB approved study with a university supported and approved registry. We performed a comparison of A2ALLTx to ADDLTx. Fisher’s exact test, χ2 test and t-test were used to individually compare age, gender, ethnicity, primary liver disease, waiting time, pretransplant CTP/ MELD score, perioperative mortality, acute and chronic rejection, and posttransplant complications. Graft survival and patient survival were estimated using Kaplan-Meier analysis and compared with a log-rank test. Variables statistically different were tested for inclusion in multivariate Cox proportional hazard models.
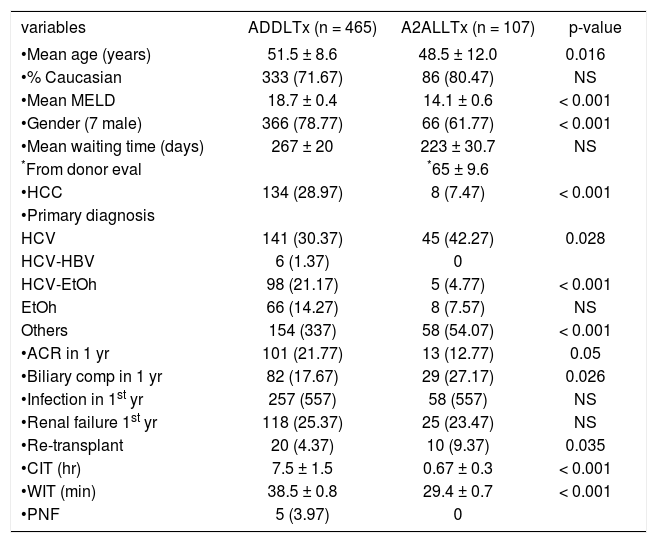
ResultsADDLTx and A2ALLTx demographics (Table 1)From March 1998 to October 2009, 572 liver transplants were performed in S44 adult patients including liver retransplant patients (pediatric and multiple organ transplants are excluded in this comparison). The groups studied included 465 grafts for ADDLTx (81.3%) and 107 grafts for A2ALLTx (18.7%). The predominant ethnicity was Caucasian (71.6-80.4%). Univariate analysis comparing ADDLTx to A2ALLTx showed no difference in ethnicity, waiting time or perioperative mortality. Recipients receiving A2ALLTx were younger (48.5 vs. 51.5; p = 0.016), were less likely to be male (61.7% vs. 78.7%: p = 0.001) and less likely to have hepatocellular carcinoma (HCC) (7.4% vs. 28.9%: p = 0.001). The mean follow-up time for the A2ALLTx group was 1660 ± 1400 days compared to 1379 ± 1115 days for the ADDLTx group (10 years post-transplant).
Patient demographics.
| variables | ADDLTx (n = 465) | A2ALLTx (n = 107) | p-value |
|---|---|---|---|
| •Mean age (years) | 51.5 ± 8.6 | 48.5 ± 12.0 | 0.016 |
| •% Caucasian | 333 (71.67) | 86 (80.47) | NS |
| •Mean MELD | 18.7 ± 0.4 | 14.1 ± 0.6 | < 0.001 |
| •Gender (7 male) | 366 (78.77) | 66 (61.77) | < 0.001 |
| •Mean waiting time (days) | 267 ± 20 | 223 ± 30.7 | NS |
| *From donor eval | *65 ± 9.6 | ||
| •HCC | 134 (28.97) | 8 (7.47) | < 0.001 |
| •Primary diagnosis | |||
| HCV | 141 (30.37) | 45 (42.27) | 0.028 |
| HCV-HBV | 6 (1.37) | 0 | |
| HCV-EtOh | 98 (21.17) | 5 (4.77) | < 0.001 |
| EtOh | 66 (14.27) | 8 (7.57) | NS |
| Others | 154 (337) | 58 (54.07) | < 0.001 |
| •ACR in 1 yr | 101 (21.77) | 13 (12.77) | 0.05 |
| •Biliary comp in 1 yr | 82 (17.67) | 29 (27.17) | 0.026 |
| •Infection in 1st yr | 257 (557) | 58 (557) | NS |
| •Renal failure 1st yr | 118 (25.37) | 25 (23.47) | NS |
| •Re-transplant | 20 (4.37) | 10 (9.37) | 0.035 |
| •CIT (hr) | 7.5 ± 1.5 | 0.67 ± 0.3 | < 0.001 |
| •WIT (min) | 38.5 ± 0.8 | 29.4 ± 0.7 | < 0.001 |
| •PNF | 5 (3.97) | 0 |
Represents patient demographics from 46S ADDLTx and 107 A2ALLTx performed at VCU-MCVH from March 1998 to October 2009.
One of the major advantages of A2ALLTx is to shorten the waiting time,10 although at our institution no significant difference was found, as documented in Table 1. The explanation can be found when reviewing this group case by case. At the beginning of this program, most patients on the list were offered this technique. Most patients who underwent A2ALLTx during the first 3 years of the program had a long waiting time before the transplant was performed (mean 223 ± 30.7 days). When the time between the donors’ first visit and the transplant operation was calculated, it was found that recipient’s waiting time was just 65 ± 9.6 days. This was the average time from the donor’s first visit to evaluation and then transplant.
ImmunosuppressionThe same immunosuppressive regimen was used for both groups. All patients received 1.S g of mycophenolate mofetil (MMF) administered orally 4-6 h before OLT. During the first week after OLT, the MMF dose was 3 g/day if operative blood loss < 10 uRBC and 2 g/day if ≥ 10 uRBC. MMF was reduced to 2 g/day after 7 days and to 1 g/day by 6 months. On postoperative day 2 (changed to postoperative day 3 in 2001), neoral (8 mg/kg/day) or tacrolimus (0.10 mg/kg/day) was administered by mouth, twice per day. Prednisone was tapered to 20 mg/day by day 18, 7.S mg/day by 3 months and S mg/day by 6 months.11 Sirolimus was added to the protocol in 2000 for patients with serum creatinine ≥ 2 mg/dL, combined with half dose, delayed initiation of tacrolimus or cyclosporine (on day S postoperative) and steroids tapered off by 1-3 months. A second major ‘learning curve’ point was the need for less immuno-supression, learned after the first 23 living donor grafts.8 This led us to keep tacrolimus trough levels at a target of 7-10 ng/mL by TDx assay (from 10 to 15 ng/mL), taper MMF over 2 months along with steroids and to delay starting calcineurin postop until the morning of day 3 in living donor and deceased donor recipients.
Cold ischemia time and primary nonfunctionCIT was different, and as expected shorter in A2ALLTx:40.2 ± 18 versus 450 ± 90 min (p = 0.001). Five ADDLTx patients (3.9%) had primary nonfunction (PNF), with two patients successfully re-transplanted within 5 days, two patients dying while awaiting retransplant and one was not a candidate for re-transplant. There were no cases of PNF in the A2ALLTx group. The mean graft-to-recipient body weight volume ratio (calculated by actual right lobe weight) was 1.1 ± 0.3% in the A2ALLTx group.
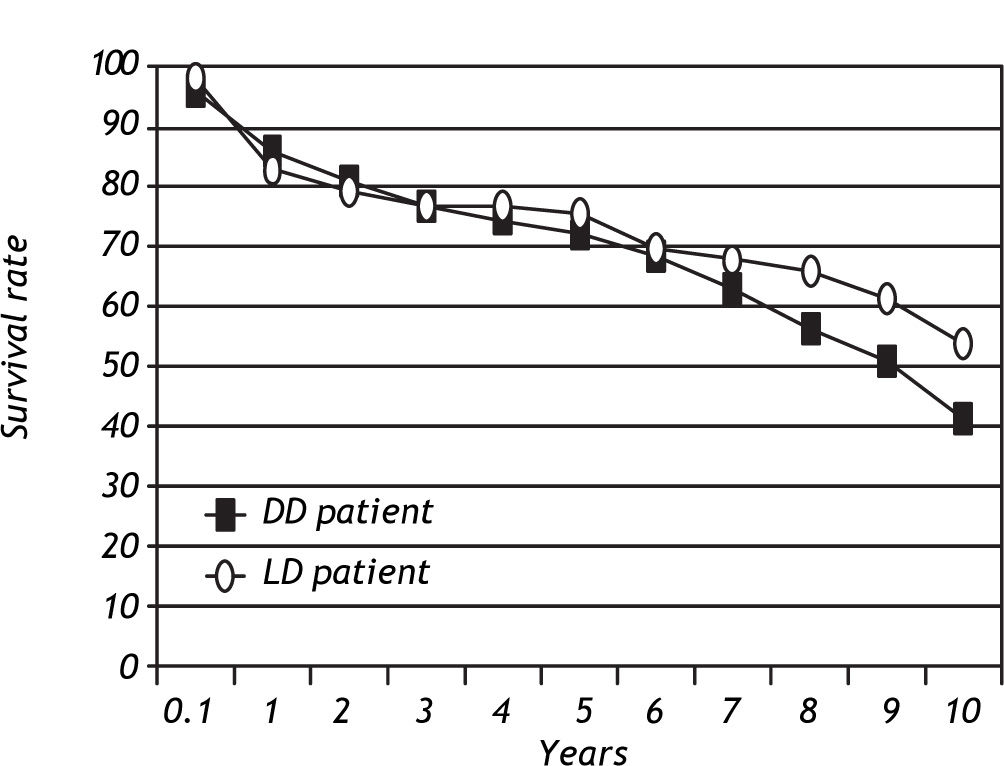
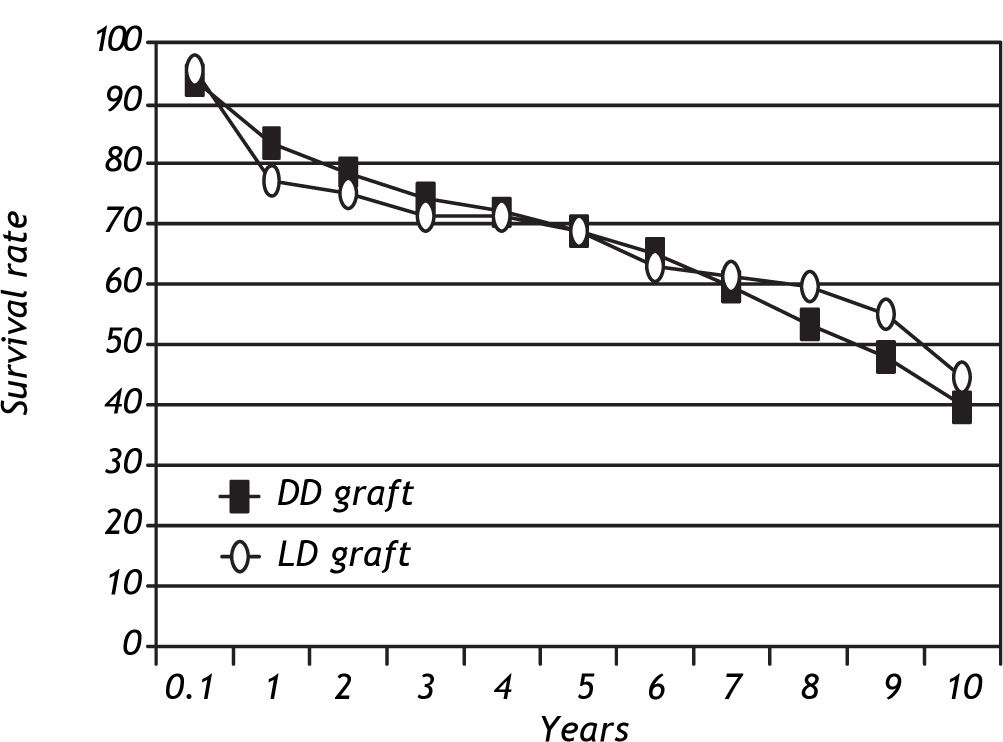
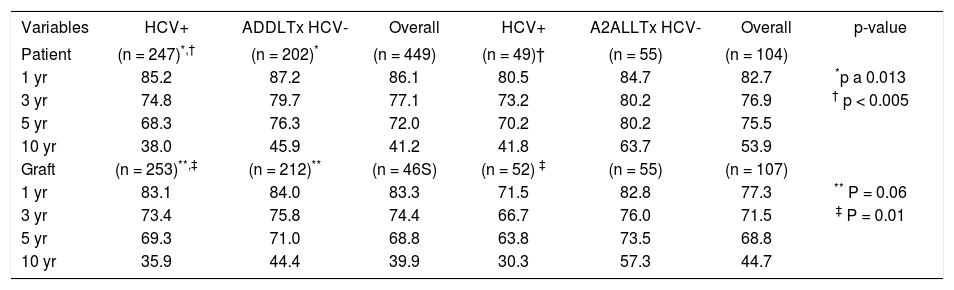
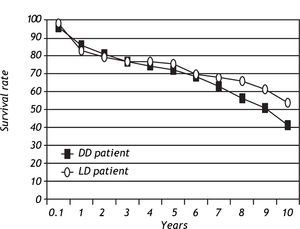
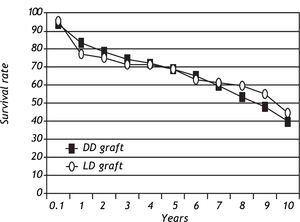
Patient and graft survivalData regarding overall patient and graft survival are shown in Figures 1 and 2. Patient survival at 1, 3 and 5 years was 86.1%, 77.1% and 72.0% in the ADDLTx group, and 82.7%, 76.9% and 75.5% in the A2ALLTx group. Graft survival at I, 3 and 5 years was 83.3%, 74.4% and 68.8% in the ADDLTx group, and 77.3%, 71.5% and 68.8% in the A2ALLTx group. No statistical differences in patient or graft survival were observed between the groups at S years, with both groups achieving a 5-year patient survival of 70% or greater. There was a nonsignificant trend of better graft survival at 5 years in A2ALLTx. Because HCV was the primary diagnosis in the majority of patients coming to transplantation, we also subdivided both groups into HCV-posi-tive (+) and HCV-negative (-) subgroups. When comparing patient and graft survivals (Table 2) between HCV+ and HCV-patients in ADDLTx and A2ALLTx groups, no significant survival differences were found, but important clinical differences are explained later under the HCV infection section. As previously described by our institution,12 the recurrence of biopsy-proven HCV was similar in the A2ALLTx and ADDLTx groups. The retransplantation rate was statistically different and higher in the HCV+ A2ALLTx subgroup (Table 2) in this HCV subgroup analysis. Eleven of 253 HCV+ ADDLTx patients were retransplanted; 3 of the 11 were for recurrent HCV. Six of 52 HCV+ A2ALLTx patients were retransplanted, one was for recurrent HCV.
Patient and graft survival by HCV diagnosis.
| Variables | HCV+ | ADDLTx HCV- | Overall | HCV+ | A2ALLTx HCV- | Overall | p-value |
|---|---|---|---|---|---|---|---|
| Patient | (n = 247)*,† | (n = 202)* | (n = 449) | (n = 49)† | (n = 55) | (n = 104) | |
| 1 yr | 85.2 | 87.2 | 86.1 | 80.5 | 84.7 | 82.7 | *p a 0.013 |
| 3 yr | 74.8 | 79.7 | 77.1 | 73.2 | 80.2 | 76.9 | † p < 0.005 |
| 5 yr | 68.3 | 76.3 | 72.0 | 70.2 | 80.2 | 75.5 | |
| 10 yr | 38.0 | 45.9 | 41.2 | 41.8 | 63.7 | 53.9 | |
| Graft | (n = 253)**,‡ | (n = 212)** | (n = 46S) | (n = 52) ‡ | (n = 55) | (n = 107) | |
| 1 yr | 83.1 | 84.0 | 83.3 | 71.5 | 82.8 | 77.3 | ** P = 0.06 |
| 3 yr | 73.4 | 75.8 | 74.4 | 66.7 | 76.0 | 71.5 | ‡ P = 0.01 |
| 5 yr | 69.3 | 71.0 | 68.8 | 63.8 | 73.5 | 68.8 | |
| 10 yr | 35.9 | 44.4 | 39.9 | 30.3 | 57.3 | 44.7 |
Shows patient and graft survival for ADDLTx and A2ALLTx based on HCV diagnosis (positive [+] or negative [-]) along with overall survival.
A team of liver pathologists and hepatologists evaluated the LBx’s. Banff 1997 criteria13 were used to diagnose severity classified as: no acute rejection, mild acute rejection, moderate acute rejection and severe acute rejection. The incidence of acute rejection (of any severity) at 1 year was significantly higher in the ADDLTx group (21.7% vs. 12.7%; p = 0.05). Severity of rejection based on the need of thymoglobulin (ATG) for severe rejection versus steroid (St) use (3-day boluses) for mild or moderate rejection was (A2ALLTx group: 8%, 4.7%; and ADDLTx group: 8.4%, 14.4%, respectively). A2ALLTx group had less rejection but episodes were severe at a similar rate for both groups. The diagnosis of chronic rejection at 3-5 year follow-up was similar in both groups 2.0% in A2ALLTx versus 1.1% in ADDLTx group.
Biliary complications in year 1 were more common in the A2ALLTx patients with an incidence of 27.1% versus 17.6% in the ADDLTx group (p ≤ 0.026). In the A2ALLTx group, biliary complications consisted of leak (most commonly a cut surface leak with operative drain left in place for treatment) 8%, stricture 11% and both 4%. Of the patients that had a leak, 93% did not require surgical or radiologic intervention due to liberal use of operative drains and anastomotic stents (learned after the first 10 A2ALLTx recipients).7 One important complication in the A2ALLTx group was seen in two patients (2%), who had excluded posterior segment bile ducts (these two aberrant bile ducts were 1-2 mm diameter ducts entering the common hepatic duct just above the cystic duct, one was reimplanted with subsequent stricture and the second was considered to small to implant and clipped). One patient had multiple session intraluminal alcohol ablations of the duct but subsequently required retransplantation and the second patient developed a biliary-pleural fistula controlled by chronic drainage which subsequently resolved. In the A2ALLTx recipient group with biliary complications (18/104 patients) there were five retransplants; one retransplant specifically due to intrahepatic sepsis. There were five deaths (three deaths after retransplant), at days 80, 142, 199, 212 and 442. No deaths were directly related to biliary sepsis.
HCV infection and liver transplantationHCV was the most common etiology of end-stage liver disease leading to transplantation in both groups. In the ADDLTx group, 253 (54.5%) of 465 transplants and 51 (48.2%) of 107 liver transplants in the A2ALLTx group were HCV + . Histological confirmation of recurrence of HCV, within the first year, was observed in 37% of the ADDLTx group, and in 13% of the A2ALLTx group (p = 0.07). Fibrosis was present in 27% of surviving living donor liver transplant recipients at 36 months, compared to 36% of deceased donor liver transplant patients (p = 0.18).12 As shown in Table 2, patient survival, graft survival and retransplant rates were not significantly different at 1-, 3-and 5-year post-transplantation. However, unlike the HCV-subgroups, the HCV+, A2ALLTx patients and graft survivals were 12-15% lower at 1 and 3 years, compared to the ADDLTx patient and graft survivals.
Donor outcomeThere were more male (60%) than female donors (40%). Mean donor age in the A2ALLTx group was 37.3 ± 9.0 years old, similar to the ADDLTx (37.2 ± 16.6 donors (NS). There was no perioperative or longterm mortality in donors at our institution (14). Complications included: 4 incisional hernias (4%), 5 brachial plexus neuropraxis (5%), 1 prolonged ileus, 3 nonfatal postdonation pulmonary embolism, 1 right hepatic vein clamp leak, 7 pleural effusions and 2 medically treated depressions due to poor recipient outcome. Two, 10 F drains, were placed introperatively along the cut surface of the liver on all donors. Only 3 donors required one JP drain to be left in for greater than 7-10 days because of bile staining (3 weeks in two patients, and 4.5 weeks in one patient, without further sequelae).
A2ALLTx experience over time in our institutionEvaluating just the A2ALLTx recipient group over time, we compared the first 23 cases (learning curve time designated by RAF, due to significant surgical, medical, and program policy changes)1versus the subsequent 82 cases of A2ALLTx recipient outcomes. Patient, graft survival and retransplantation rate are shown in Table 3. One-, 3-and 5-year patient and graft survival improved in the more recent group (p = 0.05). Sepsis was the most common complication in the A2ALLTx group; this was more common in the first era (57%) than in the second era (10%) (p ≤ 0.001). The most common cause of death in the first era A2ALLTx group was sepsis, five (36%) out of fourteen patients versus two (11%) out of 19 patients deaths in the last 82 patients were related to sepsis. Changes in the immunosu-pression regimen (wean steroids to 5 mg/day by 1 month and delay calcineurin use at half the dose until post-operative day 3) and recipient selection (no UNOS 2A recipients or MELD > 25 recipients) followed evaluation of these 23 cases resulting in significant improvement in survival. This learning curve, with similar case numbers and lessons of importance, was independently described by the nine US Adult to Adult Living Donor Liver Transplant centers as part of an NIH, ASTS, DHSS grant supported consortium studying A2ALLTx.15
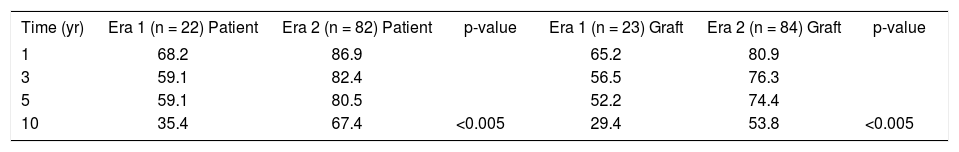
Comparison of two eras using A2ALLTx.
| Time (yr) | Era 1 (n = 22) Patient | Era 2 (n = 82) Patient | p-value | Era 1 (n = 23) Graft | Era 2 (n = 84) Graft | p-value |
|---|---|---|---|---|---|---|
| 1 | 68.2 | 86.9 | 65.2 | 80.9 | ||
| 3 | 59.1 | 82.4 | 56.5 | 76.3 | ||
| 5 | 59.1 | 80.5 | 52.2 | 74.4 | ||
| 10 | 35.4 | 67.4 | <0.005 | 29.4 | 53.8 | <0.005 |
Compares the two eras of A2ALLTx at VCU-MCVH. Era 1 spans the first 23 A2ALLTx cases (designated as the learning curve by RA Fisher) and Era 2 spans the remaining A2ALLTx cases performed at VCU-MCVH.
Recipient selection changed with time and experience. At the beginning, patients with UNOS listing status 2A were transplanted with A2ALLTx. Four out of 23 patients were UNOS status 2A at transplant time and this small group of patients showed a survival of 50% at 1 month, 25% at 1 year and 0% at 3 years. Survival of this group was far lower than the contemporaneous group of UNOS status 2B-3 (n = 19 patients), which showed 1-month, 1-year, 2-year and 5-year, survival of 100%, 73.7%, 73.7% and 68.4%, respectively.
All the patients in the UNOS 2A group required longer ICU stay and died of sepsis with concomitant acute renal failure. Transplant candidates with UNOS status 2A (> 2S MELD points) requiring ICU care were no longer accepted as candidates for A2ALLTx in the subsequent 82 cases.
Biliary duct anastomosis was changed with experience. The first group of patients (n = 23) all received ‘Roux-en-Y’ biliary enteric anastomosis (the first 10 recipients without biliary internal stents). The technique was changed to a duct-to-duct anastomosis with internal transampulary silastic stents in the second period of this program.16 Of the total A2ALLTx, 77 have been fully analyzed for biliary tract morbidity with a >3 year follow up. Of these S0 cases (6S%) were Roux-en-Y and 27 cases (3S%) were duct to duct. Eight of the S0 in the Roux-en-Y group (16%) experienced anastomotic strictures versus six of the 27 in the duct to duct group (22.4%), with one of the 8 in the Roux-en-Y group requiring surgical revision and four of the six in the duct to duct group requiring surgical revision.
There were no vascular complications leading to graft loss and only one graft lost due to a parenchy-mal fracture in the first 23 living donor recipients. This case is the subject of a previous report.17 In the second era of 82 A2ALL recipients, there was one immediate outflow venous thrombus complication leading to graft loss with successful salvage with re-transplant. One noncompliant recipient developed rejection and CMV hepatitis with late hepatic artery thrombosis at 6 months requiring retransplantation with a deceased donor allograft.
A Cox proportional model identified non-white recipients, HCV, older donors, white donors, DCD donors, increased ICU time, cholestasis, infection and ARF in the first year as significant predictors of reduced graft survival. Donor type, HCC, recipient age, donor gender, recipient gender, MELD, CIT, WIT, ARF during initial admission, LOS and biliary complications and ACR in the first year were also tested but where not independent predictors of graft loss.
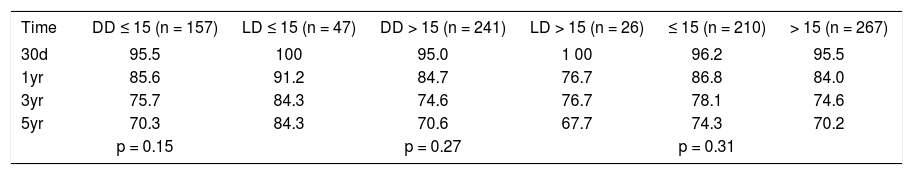
The transplant center where this study was conducted is a medium size liver transplant program. Donor and recipient characteristics; graft and patient survivals, were similar to those medium and high volume centers, where the volume of practice is associated with a center effect predictive of better and less variable transplant outcome, as defined by Axelrod et al.18 SRTR/URREA data highlighted the significant survival advantage of liver transplant, compared to pretransplant death, when the recipients MELD > 17. Recipients with a MELD score < 15 had no demonstrable survival benefit from liver transplant.19 Also, the ratio between MELD (> 15, < 15) and adult living donor liver graft volume was used to accurately predict postoperative recipient recovery.20 Patient survival of 477 adult living donor and deceased donor liver recipients, (out of the 572 total liver recipients), who had prospective data collected to calculate MELD scores, was analyzed by immediate actual pretransplant MELD ≤ 15 and MELD >15 (Table 4). There was no difference in 30-day, 1-year, and 2-year patient survival of living donor liver transplant recipients compared to deceased donor liver recipients in either MELD group (Table 4).
MELD stratified patient survival.
| Time | DD ≤ 15 (n = 157) | LD ≤ 15 (n = 47) | DD > 15 (n = 241) | LD > 15 (n = 26) | ≤ 15 (n = 210) | > 15 (n = 267) |
|---|---|---|---|---|---|---|
| 30d | 95.5 | 100 | 95.0 | 1 00 | 96.2 | 95.5 |
| 1yr | 85.6 | 91.2 | 84.7 | 76.7 | 86.8 | 84.0 |
| 3yr | 75.7 | 84.3 | 74.6 | 76.7 | 78.1 | 74.6 |
| 5yr | 70.3 | 84.3 | 70.6 | 67.7 | 74.3 | 70.2 |
| p = 0.15 | p = 0.27 | p = 0.31 |
Demonstrates patient survival when MELD score is taken into account. This represents ADDLTx, A2ALLTx performed at VCU-MCVH.
At our institution the A2ALLTx program was started in 1998. This report covers a 10-year experience and a prospective comparison of deceased donor adult LTx (i.e. ADDLTx) to A2ALLTx. Our data show that patients (donors and recipients) undergoing A2ALLTx tend to be younger, have lower MELD scores (calculated for all recipients from 1998 to 2002 prior to MELD’s institution), which gives the recipient the needed edge to receive and tolerate such an operation (Table 1). Along with expected shorter waiting time, CIT and scheduled operations, similar or better outcomes to the adult deceased donor liver recipient was our hope. MELD score by itself was a significant variable in a logistic regression model of 1-year patient survival but by itself is not a valuable predictor of survival time based on Cox regression analysis. Perhaps, the variation in outcomes is so great, and so dependent on post-transplant complications, that pretransplant status using MELD is not a significant predictor by itself, given the limited sample size of this study. Heartening was the finding that survival rates were similar between living donor and deceased donor groups for MELD ≤ 15 and >15. Thus, a ‘less sick’ living donor patient is not endangered by his or her ‘living donor decision’ compared to a similar ‘less sick’ deceased donor recipient. However, our sample of patients with prospective MELD scores was too small and too dependent on measurable but beyond the scope of this study post-transplant factors to quantify the hoped for average improved outcomes in the living donor liver recipient (A2ALLTx) group. Most importantly, the question of: “Is it riskier to wait on the waiting list for a deceased donor or accept the risk of receiving a partial liver graft from a living donor at an earlier time point?” has been definitely answered by the NIH sponsored A2ALL consortium. Taking the A2ALLTx option significantly reduces the risk of dieing while waiting on the waiting list.21
Our data revealed that A2ALLTx patients had fewer episodes of ACR (9.6% vs. 21.7%) along with a decreased number of antirejection treatments than their concurrent ADDLTx counterparts. Although biliary complications were greater in the A2ALLTx group (27.1% vs. 17.6%), 92% of the biliary complications were resolved by endoscopic or radiologic, nonoperative intervention and did not result in graft failure.
Our results showing significantly fewer episodes of ACR in the A2ALLTx are in concordance with other center experiences.22 When acute rejection severity was evaluated based on the type of treatment needed (steroids vs. ATG), no statistical difference was found. The immunosuppressant protocol was the same used for A2ALLTx and ADDLTx at our institution and included a significant reduction in prednisone and a delay in calcineurin use, and the use of sirolimus starting in year 2000 in patients with an elevated creatinine (≥ 2 mg/dL) presumed secondary to hepatorenal syndrome. As we described previously,1,8 less aggressive immunosuppression seems to be well tolerated in the A2ALLTx group without increasing the ACR rate and minimizes septic complications and mortality as evidenced by the improved survival after the initial learning curve. Our single center rejection results seemed at odds with the larger, NIH A2ALL cohort study report that incidence and severity of acute cellular rejection was no different in A2ALLTx compared to ADDLTx.23 A surprising finding of the A2ALL cohort study was that 50 minutes of cold ischemia time increased the risk of ACR equivalent to a DDLT allograft with 380 minutes of cold ischemia time.23 When the cohort data center blinding was unlocked for study publication, we learned that we were grouped in the center effect showing significant less biopsy proven ACR in A2ALLTx compared to ADDLTx and the cold ischemia time in our center when compared to other centers, in the cohort study graft subgroup (A2ALLTx vs. ADDLTx), was significantly shorter in the right lobe liver grafts. In fact the expected incremental difference in rejection was proportional to the cold ischemia time difference (Table 1). These findings were unplanned and a result of programmatic decisions in 1998 to perform donor and recipient operations in adjoining operating rooms simultaneously with 2 experienced surgical teams communicating to shorten anesthesia time for donor and recipient.
HCV recurrence post-A2ALLTx continues to be a topic under discussion. Early reports suggested a worse outcome for HCV patients undergoing A2ALLTx reflected by a more rapid and more severe HCV recurrence in the A2ALLTx group.24,25 One theory proposed is that regeneration of the small A2ALLTx graft induces HCV recurrence and fibrosis. However, these preliminary reports did not standardize immuno-supression and liver biopsy protocols between living donor and deceased donor transplant grafts.24,25 On the other hand, we12 and other groups26,27 have reported similar outcomes in HCV+ recipients from living and deceased donors. A recent report from Gaglio and associates and Russo, et al.27,28 in retrospective evaluations strongly suggest that performing A2ALLTx in recipients with chronic HCV is both, safe, effective and results in a similar short-term outcome as in patients who undergo ADDLTx.
This 10-year prospective study (with histologic monitoring) comparing A2ALLTx to ADDLTx revealed no difference in the frequency or severity of recurrence of HCV injury in the graft, or need for retransplantation. HCV recurrence has been convincingly shown to be related to ACR episodes and bolus steroid treatments.29 The increase in the number of ACR and rejection treatment episodes in the ADDLTx group might explain the higher (but not significantly different) increase in the HCV recurrence in the deceased donor group in our experience. Also, as we reported previously,12 the higher HCV recurrence as measured by the increased biochemical activity at the first year post-A2ALLTx tends to revert over time (after 36 months). The initial learning curve 16% to 23% lower 1-and 3-year patient and graft survival in the HCV+ living donor recipient subgroup was not significant due to small numbers but importantly reflects the learning curve of ‘too little too late’ in the transplantation of UNOS status 2A patients with HCV as learned simultaneously by the University of Colorado group.30 This experience is responsible for our programmatic practice that excludes recipients with chronic liver disease, not acute liver failure, with MELD score > 25 from A2ALLTx. Finally, our HCV findings reported in a prospective 6 year study of A2ALLTx to ADDLTx, first published in 20051 and now updated 10 year follow up, are corroborated by the retrospective study of hepatitis C virus infected transplant recipients in the 9 center A2ALL cohort study. Allograft and patient survival and the development of advanced fibrosis were compared among 181 A2ALLTx recipients and 94 ADDLTx recipients. The 3 year graft and patient survival in HCV infected recipients of A2ALLTx and ADDLTx, after the initial learning curve of > 20 A2ALLTx at each center, were not significantly different. The most important predictor of graft loss in HCV infected patients were: 1) < 20 living donor liver transplant experience; 2) pretransplant HCC and 3) higher MELD at transplantation.31
From the initiation of the A2ALLTx program at our center, yearly donor follow-up after the first year was offered to all donors, to include surgical, medical, financial and social work follow-up and psychological support as needed for a planned 10 years. In particular, biliary health has been documented by yearly laboratory study (Tbili, alk phos) and MRCP as indicated.14 Quality-of-life instruments were not prospectively studied in our donors and, as in most of the United States A2ALLTx experience, are a shortcoming of a valuable part of the psychosocial follow-up.32 Fortunately, a prospective quality of life assessment specifically designed for adult liver donors will be an important part of the second phase of the National Institutes of Health-sponsored A2ALLTx cohort study beginning in 2009.
Finally, A2ALLTx is as durable a liver replacement technique as the ADDLTx at 10-year follow-up for patients with or without HCV infection.
AcknowledgementThe authors would like to acknowledge Jose M. Rodriguez for his technical and typographical expertise.
Abbreviations- •
A2ALLTx: Adult to Adult Living Donor Liver Transplantation
- •
ADDLTx: Adult Deceased Donor Liver Transplantation
- •
CTP/MELD: Child-Turcotte Pugh Score/Model for End Stage Liver Disease Score
- •
CIT: Cold Ischemia Time
- •
HCV: Hepatitis C Virus
- •
LTx: Liver Transplant
- •
RL: Right Lobe
- •
IUS: Intraoperative Ultrasonography
- •
MHV: Middle Hepatic Vein
- •
RHV: Right Hepatic Vein
- •
LBx: Liver Biopsies
- •
HCC: Hepatocellular Carcinoma
- •
MMF: Mycophenolate Mofetil
- •
PNF: Primary Nonfunction
- •
ACR: Acute Cellular Rejection
- •
IRB: Investigational Review Board
- •
UNOS: United Network of Organ Sharing
- •
OLT: Ortotopic Liver Transplant
- •
ARF: Acute Renal Failure