The interleukin-2 receptor antagonist; basiliximab is used to allow delayed introduction of Calcineurin inhibitors (CNI) after liver transplantation and thus delay their renal insult. However, there is only little evidence for the safety and the efficacy of this regimen. This study aimed to evaluate the effectiveness and safety of basiliximab induction in liver transplantation.
Materials and methodsThis study included 89 patients who were classified into two groups: standard triple immunosuppression (IS) regimen of steroid, tacrolimus (TAC) and mycophenolate mofetil (MMF) (n = 47) and induction IS regimen of basiliximab, low dose steroids and MMF with delayed introduction of CNI (n = 42). All patients were followed after liver transplantation for at least six months or until death.
ResultsThere were no significant differences in patient survival, graft dysfunction, infection rate or type, or wound healing between both groups. The acute rejection rate was equivalent in both groups. Renal dysfunction in the first six months post-transplant was less in the basiliximab group in comparison to the other group (7.1% and 19.1% respectively).
ConclusionBasiliximab-induced IS protocol is a safe regimen that reduces medium-term renal dysfunction and achieves similar survival without increasing the acute rejection or infection rate in liver transplantation recipients.
The calcineurin inhibitors (CNIs): cyclosporine and tacrolimus, have had a revolutionary effect on the overall success of liver transplantation through reduction in early immunological injury and acute rejection rates [1,2]. Although CNIs have markedly improved survival after liver transplantation; their use is associated with an increase in renal failure and adverse effects resulting in significant morbidity after liver transplantation [3]. Renal dysfunction within 3–6 months after surgery is a predictive factor for long-term renal insufficiency [4]. CNIs also contribute to the development of diabetes mellitus, dyslipidemia, hypertension, and oxidative stress; all of which contribute to cardiovascular morbidity [5].
The interleukin-2 receptor antagonist; basiliximab (Simulect®) is a chimerized monoclonal antibody; its effects are sustained for 1–2 months post administration [6]. As the risks associated with basiliximab are low, it is used most often in adults with renal function impairment to allow delayed introduction of CNI and thus delay their renal insult until renal function improves [7]. However, there is only little evidence for the safety and efficacy of this CNI free regimen. Hence, this study was conducted to evaluate the effectiveness and safety of basiliximab induction in patients undergoing liver transplantation as a means to decrease early post-transplant renal impairment and CNI adverse effects.
2Patients and methodsThis was a randomized controlled trial. The study included 89 adult patients aged 18 years or older who underwent living donor liver transplantation (LDLT) in the National Liver Institute, Menoufiya University, Egypt from May 2014 to May 2016. All patients were followed up after LDLT for at least six months or until death.
The study was conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board of National Liver Institute (NLI IRB 00003413), Menoufiya University, Egypt (approval no. 00111/2014). Written informed consents were obtained from both donors and recipients regarding surgery and research. A special consent was taken from patients who were given basiliximab as an induction immunosuppression regimen. Patients were classified according to the immunosuppressive regimen into two groups: standard triple immunosuppression (IS) regimen of steroid, tacrolimus (TAC) and mycophenolate mofetil (MMF) (n = 47) and induction IS regimen of basiliximab, low dose steroids and MMF with introduction of small dose of CNI on day 3–6 (n = 42).
In the first regimen, methylprednisolone was administrated as a 500 mg i.v. bolus at the time of graft reperfusion. It was then administrated at 1 mg/kg per day from day 1 to day 3, at 0.5 mg/kg per day until day 6 and at 0.3 mg/kg per day thereafter as oral prednisolone. Steroids were slowly discontinued at approximately 3 months after transplantation. TAC was administrated orally twice daily at escalating dose maintaining trough levels of 10–12 ng/mL during the first month, 7–10 ng/mL until the end of third month, 5–7 ng/mL thereafter.
The Second regimen comprised induction with basiliximab (simulect®) given at a dose of 20 mg IV intraoperative. A second dose was given at POD4. Steroids were given at a lower dose. IV methylprednisolone 40 mg/day from Day 1 to day 3, then 20 mg/day until day 6. Thereafter, change to oral prednisolone reducing the dose to 0.3 mg/kg per day. Steroids were slowly discontinued at approximately 3 months after transplantation. MMF and TAC may be started between days 3–10 according to renal function state, at a low dose maintaining trough levels of 7–10 ng/mL until the end of third month, then 5–7 ng/mL thereafter.
The primary endpoint of the study was the patient mortality in the first six months after liver transplantation. Secondary endpoints included the incidence of acute rejection, development of renal impairment and other adverse events such as infectious complications, impaired wound healing, or hepatic artery thrombosis within the first six months after liver transplantation.
Acute rejection was diagnosed with a biopsy proven acute rejection according to Banff criteria [8]. Renal impairment was considered if creatinine >1.5 mg/dL or increase more than 50% from baseline after exclusion of other causes of renal impairment and correction of fluid deficit.
2.1Statistical analysisData analysis was performed using SPSS software for Windows, version 22 (SSPS Inc, Chicago, IL, USA). Qualitative data were expressed as frequency and percentage and analyzed with the chi-square or Fisher exact tests. Quantitative data were expressed as mean and standard deviation and analyzed using Student’s t-test for parametric data and Mann–Whitney test for nonparametric data. Cumulative survival curves were estimated using the Kaplan–Meier method and compared with the log–rank test. Two-sided p-values were reported. P value <0.05 was considered statistically significant.
3ResultsEighty-nine patients were included in the study; their mean age was 46.67 ± 7.89 years. They consisted of 79 males (88.7%) and 10 females (11.2%) (Table 1).
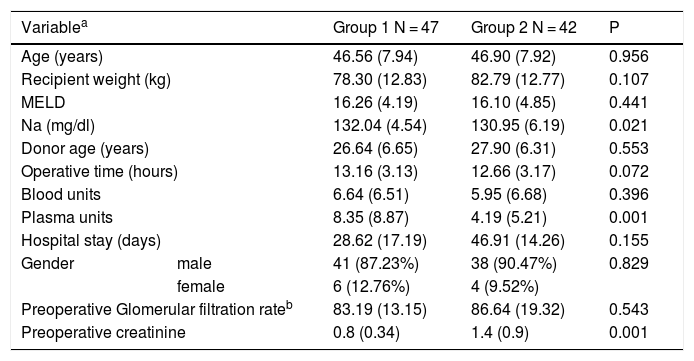
Baseline characteristics of the included patients.
| Variablea | Group 1 N = 47 | Group 2 N = 42 | P | |
|---|---|---|---|---|
| Age (years) | 46.56 (7.94) | 46.90 (7.92) | 0.956 | |
| Recipient weight (kg) | 78.30 (12.83) | 82.79 (12.77) | 0.107 | |
| MELD | 16.26 (4.19) | 16.10 (4.85) | 0.441 | |
| Na (mg/dl) | 132.04 (4.54) | 130.95 (6.19) | 0.021 | |
| Donor age (years) | 26.64 (6.65) | 27.90 (6.31) | 0.553 | |
| Operative time (hours) | 13.16 (3.13) | 12.66 (3.17) | 0.072 | |
| Blood units | 6.64 (6.51) | 5.95 (6.68) | 0.396 | |
| Plasma units | 8.35 (8.87) | 4.19 (5.21) | 0.001 | |
| Hospital stay (days) | 28.62 (17.19) | 46.91 (14.26) | 0.155 | |
| Gender | male | 41 (87.23%) | 38 (90.47%) | 0.829 |
| female | 6 (12.76%) | 4 (9.52%) | ||
| Preoperative Glomerular filtration rateb | 83.19 (13.15) | 86.64 (19.32) | 0.543 | |
| Preoperative creatinine | 0.8 (0.34) | 1.4 (0.9) | 0.001 | |
Group 1; conventional immunosuppression group, Group 2; basiliximab group.
There were no statistically significant differences between both groups regarding age, recipient weight, preoperative MELD, donor age, glomerular filtration rate, operative time, transfused blood units and hospital stay (Table 1).
There were no significant differences in patient survival, graft dysfunction, infection rate or type, or wound healing between both groups (Table 2).
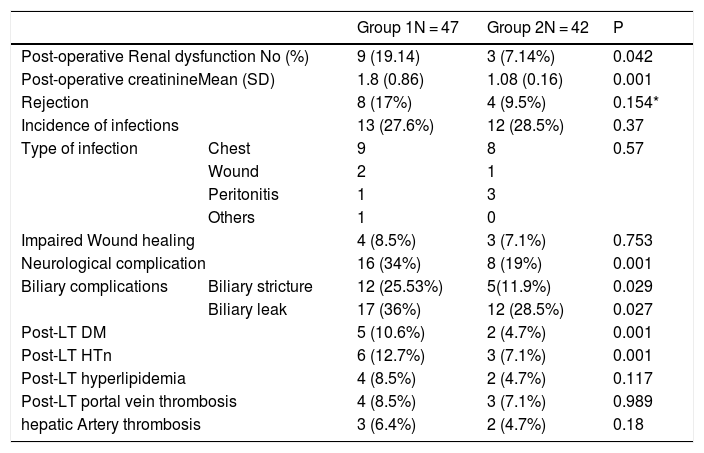
Post-LT complications.
| Group 1N = 47 | Group 2N = 42 | P | ||
|---|---|---|---|---|
| Post-operative Renal dysfunction No (%) | 9 (19.14) | 3 (7.14%) | 0.042 | |
| Post-operative creatinineMean (SD) | 1.8 (0.86) | 1.08 (0.16) | 0.001 | |
| Rejection | 8 (17%) | 4 (9.5%) | 0.154* | |
| Incidence of infections | 13 (27.6%) | 12 (28.5%) | 0.37 | |
| Type of infection | Chest | 9 | 8 | 0.57 |
| Wound | 2 | 1 | ||
| Peritonitis | 1 | 3 | ||
| Others | 1 | 0 | ||
| Impaired Wound healing | 4 (8.5%) | 3 (7.1%) | 0.753 | |
| Neurological complication | 16 (34%) | 8 (19%) | 0.001 | |
| Biliary complications | Biliary stricture | 12 (25.53%) | 5(11.9%) | 0.029 |
| Biliary leak | 17 (36%) | 12 (28.5%) | 0.027 | |
| Post-LT DM | 5 (10.6%) | 2 (4.7%) | 0.001 | |
| Post-LT HTn | 6 (12.7%) | 3 (7.1%) | 0.001 | |
| Post-LT hyperlipidemia | 4 (8.5%) | 2 (4.7%) | 0.117 | |
| Post-LT portal vein thrombosis | 4 (8.5%) | 3 (7.1%) | 0.989 | |
| hepatic Artery thrombosis | 3 (6.4%) | 2 (4.7%) | 0.18 | |
Group 1; conventional immunosuppression group, Group 2; basiliximab group.
HCV-related cirrhosis was the most common indication for transplantation (52%). HCC accounted for 35% of cases combined with other causes of cirrhosis. There was no statistically significant difference among all groups concerning the indication of transplantation.
Pre-transplant GFR was similar in both groups. The incidence of early renal dysfunction was less in the basiliximab group than in the conventional therapy group; although it was marginally statistically significant (7.1% vs 19.14%, p 0.04). Post-operative creatinine was significantly less in patients taking basiliximab than in other patients (1.8 ± 0.86 vs 1.08 ± 0.16, p 0.001). No patient required renal replacement therapy in both groups (Table 2).
The incidence of acute cellular rejection (ACR) was less in the basiliximab group; although it was not statistically significant (9.5% vs. 17%, p 0.15) (Table 2).
Neurological complications were less common in the basiliximab group compared with the other group. Also, the incidence of new-onset diabetes mellitus and hypertension were significantly less in the basiliximab group than the other one (Table 2).
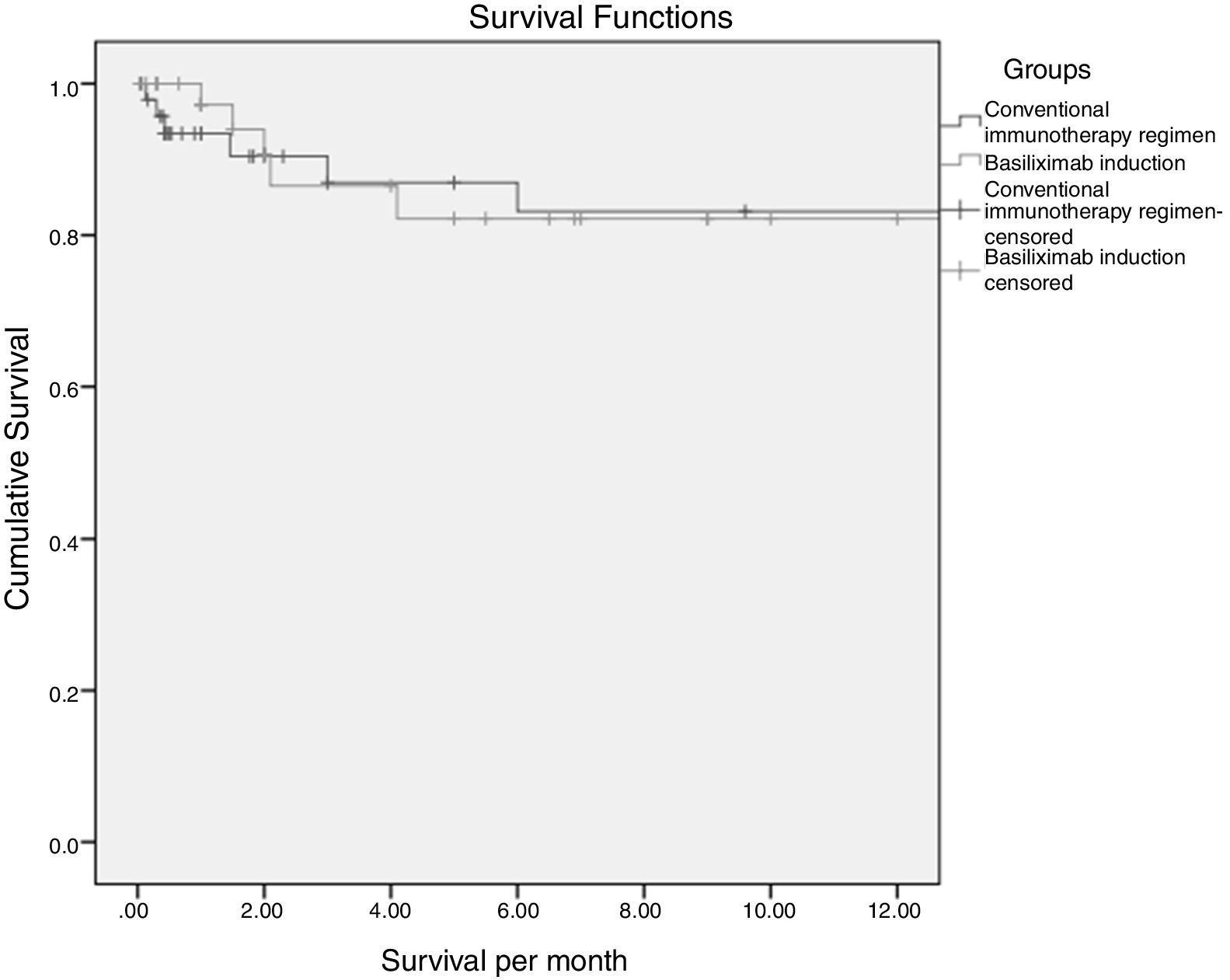
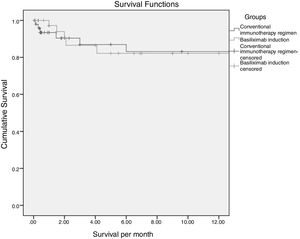
3.1SurvivalTwelve months survival was 83% and 82% in the conventional and basiliximab groups respectively (82.5% overall) (Fig. 1). This difference was not statistically significant (p 0.74). Post-LT infection and rejection were statistically significant predictors of mortality by cox regression analysis (HR 1.8, 95%CI: 1.09–3.16 and 2.3, 95% CI: 1.51–3.62 respectively).
4DiscussionIndeed, an unmet need clearly remains for identifying immunosuppressive regimens that can maintain antirejection efficacy and optimize renal function alongside a substantial reduction of CNIs adverse events [9]. Therefore, the use of CNI-sparing protocols may reduce the risk of renal failure without losing efficacy in preventing rejection or graft loss [10]. Among the different induction agents, basilixmab is the most commonly used because of ease of administration, short-term use, lack of major toxicity and no need for blood level monitoring [11,12].
This study demonstrated that there were no significant differences in patient survival, graft dysfunction, acute rejection rate (ACR), infection rate or type, or wound healing between patients taking Basiliximab and those taking conventional therapy. The incidence of early renal dysfunction was less in the basiliximab group.
Basiliximab induction prevents early renal dysfunction which is a strong predictor of chronic renal impairment in liver transplant recipients. Although it is usually a multifactorial problem, the use of CNI is the predominant cause of kidney dysfunction [13,14].
Our results are consistent with many studies. In a prospective, open-label, nonrandomized study using a similar immunosuppressive protocol; Lin et al. [15] observed a significantly lower incidence of post-transplantation renal insufficiency compared with control group (26% vs. 67%; P < 0.01) despite similar rates of ACR, CMV infection, and new-onset diabetes mellitus. Xiao and colleagues [16] reported that basiliximab-induced immunosuppressive protocol reduces medium-term mortality in high-risk patients and remarkably improves renal function in the first month after LT in patients transplanted for hepatitis B related liver cirrhosis. Schnitzbauer and colleagues [17] reported that renal function improved significantly in patients with pre-transplant renal impairment who took basiliximab in a CNI free regimen. Togashi et al. [18] also reported similar results.
Several trials investigated basiliximab in the induction therapy of liver transplantation within different regimens. They noted lower ACR rates in patients taking basiliximab compared with standard therapy with or without steroids (23% vs 41%) [18–23]. In this context; many studies reported the efficacy of basiliximab in the treatment of steroid resistant rejection [24–26].
On the other hand, many studies did not find Schmeding and colleagues did not find a significant effect of basiliximab in the reduction of the incidence of ACR [27–29].
In this study; the incidence of ACR was lower in patients taking basiliximab than other patients. In the same time, the reported incidence of ACR in this study is lower than that reported in other studies (9.5% vs 19–23%). This may be related to the small number of patients in the study. In addition, many patients who have elevated liver enzymes were managed with optimization of the immunosuppressive regimen without doing liver biopsy that may underestimate ACR incidence.
Basiliximab can be used as a sparing agent for CNI to prevent the development of neurological complications. The incidence of neurological complications in patients received basiliximab induction in our study was lower than in the other group. This may add a value to basiliximab induction as a CNI sparing to avoid neurological complications [18].
There was a concern about increased incidence of infections including CMV infection, bacterial infection and fungal infection in patients receiving basiliximab. Indeed; we did not find any significant difference in the incidence of infection between the different groups. These results are consistent with other studies including the trials mentioned above [18–23].
Although incorporating basiliximab in the immunosuppression regimen entails higher initial costs (about 10,800 Egyptian pounds or 640 dollars per vial); the resulting reduction in the incidence of ACR episodes and lower incidence of renal impairment with a decreased need for renal replacement therapy should yield considerable long-term savings. This was demonstrated in pharmacoeconomic evaluations among kidney transplant recipients [30,31].
Our observations highlight a need for long term follow up to evaluate the consequences of induction immunosuppression in liver transplant patients and define the indications and suitable patient profiles for basiliximab use. Herein; we emphasize the importance of basiliximab as a CNI-sparing agent to avoid CNI toxicity in the early post-transplant period when renal and neurological toxicities peak at that time. Basiliximab is well tolerated in our cohort with no significant major or serious adverse events.AbbreviationsAASLD American Association for the Study of Liver Diseases Acute cellular rejection Calcineurin inhibitors Hepatocellularcarcinoma Hepatitis C virus International normalized ratio Living donor liver transplantation Liver transplantation Model for end-stage liver disease Mycophenolate mofetil
The authors declare that they have no any conflicts of interest.
Compliance with ethical standardsThe study was conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board of National Liver Institute (NLI IRB 00003413), Menoufiya University, Egypt (approval no. 00111/2014). Written informed consents were obtained from both donors and recipients regarding surgery and research.
Author contributionsMohamed Hashim: Conceived and designed the study and contributed in writing the paper, Ayman Alsebaey: Analyzed the data, Amr Ragab: Data collection, Hossam Eldeen Soliman: Wrote the paper, Imam Waked: Revised and approved the paper.
Funding sourcesNo funding.