We characterised the T helper cytokine profiles on the surface of nasal mucosa of children with acute bronchiolitis caused by Respiratory Syncytial Virus, Parainfluenza Virus, Influenza Virus, Adenovirus, or without any viral identification, in order to examine whether these viral types modified cytokine responses. As an additional objective we sought to determine if T helper polarisation was associated with other demographic and environmental factors.
MethodsA prospective study of children with acute bronchiolitis was performed. Patients were recruited from the emergency department of a central hospital in Lisbon, Portugal. Demographical, epidemiological and clinical data were gathered from a questionnaire. Nasal swabs were collected for viral studies (immunofluorescence) and T cell cytokine responses (detection of expression of interleukins 4, 13, 12 and interferon-γ by real-time polymerase chain reaction assays).
ResultsRespiratory Syncytial Virus elicited lower levels of interleukin 4, when compared with samples without virus identification. A similar tendency to Th1 polarisation was found in older children, in those who attended day-care centres, and in breastfed individuals. Exposure to tobacco smoke was associated with a Th2 bias in this population.
ConclusionsThese findings show that Respiratory Syncytial Virus infection contributes to Th1 polarisation in immune response of respiratory mucosa, an effect that is similar to other environmental factors. Further studies are needed to assess immune response to other infectious causes of acute bronchiolitis.
Acute bronchiolitis is a cause of significant morbidity during the winter months among infants and young children throughout the world1. Different viruses, including Respiratory Syncytial Virus (RSV); Adenovirus; Human Metapneumovirus; Rhinovirus; Influenza; and Parainfluenza Virus, can cause this infection1. In spite of different pathogenic mechanisms described for each virus2–4, clinical presentation is similar in all viral types1,5.
Different clinical outcomes of acute bronchiolitis could be related to virus-induced changes in expression of Th1 and Th2 cytokines4,6. Higher levels of IFN-γ (Th1 bias) were found to be associated with less severe illness in RSV bronchiolitis7,8. Increased severity was, however, found in infants with gain-of-function polymorphisms in IL-4 gene (suggestive of Th2 bias)9. Similar findings were described in animal models10.
Recent studies have assessed cytokine responses against RSV, Human Metapneumovirus and Influenza Virus6,11, suggesting that different responses to pathogenic stimuli could occur, rather than similar responses to a common pathway for all agents2,6,11. The role of Th1 and Th2 cytokines in infants infected with other respiratory agents requires more extensive research.
We studied a sample of previously healthy children visiting the emergency department of a paediatric hospital in Lisbon with acute bronchiolitis, comparing clinical presentation and Th cytokine expression in patients infected by different viruses or without virus identification. Additionally we studied different demographic and clinical factors in order to understand if they influenced genetic expression of considered cytokines.
Material and methodsPatientsWe studied a sample of children who were brought to the emergency department of the Sao Francisco Xavier Hospital in Lisbon, Portugal, for a first episode of bronchiolitis, between October 2004 and April 2005, a time range that includes the respiratory infection season in Portugal. The studied population was included in a cohort that was subsequently followed until 36 months old, in order to evaluate progression to recurrent wheezing. Age for enrolment was less than 24 months. Exclusion criteria included significant chronic disease (respiratory, cardiac, immunological, neuromuscular, and gastrointestinal); age of birth of less than 32 weeks; birth weight lower than 1500g; and therapy with glucocorticoids (systemic or inhaled) during the previous two weeks.
On arrival at the emergency department, children were evaluated by a paediatrician or paediatric resident using predefined clinical criteria. A questionnaire was completed, with the following data being collected: age; gender; number of co-habitants (crowded condition was considered if five or more persons were living in the same house); existence of siblings; attendance to day-care centres (considered in children older than 120 days, according to country’s legislation); exposure to tobacco smoke in the household (according to parental information and categorised in “yes” or “no” regardless of quantity or type of product); family history of atopic disease (asthma, rhinitis, eczema in parents or siblings); gestational age; smoking habits of mother during pregnancy (regardless of quantity or type of consumption); breastfeeding (considered if mothers referred exclusive breastfeeding until three months of age); and personal history of atopic eczema. On observation, the following data were registered: presence of fever (defined as body temperature≥37.8°C), respiratory rate, presence of suprasternal, intercostal, and infracostal retractions, presence of wheeze and crackles on pulmonary auscultation, general status, skin colour and haemoglobin transcutaneous saturation of oxygen (SpO2). Each child was given a severity score, ranging from 0 to 18, adapted from Wang et al.12. A respiratory rate of less than 30 cycles per minute (cpm) was given 0 points; between 30 and 45cpm, 1 point; from 46 to 60, 2 points; and higher than 60, 3 points. Absence of costal retractions received 0 points; presence of infracostal retractions, 1 point; suprasternal and intercostal retractions, 2 points, and global thoracic retractions associated with nasal flare, 3 points. If pulmonary auscultation was normal, 0 points were given; presence of expiratory wheezing, 1 point; inspiratory and expiratory wheezing, 2 points; and diminished or absent murmur, 3 points. If general status was normal, 0 points were given; feeding difficulties received 1 point; irritability, 2 points; lethargy or coma, 3 points. Normal skin colour received 0 points; pallor, 1 point; peripheral cyanosis, 2 points; central or generalised cyanosis, 3 points. A SpO2 higher than 95% was classified with 0 points; between 93 and 95%, 1 point; lower than 93%, 2 points; respiratory failure received 3 points. These data were registered on the day of illness when the highest score for each patient was reached.
Detection of virus and cytokinesTwo nasal swabs were collected from each patient. After gently flushing both nostrils of the children with 1ml of normal saline, a wood and cotton swab (Eurotubo™ – Rubi, Spain) was introduced into each nare. The first swab was tested for respiratory virus and the other was used to study genetic expression of four immunoregulatory cytokines: two representing Th1 bias - Interleukin-12 (IL-12) and interferon-γ (IFN-γ), and two representing Th2 activity - IL-4 and IL-13.
Virulogical determination was done in the Bacteriology Laboratory of the participant hospital, using the commercial Viral Respiratory KitTM (Bartels Trinity Biotech Company) according to the manufacturer’s instructions. This test detects, by indirect immunofluorescency, four viral antigens: RSV, Parainfluenza Virus, Influenza Virus, and Adenovirus.
Genetic expression of cytokines associated with Th bias was determined by Real-Time Polymerase Chain Reaction (RT-PCR) in the Biochemistry Laboratory of the Centro de Histocompatibilidade do Sul, Lisbon, Portugal. The laboratory procedures were coordinated by Dr Dario Ligeiro.
After biological product was collected, it was preserved in a specific solution (RNAlater™ by Ambion Inc.) and refrigerated at −20°C, until transported to laboratory. RNA was extracted from samples by use of the Genelute Mammalian Total RNA™ kit by Sigma. Reverse transcription was performed on the extracted RNA, applying the commercial system High Capacity cDNA Archive Kit™ (Applied Biosystems) as described by the manufacturer. Finally, RT-PCR was performed in an Applied Biosystems 7900HT Fast RT-PCR technology, with Taqman PCR Universal Master Mix™ (Applied Biosystems). Gene expression of different cytokines was compared with the expression of a housekeeping gene, RNA18S, of constant and known activity in all cellular populations. We used the ΔΔCt method to compare fold changes between the expression of studied cytokine and the expression of the housekeeping gene. A total of 40 amplification cycles were performed for each sample. Fluorescence was emitted by a probe in the cycle in which gene expression was detected. It is relevant to note that the lower the amplification cycles needed the higher expression of a given gene to be present on the original sample. This means that, for each measure, lower numeric values of this variable correspond to higher genetic expression on the original sample. Since values originate from division between numbers of amplification cycles, no unit is presented.
Ethical aspectsWritten, witnessed, informed consent was obtained from parents or guardians of all patients. The Ethical Commission of the Hospital approved the study protocol.
Statistical analysisResults were initially studied by descriptive statistics. Comparisons used Fisher’s exact test (2-tailed), analysis of variance or the Mann-Whitney U test as appropriate. Correlations were analysed using Spearman’s coefficient. p<0.05 was considered significant.
ResultsPatients’ characteristicsBetween October 2004 and April 2005, 143 children with the diagnosis of acute bronchiolitis were included in this study. Their age on consultation was 200±147 days (range, 18–672 days); 82 (57.3%) children were male. Twenty-six (18.2%) lived in crowded conditions. Fifty-seven (39.9%) children had no siblings. Of the 93 children older than 120 days old, 50 (53.8%) attended child-care centres. In 82 cases (57.3%) cohabitants referred smoking at home. Seventy-eight children (54.5%) had family history of atopic disease. Twenty-one patients (14.7%) were born between 32 and 36 weeks of gestation. In 24.5% of children (35/139), their mothers smoked during pregnancy. Ninety patients (62.9%) were or had been breastfed. In 21 cases (14.7%) there was personal history of atopic eczema.
On the first episode, 53 (37.1%) children had fever. Severity score was 7.9±3.9 (range, 1–17).
Detection of virusAmong the 143 patients, 52 swabs (36.4%) were negative for respiratory virus antigen, 74 (51.7%) were RSV positive and 17 (11.9%) were positive for other viral types (nine children had Parainfluenza Virus infection, six Influenza Virus, and two Adenovirus). There were no cases of simultaneous detection of more than one viral type.
Detection of cytokinesIt was possible to detect genetic expression of IL-4 in 82 patients (57.3%), with a mean of 26.768 and a standard deviation of 4.360 (range 12.710–31.876). IL-13 genetic expression was detected in 104 samples (72.7%): mean±SD 23.535±3.490 (range 12.390–29.899). IL-12 was detected in 118 (82.5%) patients: mean±SD 19.875±3.681 (range 9.971–27.213). IFN-γ was detected in 117 samples (81.8%): mean±SD 20.765±4.113 (range 9.428–29.880). The number of cytokines measured did not match with the number of patients’ samples since some results were inconclusive, due to procedure failures.
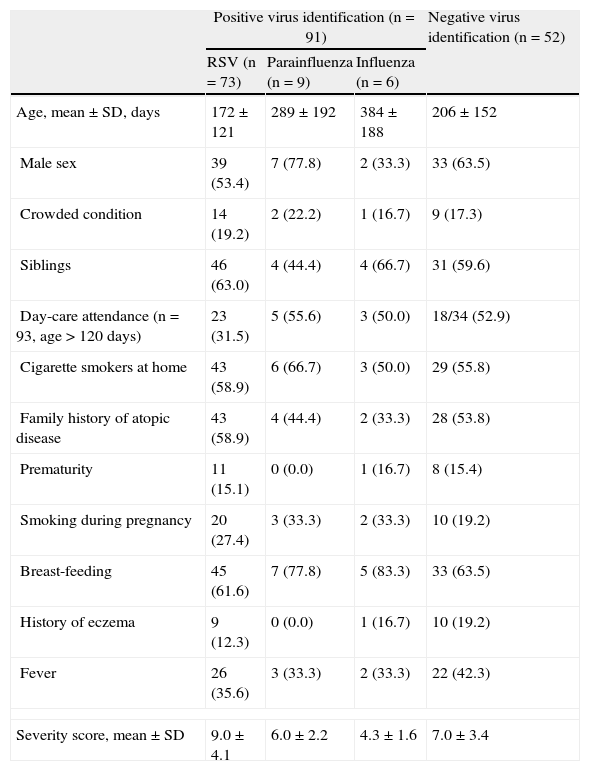
EpidemiologyTo determine the comparability of children infected with different respiratory viruses, we examined the frequency of each risk factor in our study population. By ANOVA with Bonferroni comparison, as shown in Table 1, there were no differences in considered demographic and epidemiological characteristics between groups.
Distribution of patients’ characteristics according to virus identification. Significant differences in bold type. N (%)
| Positive virus identification (n=91) | Negative virus identification (n=52) | |||
| RSV (n=73) | Parainfluenza (n=9) | Influenza (n=6) | ||
| Age, mean±SD, days | 172±121 | 289±192 | 384±188 | 206±152 |
| Male sex | 39 (53.4) | 7 (77.8) | 2 (33.3) | 33 (63.5) |
| Crowded condition | 14 (19.2) | 2 (22.2) | 1 (16.7) | 9 (17.3) |
| Siblings | 46 (63.0) | 4 (44.4) | 4 (66.7) | 31 (59.6) |
| Day-care attendance (n=93, age>120 days) | 23 (31.5) | 5 (55.6) | 3 (50.0) | 18/34 (52.9) |
| Cigarette smokers at home | 43 (58.9) | 6 (66.7) | 3 (50.0) | 29 (55.8) |
| Family history of atopic disease | 43 (58.9) | 4 (44.4) | 2 (33.3) | 28 (53.8) |
| Prematurity | 11 (15.1) | 0 (0.0) | 1 (16.7) | 8 (15.4) |
| Smoking during pregnancy | 20 (27.4) | 3 (33.3) | 2 (33.3) | 10 (19.2) |
| Breast-feeding | 45 (61.6) | 7 (77.8) | 5 (83.3) | 33 (63.5) |
| History of eczema | 9 (12.3) | 0 (0.0) | 1 (16.7) | 10 (19.2) |
| Fever | 26 (35.6) | 3 (33.3) | 2 (33.3) | 22 (42.3) |
| Severity score, mean±SD | 9.0±4.1 | 6.0±2.2 | 4.3±1.6 | 7.0±3.4 |
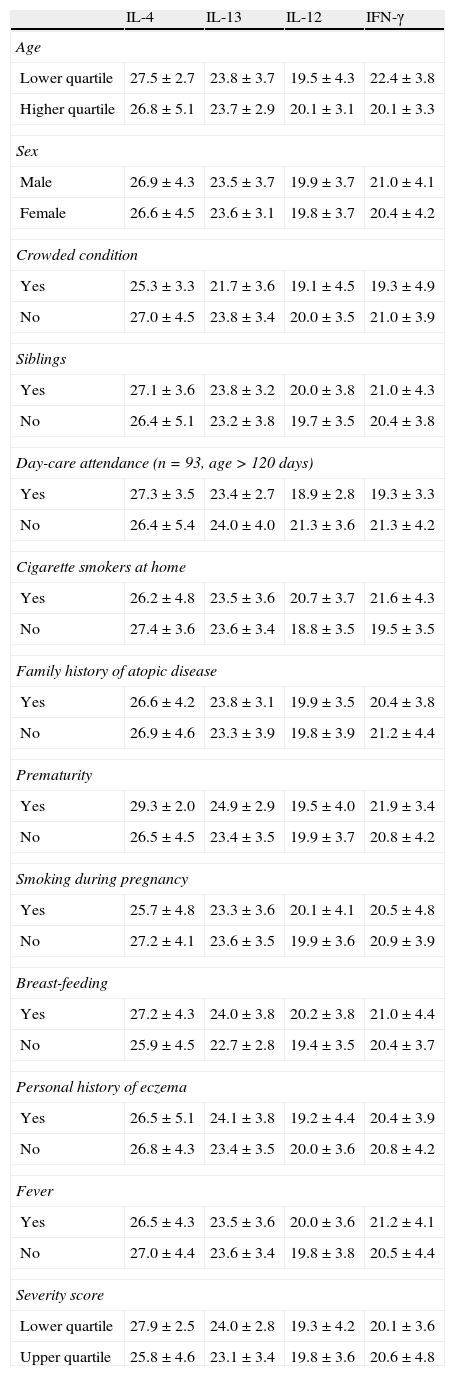
We further investigated any possible factors which could be related with different genetic expression of cytokines on the surface of the nasal mucosa. As shown in Table 2, the following differences were found: older children had higher genetic expression of IFN-γ (p=0.019); children in day-care had also Th1 polarisation, shown in higher expression on IL-12 (p=0.001) and IFN-γ (p=0.020); in breastfed children a decrease in Th2 cytokine (IL-13) expression was identified (p=0.040). Conversely, children living in smoking environments had significantly lower expression of IL-12 (p=0.008) and IFN-γ (p=0.006), representative of Th2 bias. All other items considered showed no difference in expression of the cytokines studied.
Relation between genetic expression of cytokines on the surface of nasal mucosa and sample data. Significant differences in bold type.
| IL-4 | IL-13 | IL-12 | IFN-γ | |
| Age | ||||
| Lower quartile | 27.5±2.7 | 23.8±3.7 | 19.5±4.3 | 22.4±3.8 |
| Higher quartile | 26.8±5.1 | 23.7±2.9 | 20.1±3.1 | 20.1±3.3 |
| Sex | ||||
| Male | 26.9±4.3 | 23.5±3.7 | 19.9±3.7 | 21.0±4.1 |
| Female | 26.6±4.5 | 23.6±3.1 | 19.8±3.7 | 20.4±4.2 |
| Crowded condition | ||||
| Yes | 25.3±3.3 | 21.7±3.6 | 19.1±4.5 | 19.3±4.9 |
| No | 27.0±4.5 | 23.8±3.4 | 20.0±3.5 | 21.0±3.9 |
| Siblings | ||||
| Yes | 27.1±3.6 | 23.8±3.2 | 20.0±3.8 | 21.0±4.3 |
| No | 26.4±5.1 | 23.2±3.8 | 19.7±3.5 | 20.4±3.8 |
| Day-care attendance (n=93, age>120 days) | ||||
| Yes | 27.3±3.5 | 23.4±2.7 | 18.9±2.8 | 19.3±3.3 |
| No | 26.4±5.4 | 24.0±4.0 | 21.3±3.6 | 21.3±4.2 |
| Cigarette smokers at home | ||||
| Yes | 26.2±4.8 | 23.5±3.6 | 20.7±3.7 | 21.6±4.3 |
| No | 27.4±3.6 | 23.6±3.4 | 18.8±3.5 | 19.5±3.5 |
| Family history of atopic disease | ||||
| Yes | 26.6±4.2 | 23.8±3.1 | 19.9±3.5 | 20.4±3.8 |
| No | 26.9±4.6 | 23.3±3.9 | 19.8±3.9 | 21.2±4.4 |
| Prematurity | ||||
| Yes | 29.3±2.0 | 24.9±2.9 | 19.5±4.0 | 21.9±3.4 |
| No | 26.5±4.5 | 23.4±3.5 | 19.9±3.7 | 20.8±4.2 |
| Smoking during pregnancy | ||||
| Yes | 25.7±4.8 | 23.3±3.6 | 20.1±4.1 | 20.5±4.8 |
| No | 27.2±4.1 | 23.6±3.5 | 19.9±3.6 | 20.9±3.9 |
| Breast-feeding | ||||
| Yes | 27.2±4.3 | 24.0±3.8 | 20.2±3.8 | 21.0±4.4 |
| No | 25.9±4.5 | 22.7±2.8 | 19.4±3.5 | 20.4±3.7 |
| Personal history of eczema | ||||
| Yes | 26.5±5.1 | 24.1±3.8 | 19.2±4.4 | 20.4±3.9 |
| No | 26.8±4.3 | 23.4±3.5 | 20.0±3.6 | 20.8±4.2 |
| Fever | ||||
| Yes | 26.5±4.3 | 23.5±3.6 | 20.0±3.6 | 21.2±4.1 |
| No | 27.0±4.4 | 23.6±3.4 | 19.8±3.8 | 20.5±4.4 |
| Severity score | ||||
| Lower quartile | 27.9±2.5 | 24.0±2.8 | 19.3±4.2 | 20.1±3.6 |
| Upper quartile | 25.8±4.6 | 23.1±3.4 | 19.8±3.6 | 20.6±4.8 |
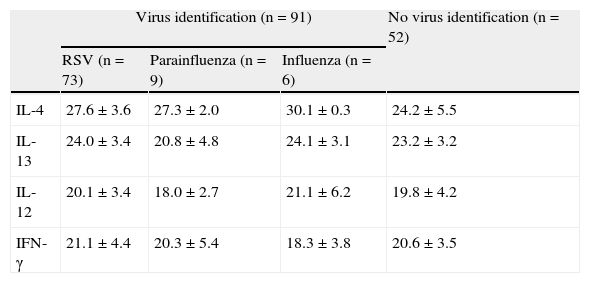
The main purpose of this study was to compare immune response of nasal mucosa (in terms of Th1 versus Th2), when exposed to different respiratory viral agents. These results are represented in Table 3, where it is possible to acknowledge a lower expression of a Th2 cytokine, IL-4, in RSV positive cases comparing with negative samples (p=0.019). A similar trend was detected in Parainfluenza and Influenza Virus positive samples but without statistical significance. Due to the limited number of Adenovirus identified, these could not be included in the comparison. Other determinations showed no differences, namely when IL-12, IL-13 and IFN- γ expression were compared.
Genetic expression of cytokines in acute bronchiolitis, according to identified viruses. Significant differences in bold type.
| Virus identification (n=91) | No virus identification (n=52) | |||
| RSV (n=73) | Parainfluenza (n=9) | Influenza (n=6) | ||
| IL-4 | 27.6±3.6 | 27.3±2.0 | 30.1±0.3 | 24.2±5.5 |
| IL-13 | 24.0±3.4 | 20.8±4.8 | 24.1±3.1 | 23.2±3.2 |
| IL-12 | 20.1±3.4 | 18.0±2.7 | 21.1±6.2 | 19.8±4.2 |
| IFN-γ | 21.1±4.4 | 20.3±5.4 | 18.3±3.8 | 20.6±3.5 |
This article characterises Th cytokine expression at the surface of nasal mucosa of small children with acute bronchiolitis caused by RSV, Influenza, Parainfluenza Virus, or with no viral identification. A unifying Th-related hypothesis was unable to explain the pathogenesis and clinical presentation of viral acute wheezing respiratory illnesses in this population. In addition to immunological mechanisms and the role of inflammation, other anatomical, structural, and developmental factors need to be assessed.
Our population included 143 children, which led to a small number of viral identification - a limitation of the present study. The methodology applied to acknowledge smoking habits and breastfeeding (questionnaire) could also lead to incorrect estimation of this data. When this prospective study was conducted (2004–2005), Human Metapneumovirus and Rhinovirus infections could only be diagnosed by PCR, unavailable in the participant hospital, so these viruses were not studied. We considered this to be a major limitation of this study and a suggestion for further investigations. Expression of Th1 and Th2 cytokines was studied in a biological product of unknown cellular type. We think that further research is needed in order to identify cell populations which are most likely to be responsible for the detected immunological profiles.
The use of a simple severity score, based on clinical data and measure of SpO2 allowed us to standardise criteria and carefully assess and register observational data. These procedures were beneficial to patients. We did not use data on hospitalisation, since these depend on other factors than the clinical severity (clinical subjectivity, socio-economic features).
Immune response, namely cytokine activity at the surface of nasal mucosa, is very difficult to determine, as these molecules have an excessively short half-life and are very liable in vitro. We preferred to determine the expression of codifying genes, applying RT-PCR methodology. By this method we could measure in a non-invasive way the local immune response to different stimuli, namely respiratory virus. Other authors studied concentration of cytokines either on local fluids or sera13, but we considered the chosen method more reliable, as these molecules are unstable in the mucosa and serum values do not accurately reflect local responses14. In the present study it was not possible to determine, in the same patients, both genetic expression and protein concentrations of at least one or two cytokines, which could contribute to further validation of our findings.
Intuitively, it would seem that assessments of levels of cytokines in infants with lower respiratory tract infection symptoms would benefit from samples obtained from the lower airways. Since it was obviously impossible to obtain tracheal aspirates in this population, only nasal washes were obtained. However, previous observations indicate that our findings are biologically meaningful15.
Demographical and epidemiological characteristics of our population were similar to those expected in children living in the urban area to which the hospital gives assistance. More than half of the children attended day-care centres, almost 60% lived in smoking environments, and one in four mothers admitted smoking during pregnancy. These are realities that need to be faced by local health services16.
Acute bronchiolitis occurred with fever in only 37% of patients, which highlights the incidence of afebrile RSV infection1,5. We also admit that, in some children, this acute episode of wheezing was not due to infective agent but had other cause (atopy, malformation or functional, among others)1.
RSV was the most frequently identified agent in our sample, with small percentages of Parainfluenza, Influenza and Adenovirus. Other non-studied agents such as Rhinovirus and Human Metapneumovirus could be responsible for a number of cases1,5.
We did not find statistically significant differences between virus-positive or virus-negative patients in relation to age, gender, existence of siblings, day-care centre attendance, prenatal or postnatal contact with smoking, crowded life conditions, breastfeeding, and severity of illness. These were considered as risk factors by other authors but were not confirmed in the present study1,5,17. Small numbers of Parainfluenza and Influenza Virus identifications could impair detection of further differences.
One of the positive aspects of this study was its contribution to acknowledge the profile of cytokines at nasal mucosa during acute bronchiolitis. We found very subtle differences, not suggestive of unequivocal inversion of Th1/Th2 balance but, nevertheless, with statistical significance that deserve further studies.
It was possible to determine cytokine expression in a significant percentage of samples (57–82%). These high rates of positivity, in spite of the vulnerability of biological products allow us to consider RT-PCR as a useful method for this determination15,18.
Different expressions of cytokines at the surface of upper airways are probably related to previous conditioning factors, either intrinsic (genetic, immune, among others) or extrinsic (smoking, viral type).
To obtain an overview of these immunological phenomena, we studied by molecular biology techniques, the activity of two cytokines which participate in Th2 pathways (IL-4 and IL-13) and another two which take part in Th1 pathways (IL-12 and IFN-γ).
We found increased activity of one Th1 cytokine (IFN-γ) in older children, and of two Th1 cytokines (IL-12 and IFN-γ) in those that attended day-care centres. A decrease in expression of a Th2 cytokine (IL-4) was identified in breastfed children. Th2 predominance, with decreased expression of Th1 cytokines, IL-12 and IFN- γ, was found in children who lived in tobacco smoking environments.
Production of IL-12 and IFN-γ, cytokines with important antiviral activity6,7 is greatly dependent on immune maturation, being higher in older children19. Their synthesis is also related to previous antigenic stimulation by microbial agents, that are more prevalent in a day-care centre environment19,20. Breastfeeding also contributed, in our study, to lower expression of IL-13, cytokine that has Th2 functions21. Exposure to tobacco had an opposite effect, lowering production of IL-12 and IFN-γ, leading to Th2 bias. This could be explained by direct action of tobacco metabolites on the immune system, where they probably inhibit gene expression22,23.
We found no statistical relations between cytokine expression and gender, crowded conditions, presence of siblings, family or personal history of atopy, exposure to smoking during pregnancy, presence of fever or severity of bronchiolitis. In theoretical terms, these factors could modify immune response2,8,19,20. Our study probably lacked power to demonstrate such differences.
In the present study, we found lower expression of IL-4 in children infected by RSV during acute bronchiolitis than in virus-negative patients. IL-4 takes part in immunological reactions of Th2 type, stimulating directly IgE production. We believe this inhibition of IL-4 corresponds, in this context, to relative stimulation of Th1 pathways, induced by viral metabolism. Other authors found similar results in RSV infection2,5,7,13,14. On the contrary, others described opposite changes, with elevation of IL-4 and lowering values of IFN-γ, in RSV positive patients, suggestive of Th2 induction4,6,10. To complicate furthermore, others found simultaneous stimulation of IL-4 and IFN-γ24.
The diversity and apparent discordance in results between different studies show the complexity of immune response to RSV. Some authors attribute differences in immune response to previous host genetic predisposition: in some cases that response is directed to viral protein F, a Th1 bias is obtained; when response is directed to viral protein G, immune response is predominantly Th2 type25.
We cannot also exclude the possibility that a previously existing relative deficiency of IL-4 contributes to higher susceptibility of infection by RSV26.
The role of IFN-γ in respiratory infections has been a long-standing source of interest2,4,7–9,11. Although some studies identified an association between IFN-γ production and protection14,27, others did not13,22. The majority of the literature on IFN-γ and respiratory viruses focused on the effects of this cytokine during RSV infection and responses to be decreased compared with infections with other viruses22,26,27. Our study is not in agreement with these observations, since there was no statistical difference in expression of IFN-γ between virus negative and positive cases.
Unfortunately, our study lacked power to analyse the clinical role of cytokines in association with individual viruses. It is also possible that other (unmeasured) cytokines, such as IL-10 and TGF-β, may play a role common to the pathogenesis of all viral respiratory diseases in infancy.
ConclusionsDuring an episode of acute bronchiolitis, RSV was associated with marginally higher Th1-cytokine responses, when comparing with cases without viral identification. However, no association was found between IFN-γ or Th bias with wheezing or disease severity. These findings indicate that a unifying mechanism of illness for respiratory viruses based only on immune response is unlikely; therefore, in the pathogenesis of bronchiolitis, non-immune host-dependent factors should be considered.
The authors thank Dr Dario Ligeiro for the laboratory proceedings of RT-PCR.