Seasonal variation of Dermatophagoides allergens and its influence in allergic respiratory airway diseases has not been investigated in Andean cities. The objective of this study was to evaluate those parameters in a city located in the Andean mountains.
MethodsDer p1 and Der f1 were measured in dust samples from mattresses in 13 houses in Quito (2800m above sea level). Samples were collected monthly from August 2004 to July 2005. Patients presenting to a local outpatient allergy clinic with asthma and rhinitis and isolated allergy to Dermatophagoides were analysed to determine if a correlation existed between seasonal Der allergen levels and the number of patients presenting with allergies.
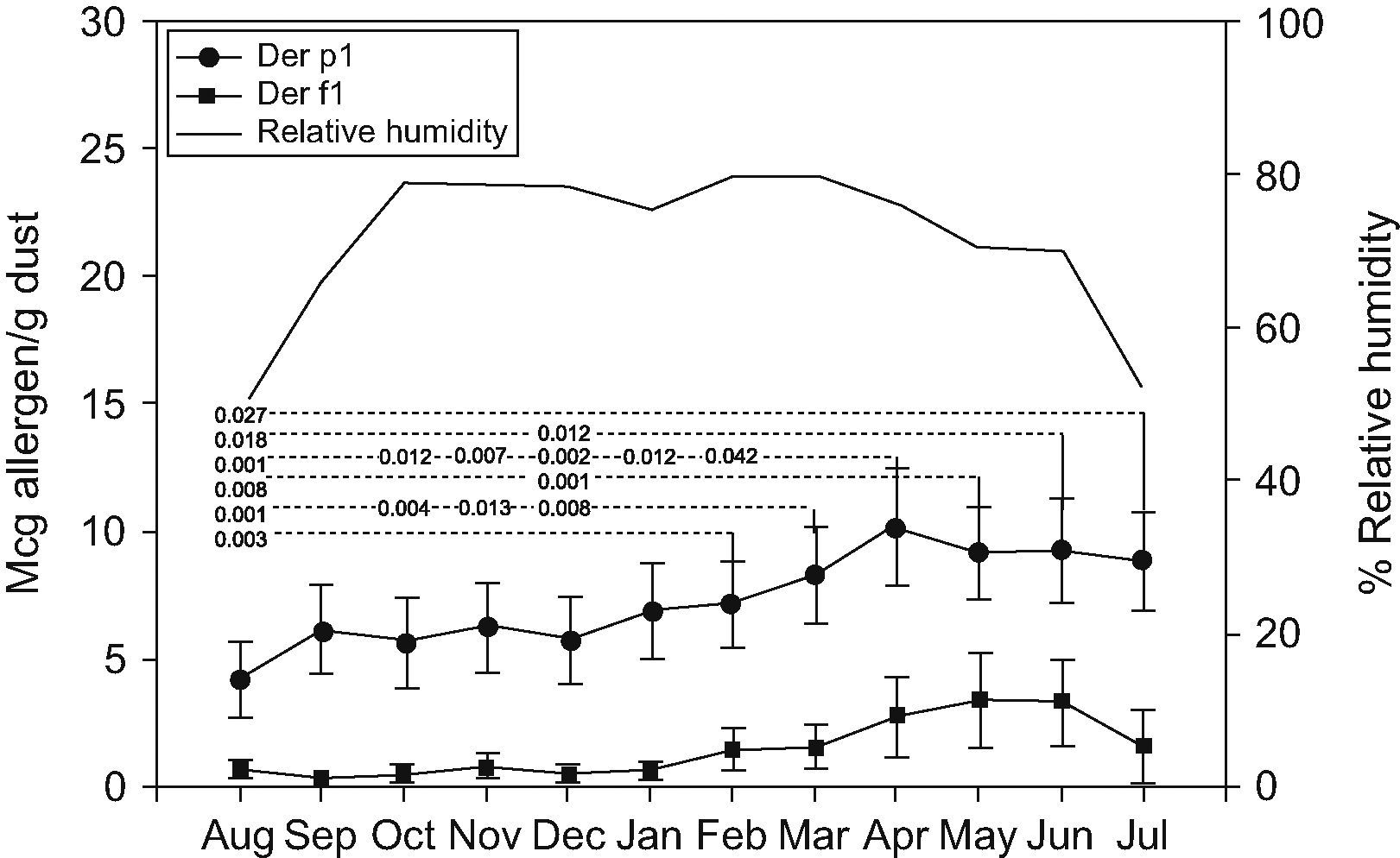
ResultsHigh levels of dust mites and humidity were observed throughout the year. The highest geometrical mean values of allergens were detected in April (Der p1, 10.15μg/g) and May (Der f1, 13.03μg/g), whilst the lowest levels were detected in August (Der p1, 4.26μg/g), and September (Der f1, 1.4μg/g). Of the 361 patients examined, 182 were allergic to Dermatophagoides, (45.6% asthmatics, 97.8% rhinitics, and 43.4% with both diseases). Patient presentation spiked in August, and from February to May. However, there was not a significant correlation between mite allergen concentrations and humidity or the number of patients presenting with allergies.
ConclusionsDust samples from mattresses in Quito revealed high concentrations of Der p1 and Der f1. We observed a trend towards increased presentation of asthmatic and rhinitic patients in the months with highest levels of allergens.
Prolonged exposure to house dust mite allergens can lead to the development of allergic rhinitis and bronchial asthma in sensitised subjects. Studies conducted in The Alps in Europe1–3 and in the Rocky Mountains of North America4,5 have shown low occurrences of these arthropods above 1000m above sea level (a.s.l) and no mites above 1600m. In contrast, studies on domestic mite fauna in high altitude (2500–2800m) Andean cities demonstrated an abundant presence of mites and their allergens in dust samples collected in this region.6–10 We have previously demonstrated that Dermatophagoides pteronyssinus and D. farinae are important sensitising agents in patients with allergic respiratory diseases in Quito11 and these arthropods were more significant sensitisers than pollens or other allergens.12
Discrepancies in the prevalence of mites in high altitude areas of the Northern hemisphere and South American Andean regions could be explained by climatic differences, especially differences in the ambient humidity. Andean cities and villages have a moderate year-round temperature, high humidity and abundant rainfall. Coupled with reduced usage of heating and cooling systems, this environment is particularly well suited to the growth of mites.
The data supporting seasonal increases of mite numbers and their allergen levels analogous to relative humidity have been conflicting.13–15 However, asthmatic patients exhibit bronchial hyperreactivity in parallel to seasonal mite allergen exposure.16,17 In high altitude Andean cities, seasonal variation of Dermatophagoides allergens and its influence in allergic respiratory airway diseases have yet to be investigated. Due to minimal climate changes throughout the year, and the subsequently stable mite population, we hypothesised that mite allergens affect patient allergies differently in this region. Quito, the capital city of Ecuador, is located in the Andes Mountains at 2800ma.s.l., has an annual mean relative humidity of 75% and `average temperature of 15°C. To determine risk factors for asthma and respiratory allergies among the citizens of Quito, and as an exploratory pilot study, we investigated the monthly levels of Dermatophagoides allergens Der p1 (D. pteronyssinus) and Der f1 (D. farinae) found in mattresses, and the correlation of those levels with the number of allergic patients attended in an allergy outpatient clinic.
MethodsCollection of dust samplesIn order to verify the presence of house dust mite allergens, we randomly selected 13 non-asthmatic individuals residing separately in Quito. To obtain natural levels of allergens, we selected non-allergic households in the hope of avoiding mattresses subjected to dust mite eradication activities. Before commencing the study, approval was obtained from the institutional review board and written and oral informed consent was obtained from each subject prior to entry into the study. Dust samples were collected once monthly beginning in August 2004 and ending in July 2005. Samples were collected from the entire surface of each mattress for a period of two minutes by a single trained technician using a 1.4kW portable vacuum cleaner (Trio 2, Electrolux, Brazil) and a Mitest Collector (Indoor Biotechnologies, VA, USA). All samples were frozen immediately and for at least 48h to ensure against mite proliferation.
Der p1 and Der f1 levelsDer p1 and Der f1 levels were quantified using an ELISA technique following manufacturer’s instructions (Indoor Biotechnologies, VA, USA). The results were expressed as micrograms of allergen per gram of dust (μg/g). Information on relative humidity in Quito was obtained from http://espanol.wunderground.com/history/airport.
Diagnosis and monthly evolution in number of patients allergic to mitesTo investigate a possible correlation between Der p1 and Der f1 allergen levels and the number of Dermatophagoides allergic patients requesting medical attention, medical charts recorded in the database system of an outpatient allergy clinic of the city were audited. This centre attends patients the same day an appointment is requested. We selected both patients seen for the first time, as well as patients previously attended in the clinic for a respiratory allergic disease and requesting a new visit during the months investigated. The diagnosis of allergic rhinitis was established following current guidelines,18 while that of asthma was made according to GINA recommendations (http://www.ginasthma.org). Suspected asthmatic patients were diagnosed if outward symptoms including coughing, episodic breathlessness, wheezing, and chest tightness were observed, and tests revealed at least a 12% improvement in FEV1 following inhalation of a bronchodilator. Skin tests were conducted on the forearm volar surface using a standard battery of reactives, including extracts of D. pteronyssinus and D. farinae (Laboratorios LETI, Tres Cantos, Madrid, Spain). Other tested allergens included German cockroach, Cypress, Eucalyptus, grasses, weeds, dog and cat dander, feathers and moulds. To be included in the studied group, the patients must be sensitised to house dust mites (D. pteronyssinus and/or D. farinae) as determined by skin prick testing, whereas skin test results to the other inhalant allergens tested must be negative.
Statistical analysisNon parametric methods, such as Wilcoxon signed rank test for paired data, Kruskal-Wallis and Mann-Whitney test to compare groups, and Spearman to determine the correlation between variables, were used. Statistical analyses were performed using the StatView program (version 4.53) (Abacus Concept Inc. Berkeley, CA, USA).
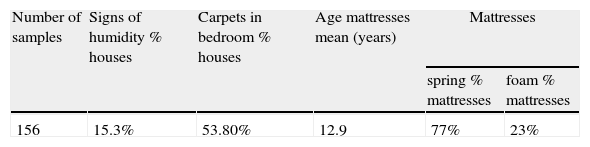
ResultsCollection of dust samplesParticipants were 8–80 years old, with a mean age of 49.6. Seven were males and six were females. The characteristics of the houses and mattresses are shown in Table 1. A total of 156 samples were collected.
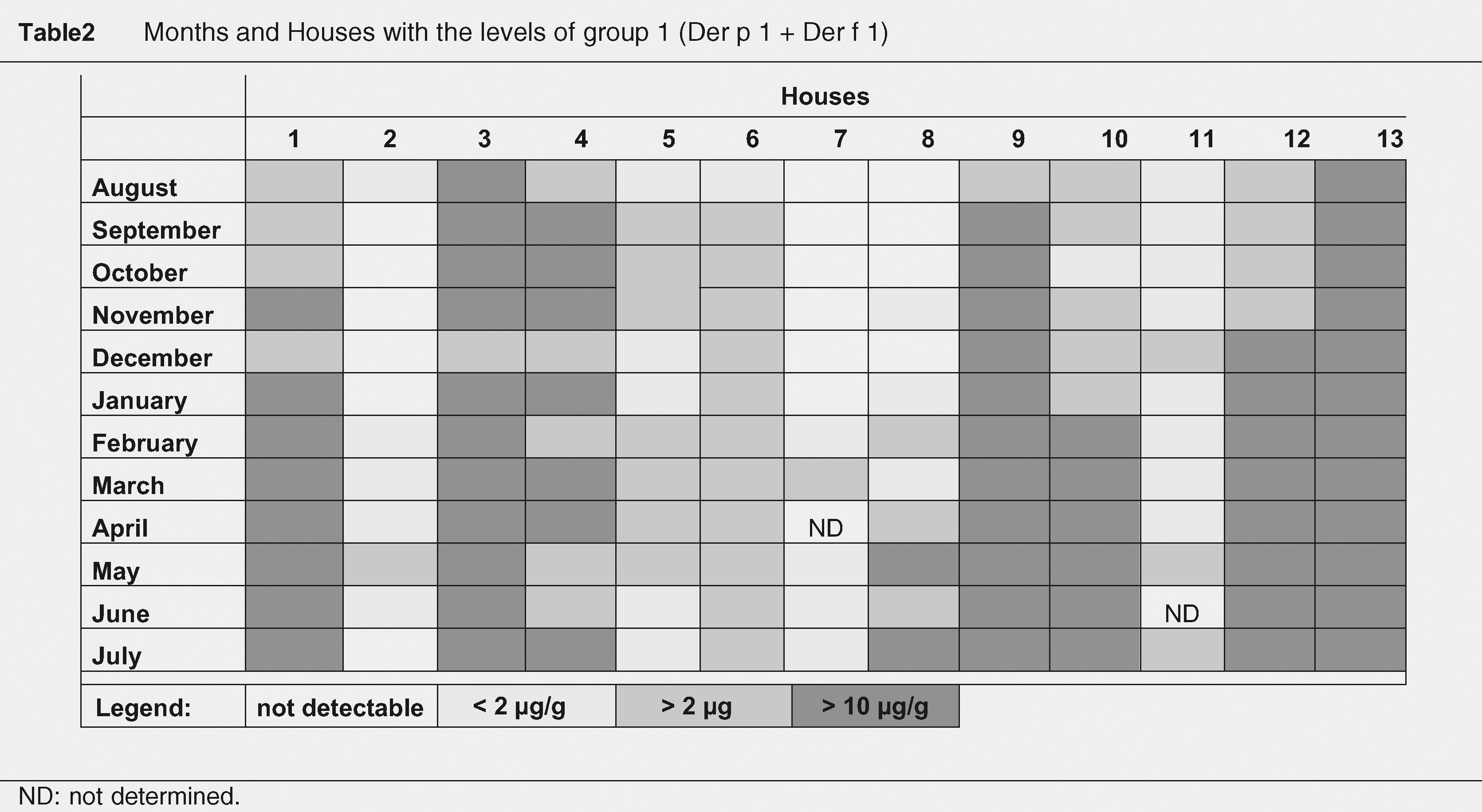
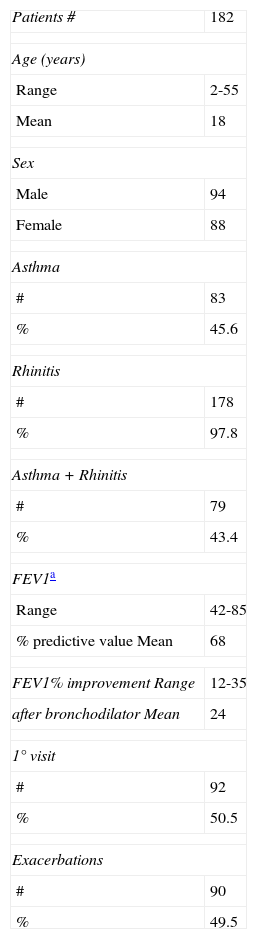
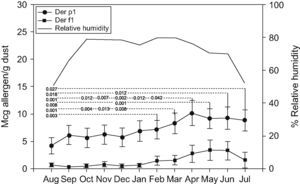
Der p1 and Der f1 levelsMonthly variations of Der p1 and Der f1 allergen levels are shown in Figure 1. We identified steady increments in the levels of mite allergens from January to April (Der p1) and from February to May (Der f1). The months and total of group 1 allergen levels per house, according to the threshold levels of 2 and 10μg/g of dust are shown in Table 2. Levels of >10μg/g were present in eight (61.5%) mattresses, and in seven (53.8%) of them they were detected in at least half of the year.
Obvious signs of humidity in the house, the age and type of mattress, age of the owners, presence of carpet in the bedroom, or location in the city, showed no significant correlation with the levels of house dust mite allergens detected (Table 1).
Der p1 levelsDer p1 allergen was detected in all 13 mattresses (>2μg/g). Levels >10μg/g of Der p1 allergen in seven (53.8%) were detected in at least half of the months studied (Table 2). The highest geometric mean value of Der p1 was detected in April (10.15μg/g) and represented a 2.3-fold increase from the lowest level (4.26μg/g) detected in August. Statistically significant differences were detected between values of Der p 1 obtained each month (Figure 1). Values obtained in August were statistically lower than those obtained from February to July; those in December were lower than those obtained from March to June. October and November levels were lower than those from March and April; and finally April values were higher than those from January and February.
Der f1 levelsDetectable Der f1 allergen levels were present in only three (23%) mattresses. Levels >10μg/g of Der f1 allergen were detected in all of them at least in one of the months studied. The highest mean value occurred in May (3.44μg/g; Geometric mean: 13.03μg/g) and represented a 10.8-fold increase from the lowest level detected in September (0.32μg/g; Geometric mean: 1.4μg/g). Non-statistically significant differences were observed.
Correlation mite allergens with relative humidityOutdoor relative humidity remains stable from October to June (70–79%) with the lowest values in July, August, and September (52%, 48.1%, and 66.3% respectively). The lowest levels of Der p1 allergen occurred in August, and of Der f1 in September. Although there was a coincidence of lowest levels of Der p1 (August) and Der f1 (September) with months in which the relative humidity is lower, the mite allergen levels did not statistically correlate with environmental relative humidity (Figure 1).
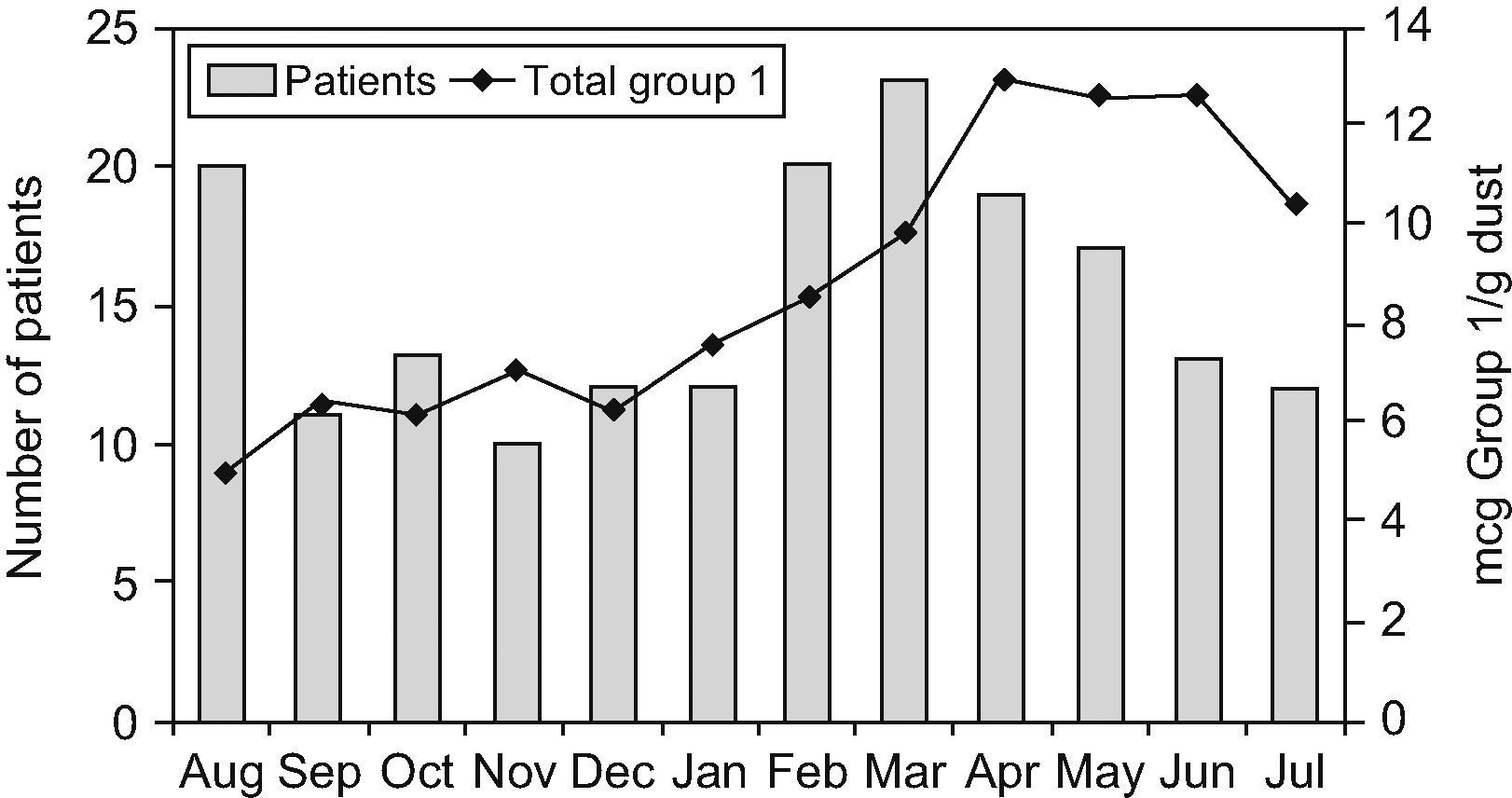
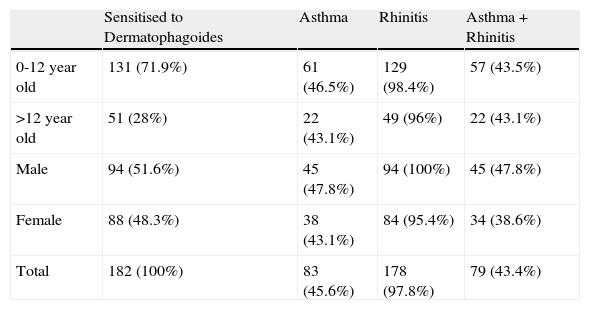
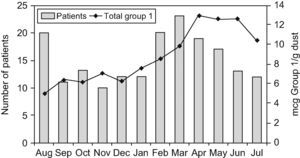
Patients attended monthly in the allergy outpatient clinicA total of 361 asthmatic and rhinitic patients were attended between August 2004 and July 2005 in the allergy outpatient clinic investigated. Of the 361 patients, 182 (57.4%) tested positive by Dermatophagoides skin prick. The final patient cohort ranged in age from 2 to 55 years, with a mean of 18 years, and males and females were equally represented (51.6% and 48.3%, respectively). One hundred and fifty-five patients (85.1%) were sensitive to both mites, 19 (10.4%) to D. pteronyssinus alone, and 8 (4.3%) to D. farinae alone. Skin test results to other common inhalant allergens tested (cockroach, grass, weed and tree pollen, animal dander, feathers and moulds) were negative. Of the 182 patients, 83 (45.6%) were diagnosed with asthma, 178 (97.8%) with rhinitis, and 79 (43.4%) with both diseases. Clinical characteristics of the patients and FEV1 measures (carried out in 52 [62.6%] of the 83 asthmatics) are shown in Table 3. Patients were classified by sex and age (Table 4). More patients presented at the clinic in August and from February to May (Figure 2), but no statistically significant differences were found between the number of allergic patients seen in those months from the other months studied, even after correction for disease, age, and sex or first and exacerbations.
Clinical Characteristics of the patients studied
| Patients # | 182 |
| Age (years) | |
| Range | 2-55 |
| Mean | 18 |
| Sex | |
| Male | 94 |
| Female | 88 |
| Asthma | |
| # | 83 |
| % | 45.6 |
| Rhinitis | |
| # | 178 |
| % | 97.8 |
| Asthma+Rhinitis | |
| # | 79 |
| % | 43.4 |
| FEV1a | |
| Range | 42-85 |
| % predictive value Mean | 68 |
| FEV1% improvement Range | 12-35 |
| after bronchodilator Mean | 24 |
| 1° visit | |
| # | 92 |
| % | 50.5 |
| Exacerbations | |
| # | 90 |
| % | 49.5 |
Number of asthmatic and rhinitic patients, divided by age and sex groups.
| Sensitised to Dermatophagoides | Asthma | Rhinitis | Asthma+Rhinitis | |
| 0-12 year old | 131 (71.9%) | 61 (46.5%) | 129 (98.4%) | 57 (43.5%) |
| >12 year old | 51 (28%) | 22 (43.1%) | 49 (96%) | 22 (43.1%) |
| Male | 94 (51.6%) | 45 (47.8%) | 94 (100%) | 45 (47.8%) |
| Female | 88 (48.3%) | 38 (43.1%) | 84 (95.4%) | 34 (38.6%) |
| Total | 182 (100%) | 83 (45.6%) | 178 (97.8%) | 79 (43.4%) |
We did not identify a positive correlation between the monthly levels of dust mite allergens and the number of patients attended each month (Figure 2).
DiscussionThis study demonstrated a high level presence of Der p1 in the 13 mattresses examined, which remained constant throughout the 12-month study period. The results of our study could be similar to those obtained in investigations performed before in many other cities of the word. The difference is that this investigation has been done in a city located at 2800ma.s.l. Those altitudes have been previously associated with an absence of house dust mites, according to the investigations in Europe1–3 and North America4,5 and implicitly generalised to all the high altitudes of the world. Therefore, the results of this study could better fit the interest of international readers. The primarily goal of the investigation was to provide data on natural monthly variations in Der p1 and Der f1 concentrations in houses in Quito by collecting a sample population of non-allergic residents. We expected this randomised group to reflect the general housing pattern. On the other hand, we assume that the house environmental conditions were not the same in the dwellings studied. Some investigations have demonstrated that at the same time of the year mite numbers in one house can rise dramatically, while microclimate conditions in another house are such that they can be kept down to an insignificant level.19,20
In all the mattresses studied, Der p1 levels exceeded the concentrations proposed for sensitisation (>2μg/g). In eight (61.5%) mattresses Der p1 exceeded the levels proposed to induce allergic symptoms (10μg/g),21 in at least half of the year. The Der p1 allergen level showed an increase from its minimum count in August to its maximum in April, calculated as a 2.3–fold increase. This more than doubled total was considerably less than the 100–1000 fold change reported in Virginia,14 but was similar to the change obtained in Manchester,15 Boston22 and Cartagena.23 The Der f1 allergen was present in only three of the mattresses. In all of them, Der f1 levels exceeded the concentrations which have been proposed for sensitisation and for presence of symptoms. There was a 10.8 fold increase from the minimum level detected in September to the maximum level in May. It is important to note that changes in the levels of both types of Dermatophagoides allergens are considered clinically significant only when they reach a magnitude of 10-fold or higher.24 The occasional presence of Der f1 allergen in the mattresses studied might be explained by the results obtained from a previous study conducted in Quito.10 The study demonstrated that D. farinae was present in only a small percentage of the house dust samples studied (D. farinae in 15% vs. D. pteronyssinus present in 95% of the dust samples), but in high quantities (geometric mean of 696.2 mites per gram of dust).
Similar to Der p1 concentrations detected, the relative humidity remained high and stable throughout the year, decreasing slightly during July, August and September. Furthermore, in August and September we observed lower levels of Der p1 and Der f1. This fact suggested that changes in the environmental humidity might have a dramatic effect on total Dermatophagoides populations and their allergens.
On the contrary, from January to April (Der p1) and from December to May (Der f1) the incremental change in mite allergens was small but constant. The investigations of van der Heide et al.16,17 have demonstrated increases in nonspecific airway hyperresponsiveness in asthmatic patients, in the months with maximal levels of Dermatophagoides in dust samples. In the present study, we did not measure the levels of dust mite allergens in the houses of our allergic patients. Those levels could have revealed with more confidence the correlation between the evolution of their diseases and the Dermatophagoides allergen levels. It was not our intention to assume a direct relationship between the levels of Dermatophagoides allergens found in the 13 mattresses of non-asthmatics subjects and the clinical outcome of 182 allergic asthmatic/rhinitic patients. However, it is possible to use the determination of the mite allergen levels in non-allergic controls to estimate the exposition in allergic patients, since it has been demonstrated that allergic patients (asthmatic and rhinitic) and non-allergics living in the same area are exposed to the same allergen levels of these allergens.25 Therefore, we assumed that in those months with high levels of Dermatophagoides, we would see also an increase in the number of patients attended in the outpatient allergy clinic studied, due to a deterioration of the respiratory diseases in people allergic to dust mites. But, despite the fact that more allergic patients were attended in clinic between February and May, a statistically significant correlation was not found. This absence of correlation could be explained by the clinical factors that were not considered as direct statistical components of variance (i.e. the monthly levels of dust mite allergens, the limited number of patients collected, or the inclusion of non-emergency patients). We were unable to identify the exact reason why a substantial number of allergic patients were attended in August, a month with a particularly small concentration of mite allergens. It is possible that other factors different to mite allergens (e.g. virus infections), could have been the cause of the patients’ visits. Fever is one of the main symptoms that differentiate allergy from infection. We were always aware of the presence of fever and excluded those febrile patients from the study. Our group of asthmatic and rhinitic patients was exclusively sensitised to house dust mites, thereby excluding the interference of exposure to other airborne allergens. Another explanation could be that, in August, the low levels of allergens detected in our study, do not correspond with the levels (possible high) of the majority of the mattresses in Quito.
The moderate year round temperature, high humidity and abundant rainfall typical of Quito, make this environment ideal for the reproduction and growth of mites. As in similar tropical regions, the indoor climate is primarily influenced by external environmental conditions, since most of the houses keep their windows open throughout the day. In addition, the absence of heating and cooling systems in the houses we studied ensured against these artificial climate factors influencing our results. Nonetheless, bedroom environmental conditions can vary greatly in the same house and from house to house. In addition, a mattress is a model that can predict the environmental conditions in the bed. This environment is influenced by room and bed hygrothermal conditions, occupant characteristics, etc.26 Although the analysis of those factors was not the purpose of our investigation, we consider that they could have influenced in the levels of dust mite allergens found in this study. Our data lends support to the idea that altitude is not the major determinant for the presence or abundance of house dust mites in the tropics. Other climatic conditions, such as temperature and humidity, are well-established regulators of dust mite growth. Therefore, at these altitudes (2800ma.s.l.), mites and their allergens may play an important role in the pathogenesis of allergic respiratory diseases in cities of South America.
This study has several limitations. Only the relative outdoor, not the indoor, humidity was evaluated, assuming that both were the same or had only little differences.
We are aware of the limited number of mattresses analysed in this study. The small sample size is a concern regarding generalisability. Moreover, the number of isolated Dermatophagoides allergic patients attended in the outpatient clinic, during the year investigated, was too small to allow the evaluation of potential differences. Also, measuring the levels of dust mite allergens in the houses of our allergic patients, we could have more consistent results about the correlation between the evolution of their diseases and the Dermatophagoides allergen levels detected through the year. Thus, further investigations are needed before conclusions can be established.
Additionally, the analysis of the allergic patients attended was confined to persons who were evaluated as outpatients. Data of ER visits or hospitalisation for acute asthma or patients who had mild rhinitis (and may not seek medical care) were not included. This exclusion probably reduced the number of allergic persons attended, especially in those months with high levels of mite allergens.
In conclusion, during the 12 months studied, we have identified a constant presence of Der p1at high levels in all 13 mattresses examined, and the presence of Der f1 in 3 of them. In all cases, allergen levels exceeded the concentrations which have been proposed for sensitisation, and in seven (53.8%) mattresses exceeded the levels suggested the presence of symptoms. Relative humidity was stable throughout the year with a decrease occurring in July, August and September. In addition, during August and September we found lower levels of Dep1 and Der f1, respectively. April, May and June were identified as the months with the greatest geometric mean levels of both Dermatophagoides allergens. There is a trend towards more patients being attended in the months with the highest levels of mite allergens. The climatic conditions, especially high humidity, may explain the presence of dust mites and their allergens throughout the year. We should consider Dermatophagoides as an important allergenic factor at 2800ma.s.l. in the Andes Mountain cities.
Conflict of interestThe authors have no conflict of interest.