The reasons for the relative resistance of children to certain infections such as that caused by coronavirus SARS-CoV2 are not yet fully clear. Deciphering these differences can provide important information about the pathogenesis of the disease. Regarding the SARS-CoV2 virus, children are at the same risk of infection as the general population of all ages, with the most serious cases being found in infants. However, it has been reported that the disease is much less frequent than in adults and that most cases are benign or moderate (even with high viral loads), provided there are no other risk factors or underlying diseases. It is not clear why they have lower morbidity and virtually no mortality. A series of findings, relationships and behavioral patterns between the infectious agent and the child host may account for the lower incidence and a greatly attenuated clinical presentation of the disease in children.
It is not just a question of the infectious agent. The reasons for the relative resistance of children to certain infections such as that caused by coronavirus SARS-CoV2 (which causes COVID-19 disease), are still not fully clear. Many infectious processes in this age group occur differently from what is seen in adults, and deciphering these differences can provide important information about the pathogenesis of the disease. Regarding the SARS-CoV2 virus, children are at the same risk of infection as the general population of all ages, with the most serious cases being found in infants.1 However, it has been reported that the disease is much less frequent than in adults and that most cases are benign or moderate (even with high viral loads), provided there are no other risk factors or underlying diseases. The condition may even be asymptomatic. Of note in this regard is the documentation of 12 asymptomatic children with mild pneumonias.2–5
However, knowing the evidence on the "benignity" of this infection caused by such a novel virus in the childhood population should not generate a feeling of complacency or relaxation towards the disease.6
Recently, the National Health Service and the Paediatric Intensive Care Society of the United Kingdom have alerted clinicians about a small number of children admitted with symptoms of toxic shock syndrome and Kawasaki disease (KD), and their possible association to COVID-19 (not all cases being positive).7 Without ruling out a new type of presentation of COVID-19, such a relationship has not been definitively confirmed. These are most likely presentations of KD coinciding in time with the viral pandemic and having an incidence not higher than in previous years.
It is worth pointing out that toxic shock syndrome is associated to bacterial toxins and has nothing to do with a rare complication: Kawasaki shock syndrome in the context of also rare KD. Lastly, several studies have described associations of KD with different viruses that are common in children.
Are all children well protected from acquiring the infection or only from the risk of becoming ill after being infected? Children are also infected but it is not clear why they have lower morbidity and virtually no mortality.
A series of findings, relationships and behavioral patterns between the infectious agent and the child host may account for the lower incidence and a greatly attenuated clinical presentation of the disease in children.
Immune system differences between children and adultsData on the immune response to the SARS-CoV2 virus are still not entirely conclusive, although information on its SARS-CoV and MERS-CoV congeners is known and can serve to make an approximate or potentially comparative assessment.
The respiratory epithelial cells (RECs) form a major line of defense in the human body against microorganisms present in inhaled air. This first barrier from the nasopharynx to the alveolar epithelium is a constituent of the innate immune response (IIR) formed by many different cells: epithelial cells, alveolar macrophages, and innate lymphoid and dendritic cells. The most common respiratory viruses that reach and invade the lungs in children are rhinoviruses (RVs), respiratory syncytial virus (RSV), influenza, and coronavirus. They share a common RNA genome that plays an important role in the set of sensors (pattern recognition receptors) present in the IIR, and which are responsible for identifying these viruses when they target respiratory cells. These sensors generate cascades of specific inter- and intracellular molecular signals that,—with integrity of the cellular defensive barrier mentioned above,—play a key role as the first line of defense to ensure the establishment of antiviral protection in the lungs.8
The first elements encountered by SARS-CoV2 are alveolar macrophages—the most numerous pulmonary leukocytes in the first years of life—with the contribution of antiviral proteins produced mainly by RECs and neutrophils.9 The response of these viruses, and in particular of SARS-CoV2, is to activate mechanisms to evade or suppress the IIR, and thus be able to open a gate to replicate and cause the disease. It can be argued that the IIR is more important in the first years of life when adaptive processes are less developed.9 Considering the differences in the IIR between children and adults, it has been hypothesized that this non-specific response occurring after the first encounter with the virus is more robust and active in children.3 In this regard, it could be asked whether the mentioned response is particularly diligent against SARS-CoV2, for it is already known that against other viruses such as RSV, this desired protection does not occur as effectively in infants. Moreover, infants are characterized by a respiratory tract that is healthier and less punished by extensive exposure to inhalants, pollution and other respiratory processes as seen during adulthood. To achieve this protection, the infected person must be in an optimum state of health and with an adequate genetic background (for example, the HLA system which ensures the immune response with some determined anti-SARS-CoV2 immune locus), favoring specific antiviral immunity. When this protective response is altered, viral expansion will be greater, increasing damage to the lung, where there is a strong expression of angiotensin converting enzyme (ACE2) receptors. Initial good health is no longer an advantage once the lung is damaged.10
The IIR is considered to be more delayed and weakened with aging and when viral attack occurs. In an effort or as a replica update (reset), it reacts with an excessive and out-of-control inflammatory response, and pulmonary dysfunction also occurs due to altered O2 exchange, resulting in further damage to both tissue and lung function and capacity.11,12
In short, the purpose of this first defensive barrier for early control during the incubation period and the first symptoms of SAR-CoV2 infection is to inhibit viral replication, promote elimination of the virus, induce tissue repair and trigger a specific adaptive immune response (AIR).12
This next defensive activity is slower in developing, and it is not clear whether it is stronger in children than in adults with regard to the clearance of SARS-CoV2. Studies of SARS-CoV-infected mice have shown the T lymphocyte response to be particularly important, particularly that of helper CD4+ T lymphocytes, which stimulate B lymphocytes (in charge of humoral antibody response) - all of which are essential for disease protection and viral clearance. A subpopulation of CD4+ T cells, the regulatory T lymphocytes (Tregs), are relatively abundant in pediatric tissues, and possibly have a greater suppression capacity than in adults.8 Likewise, CD8+ T cells account for 80% of the inflammatory cells in the lung interstitium, and play a vital role in eliminating the virus and also in inducing immune damage. The response of these T lymphocytes to S protein and other structural proteins of the coronaviruses is persistent and long-lasting.12–14
In the event of SARS-CoV2 infection, the T lymphocyte pattern that predominates in the first few years of life could be considered more effective in rejecting the virus. It is still premature to know how long this antibody response will last, with long-term serological studies being needed to monitor the immune status of the patients. Evidence from other particularly virulent coronaviruses (SARS-CoV, MERS-CoV) shows that defensive immunity can last up to three years, and that reinfection with the same strain is highly unlikely during the next winter season.15
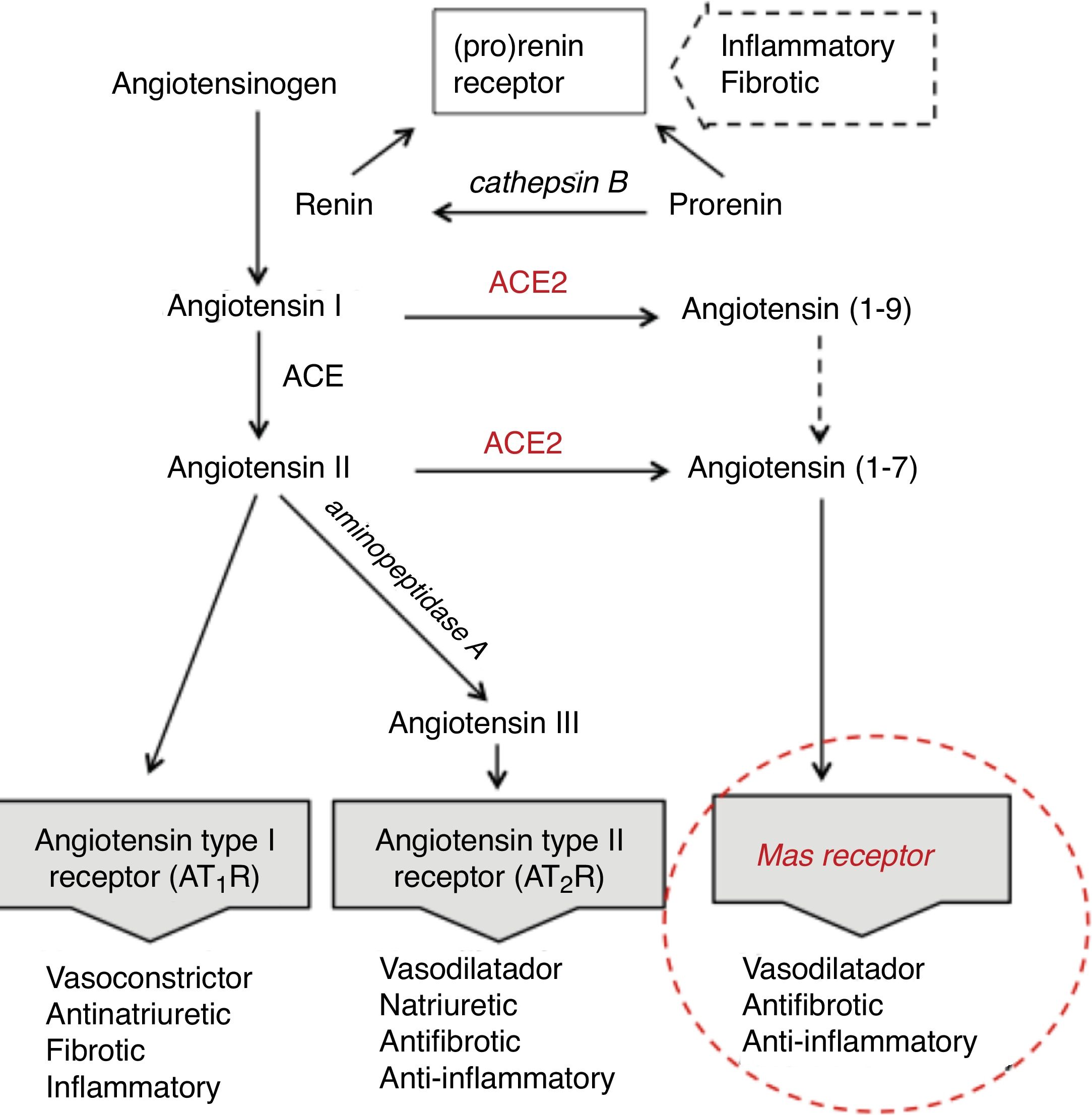
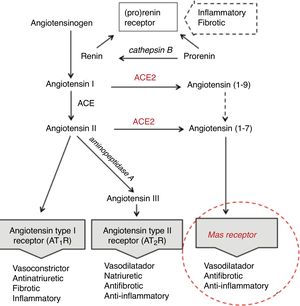
Age differences in distribution, maturation and function of SARS-CoV2 receptorsThe SARS-CoV2 coronavirus, as was previously verified with SARS-CoV and MERS-CoV, initiates the attack due to its interrelationship and affinity with the renin-angiotensin system (RAS) (Fig. 1), through a key enzyme in this system—ACE2—which in addition to its known physiological contribution has been shown to be the receptor for these viruses and to facilitate access to the target cells that will become their reservoir. The virus, by means of the spicules on its surface (with the S-protein) as a key, invades the cells expressing ACE2 and the serine protease TMPRSS2 to replicate and reproduce. This interaction is a powerful conditioning factor of the virulence of the virus.16
The expression of ACE2 is very high on the apical surface of well-differentiated epithelial cells lining the airways, and especially in the alveoli of the lung—precisely the main target of the virus and where it causes the greatest damage. Strong expression has also been demonstrated in the mouth and tongue, indicating that the oral cavity is a potential route of infection.17 Furthermore, this enzyme also plays an important role in the immune response, especially in inflammation, and is involved in the defensive mechanisms of the lung—protecting it from severe injury induced by respiratory viruses.11,18
Following cell damage induced by the virus, the expression of messenger RNA (mRNA) (responsible for transporting genetic information) and the enzymatic activity of ACE2, in an intimately related pattern, are significantly reduced.16,19,20 This reduction partly explains the poor ability to slow down the inflammatory response, as a consequence of advancing age.
The expression of ACE2 does not seem to play an essentially harmful role in the body, especially in children, rather quite the opposite. On the one hand, under normal conditions, ACE2 is involved in the RAS cascade through activation of the Mas receptor, and the function of this ACE2/Mas pathway is crucial due to its beneficial effects: vasodilatation, with anti-fibrotic, anti-proliferative and anti-inflammatory actions, thereby counteracting the actions of the ACE-angiotensin II-At1R axis (Fig. 1). However, as previously mentioned, it is the lock which the virus skillfully recognizes and uses to penetrate and damage the alveolar epithelial cells. Since the virus first needs to attach and gain access to the cell before it can replicate, the state of cell differentiation with surface location of ACE2 can have a strong impact upon the disease.19 Considering this state of cell differentiation in childhood and that the distribution of ACE2 receptors is uneven in different organs and populations, are there differences in the extent and function of ACE2 in the alveolar tissue of children and adults? Some studies report that the number and function of ACE receptors is not as robust in children as it is in adults.3 However, definitive information is not available, as it is very difficult to obtain lower airway samples from healthy children. The existing data are therefore based on studies in murine or other animal models. Studies in mice have shown that ECA2 protein expression increases with age, and that mRNA/ECA2 activity is not only regulated during development but is also impacted by aging.19,21 In extrarenal tissues such as the lung, mRNA/ECA2 expression is low during pregnancy, increases in the perinatal period and peaks in adulthood.22 An analysis of thousands of lung tissue samples from patients of different ages with a variety of lung tumor processes investigated the determinants of ACE2 expression from the cancer transcriptome data. The activity and amount of ACE2, not affected by carcinogenesis, was found to increase from 40 years of age, with peak expression between 60–80 years of age.21
Ideally, by demonstrating a more precise number and distribution of epithelial cells expressing ACE2 in cohorts such as age, it could be determined whether the pediatric population is potentially less susceptible.
Immunity to other coronaviruses and cross-immunity with SARS-CoV2Common human coronaviruses (CovH), not SARS or MERS, have been detected in respiratory secretions of a significant percentage of healthy children during the winter months. One feature to note is that in children over four years of age, the infection rates were relatively consistent, when it is well known that the pathogens that cause respiratory infections are less prominent above that age.23,24 Like other respiratory viruses, CovH can infect people of all ages, and the infection only generates relative immunity—although the type of specific protection has not been completely defined. The usual co-circulation of CovH types and co-infection in people suggests that protection is unlikely to be of high cross-reactivity, and also that the greater incidence of SARS-CoV2 infection in adults points to poor cross-immunity with previous CovH infections.24 However, serological studies evaluating the immune response to respiratory infections including CovH have shown steadily increasing seroprevalence of antibodies to CovH in both children and young adults, as well as cross-reactivity, such as between antibodies to the previous SARS-CoV and CovH.25,26
The extent to which this antibody response may mean cross-immunity to SARS-CoV2 remains unclear.
Blocking SARS-Cov2 in the context of viral co-infectionsThe respiratory system constitutes a reservoir for a group of microorganisms—both commensal and pathogenic. Children are especially prone to many viral infections, with a high burden of respiratory viruses in the upper respiratory tract (URT) mostly (approximately 90%) in infants and toddlers, and with a prominent participation of respiratory syncytial virus (RSV) and rhinoviruses (RVs).27
In addition to these two well-known viruses, molecular techniques have allowed more viruses to be identified in the URT coexisting in the same period—although this does not imply that there will be a greater tendency towards more serious processes. According to the available data, the number of viruses involved and the related symptoms are variable and not always similar—this being a peculiar aspect in the pediatric population. In a recent series of adults hospitalized with COVID-19 in Germany, no viral co-infection was demonstrated in any patient.28
In relation to CovH, four are endemic in many populations and have been involved in upper and lower respiratory tract infections in healthy children, as commented above. Current studies preceding the present pandemic showed that children carrying CovH in their respiratory tract become co-infected in up to two thirds of all cases.23
Under these conditions of co-infection, there is some evidence of synergistic and antagonistic interactions among the coexisting viruses that can play a key role in acute respiratory infection. An example is RSV growth blocked by the activity of the influenza A virus. Studies on the dynamics of these viral respiratory co-infections have observed that if a given virus initiates colonization and infection in the nasopharynx first, it is able to block or reduce the growth of a second arriving invader. That is, the level of viral load (fastest growing virus) determines which virus will be dominant. In particular, the presence of RVs recognized as the fastest growing is able to reduce the growth of the remaining viruses during a state of co-infection.29,30 The clinical course in co-infection scenarios will be determined by specific combinations of the virus involved. If the amount of more than one virus in the URT of children is usually higher in winter and early spring (RVs and RSV being the most frequent), the question that arises is whether in the current pandemic the activity and virulence of SARS-CoV2 virus is blocked or somehow reduced by other viruses already often present in the URT of this age group.
It is possible that such repeated and continuous viral exposure also sustains and keeps the immune system active when it needs to respond to SARS-CoV2 infection, added to the fact that enzyme ECA2 is more immature in this age group.5
Melatonin secretion is highest in children and young adultsMelatonin is a hormone involved in a wide range of cellular, neurophysiological and neuroendocrine processes. It does not exert a specific direct antiviral effect but indirectly possesses anti-inflammatory and anti-oxidative action, thus improving the immune response.31 All these effects afford potential mitigation of COVID-19 disease. This is a benefit to be considered not only preventively but also as an adjuvant to treatment when the disease is already established.
Respiratory viruses such as CovH and, as was demonstrated with SARS-CoV1 in its day (the molecular biology of SARS-CoV2 is still not completely documented) in its attack upon the lung, trigger oxidative stress and activation of the NLRP3 inflammasome (multiprotein complex of the innate immune system), activating a cytokine avalanche—specifically IL-1β and IL-18 - which will play a critical role.
Melatonin has been shown to inhibit coronavirus-induced cell death; exert an antioxidative and inhibitory effect upon the cytokine complex; and also prevent secondary pulmonary fibrosis.32–34
It is noteworthy that nocturnal melatonin blood levels are much higher in children aged 1−3 years and under 15 years compared to adults, with a significant drop after age 70. There is a definite negative relationship between age and melatonin secretion between the ages of 20 and 90 years.34 In fact, children typically exhibit significantly greater secretion and activity of melatonin than their grandparents, an age group characterized by more frequent and more severe COVID-19 disease.
Effect of pneumococcal vaccines upon the incidence of viral respiratory infectionsOf course, the pneumococcal vaccine does not provide any protection against coronaviruses. Children over 12 months of age have theoretically completed pneumococcal vaccination and have a robust anti-pneumococcal antibody response.
Previous studies on the benefits of vaccination with heptavalent and nine-valent conjugate vaccines have shown a significant reduction in both children and adults not only of invasive pneumococcal disease but also of influenza episodes and admissions due to viral pneumonia compared to pre-vaccination years.35–37 Furthermore, lower influenza morbidity has been reported during a 2−3 year period in people over 60 years of age who have been vaccinated with the pneumococcal polysaccharide vaccine.38
The most recent direct beneficiaries of the pneumococcal vaccine besides children are people over 65 years of age. During this pandemic, given the high mortality rate in individuals over age 65, and in order to investigate any possible indirect effects of the vaccine such as those reported for influenza virus, it could be important to know the proportion of individuals that died due to COVID-19 in this age group and who were vaccinated against pneumococcus with conjugate and polysaccharide vaccine administered five years prior to death.
Style and type of livingThe range and type of activities in children is generally relatively minor, with a more attenuated exposure to the virus, considering the main routes and risks of transmission common in adults. Children are usually infected through contact with their adult family members, with more limited exchanges than is usual for adults, and the majority—being asymptomatic or presenting only very mild symptoms—are not tested for the presence of the virus. One of the characteristics of an RNA virus such as SARS-CoV2, is its tendency to make mistakes in replication and mutation. This allows it to survive without being well recognized by the immune system, and also results in attenuation of its virulence.3
Assuming that children as secondary cases become infected in second, third or even fourth order after the initial case, would their clinical manifestations be more attenuated?
Children are more spared by SARS-CoV19 but are being stigmatized as carriers and disseminators of the infectionTo better appreciate potential infectivity in patients infected by SARS-CoV2 with mild disease (as usually happens in children), in general the concentrations of RNA and viral replication are higher in the first seven days when the viral load is greater and more active.28 After the first few days of symptoms, the viral load drops uniformly and infectivity is also significantly reduced, and the virus might not be isolated from respiratory samples after one week from symptoms onset.39 So what is the chance of transmission of active virus from the respiratory tract of the child? Some data indicate that the positivity time of the polymerase chain reaction (PCR) test is longer than in adults.3 In mild processes (such as in children), after the first 7–8 days the detection of viral RNA in the URT does not necessarily imply the presence of an infecting virus, as this depends on the threshold level of the viral load under which infectivity (virus isolation) is less likely. In this regard, a study in adult patients has shown that the virus was not detected in throat and sputum samples containing <106 copies/mL.28 Ideally, but impractical and chimerical as a widespread test, the determination of viral load would be an interesting resource to have an indirect but more concrete estimate of infectivity in the current circumstances.
Concerning the importance of the pediatric population as a transmitter, children infected with symptoms or asymptomatic, as well as adults, can potentially spread the infection. One concern, considering the significance which the disease can have, is that elderly people may become infected. As in the case of influenza, there is a high possibility of spread from children to grandparents, but with the difference that the latter are protected by a vaccine in the case of influenza. The role which children play in spreading the infection is unclear, but there are obviously no children in nursing homes for the elderly. So far, most reported children with infection have been part of a household outbreak, living with symptomatic or very subtly infected adults (almost all sick with symptoms before the children were infected)—suggesting that children are not an important reservoir.40 In the current situation with day-care centers and schools closed, it is very unlikely that children will become index cases in households. The true hazard of the asymptomatic child as a transmitter to its environment is probably being overestimated. Among over two thousand children diagnosed in China, 13% of those virologically confirmed had asymptomatic infection1—a rate that obviously minimizes the true number of asymptomatic infections, since many of them are not tested.
Although theoretically they can be as infectious as adults, asymptomatic children do not spread the virus by coughing, and generate a smaller volume of expired air than adults. In contrast, however, they have a lot of physical activity and closer social interaction. It is therefore prudent to remain cautious.
ConclusionsThe reasons for the relative resistance of children to some infections remain unclear. In relation to the very benevolent behavior of the SARS-CoV2 virus, several reasons have been proposed that are not free from argumentation and controversy, and which demand more studies, more time, and a greater notion of the magnitude and biology of this virus. The immunological interrelationship between the virus and its pediatric host has not been well recognized yet. The SARS-CoV2 virus discovered that children are not the "fishing grounds" to cast their nets, since the chances of abundant fishing—ACE2—are unfavorable. Probably, and given the extraordinary research in progress, in the time remaining until publication of this paper, light will have been shed on the questions now raised. In these months of pandemic, the observation that the incidence and severity are much lower in children does not necessarily mean that they are less susceptible to infection. The number of infected and sick children may increase in future, and with some concerns especially in children with special needs or underlying illnesses. The relevance of the childhood population in relation to the spread of infection is uncertain and no more accentuated than in adults.