The epidemiology and hospitalisation trends of primary immunodeficiency (PID) in Chile are unknown. We aimed to evaluate hospitalisation trends and demographic characteristics of PID admissions in Chile.
MethodsPID admissions between 2001 and 2010 (ICD-10 codes D70.0, D70.4, D71, 72.0, D76.1, D80-D84, E70.3, G11.3) were reviewed using national hospital discharge databases.
ResultsDuring the study period, 5486 admissions due to PID were registered (0.03% of total). 58.5% of patients were male and 66.3% were under 18 years. Median length of stay was one day (range 1–403 days). The most frequent diagnoses were hypogammaglobulinaemia (27.6%), unspecified immunodeficiency (21.9%), haemophagocytic lymphohystiocytosis (18.3%) and common variable immunodeficiency (11.2%). There was a significant increase in PID admission rate and in one-day hospitalisations during this period (β=0.2; P=0.001 and β=33; P≤0.001, respectively), however no significant variation was found for longer admissions (β=4.8; P=0.175). The increasing trend in PID admission rate was significant in patients with private, but not public insurance (β=0.53; P≤0.001 vs. β=0.08; P=0.079, respectively).
ConclusionsWe report an increasing trend in admissions due to PID in Chile over a 10-year period. Increase is mainly due to short hospitalisations, possibly accounting for improvements in IVIG access. Higher admission rates in patients with private vs. public insurance suggest socioeconomic disparities in access to PID treatment. ICD-10 coded hospitalisation databases may be useful to determine hospitalisation trends and demographic characteristics of PID admissions worldwide.
Primary immunodeficiencies (PID) group more than 250 distinct diseases that affect different components and functions of the immune system, predisposing affected individuals to recurrent infections and also to systemic inflammation, hypersensitivity reactions, autoimmunity and cancer.1,2 The incidence of PID in the general population is approximately 1:2500 live births, and the global prevalence is estimated to vary from 1:1200 to 1:500,000 depending on the population studied.3–8 The prevalence of PID depends on the type of immune defect, varying from 1:350 for the most prevalent and generally less severe forms, such as antibody deficiencies,8 to less than 1:1,000,000 for the more rare diseases.9 Although there has been an increasing trend in improving PID awareness and diagnosis, delayed diagnosis is still frequent3,7,8,10,11 and many patients probably remain undiagnosed, being subject to infections or complications that impair their quality of life and life expectancy.
Increasing trends in diagnosis of PID worldwide and better access to treatment have improved patients’ survival and quality of life. The most prevalent class of PID corresponds to antibody deficiencies, and for this patient group, immunoglobulin replacement is the standard therapy. Immunoglobulin is most frequently administered via the intravenous route (IVIG)12 even though subcutaneous immunoglobulin administration is also available worldwide.13 Treatment with IVIG has been shown to increase the life expectancy and reduce both frequency and severity of infections in PID patients,14–16 improving their quality of life.
In Chile, an increase in physician training and awareness about PID has contributed to improve the diagnosis and management of these patients.4,10 Despite this improvement, diagnosis is still frequently delayed or incomplete in many cases. Although Chile participates in the Latin American registry of PID, the contributions to this registry are incomplete and very few centres actively register their patients. Therefore, clinical and demographic data on the Chilean population living with PID is missing and studies are needed in order to evaluate the current status of diagnosis and access to treatment.
The Chilean population is a blend of European, mainly Spaniard, and American Indian ethnicities. In economic terms Chile is considered a developing country. Its 2010 gross domestic product per capita was equivalent to about one fourth of that reported in the United States, and it maintains high levels of income inequality. Its current healthcare system was established in the mid-1980s and options for insurance have been described as “mixed”, consisting of 65–70% public insurance (middle–low socioeconomic level population) and 15–20% private insurance (high socioeconomic level population). During the study period, Chile was divided in thirteen administrative regions from north to south. The Chilean population is unevenly distributed throughout the country, 12% live in the northern regions (I–IV), 62% in the central regions (V–VII and Metropolitan region) and 26% in the southern regions (VIII–XII). The capital city of Santiago, located in the Metropolitan region, concentrates 40% of Chilean population. The majority of immunologists during the period of study worked in academic and private institutions of Santiago, while very few practiced in a few southern regions (VIII and IX) and essentially none were based in the northern regions.
Patients with PID frequently require hospital admissions for diagnostic work-up, management of infectious or other complications, and treatment with IVIG. Furthermore, curative treatment with haematopoietic stem cell transplantation requires costly and prolonged hospital stays and multidisciplinary care at tertiary hospitals. During the study period no home-based therapies for immunoglobulin replacement were practiced yet in Chile, and IVIG would not be covered by insurance if administered as an outpatient therapy. Thus, all patients in Chile requiring IVIG infusions in both, public and private health systems were hospitalised every four weeks in order to receive treatment coverage from the government or health insurance companies. Hospital admission data have been previously reported to be useful to evaluate the epidemiology of PID at a local level.11,17 However, to the best of our knowledge, there are no population-based epidemiological studies centred on evaluating the characteristics and temporal trends of PID admissions in Latin America. The purpose of this study is to determine hospitalisation trends and demographic characteristics of admissions due to PID in Chile.
MethodsWe performed a retrospective review of national hospital discharge databases from the Chilean Ministry of Health to evaluate hospital admissions due to PID during the time period between January 2001 and December 2010.18 Hospital discharges due to PID were examined using the following ICD-10 codes: D70.0 (congenital agranulocytosis), D70.4 (cyclic neutropenia), D71 (functional disorders of polymorphonuclear neutrophils), D72.0 (genetic anomalies of leukocytes), D76.1 (haemophagocytic lymphohystiocytosis), D80 (hereditary hypogammaglobulinaemia), D81 (combined immunodeficiencies), D82 (Wiskott–Aldrich syndrome), D83 (common variable immunodeficiency), D84 (other immunodeficiencies), E70.3 (Chédiak–Higashi syndrome) and G11.3 (cerebellar ataxia with defective DNA repair). Other admissions coded as neutropenia (ICD-10 code D70; n=7404) were not included in this study due to the inability to differentiate congenital neutropenia from other more common causes unrelated to PID (e.g. neutropenia due to cancer chemotherapy). This database is a mandatory registry of all hospitalisations in the public and private health systems throughout the country. It provides a single main diagnosis per hospitalisation, however, no information on comorbid conditions or treatments administered during the hospital stay are provided. The database has no unique patient identifiers, and thus, does not provide data on incidence or readmissions. Paediatric admissions were defined as those occurring in patients younger than 18 years old, adult admissions were defined as those in patient 18 years old or older.
Statistical analysisAll rates were calculated per 100,000 inhabitants and are shown with 95% confidence intervals (95% CI). Simple linear regressions were used to evaluate the time trends of PID hospitalisations. Unstandardised β coefficients and 95% CI are reported for each regression. A two-sided P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics 21.0 (SPSS Inc., Chicago, IL, USA) and OpenEpi software version 2.3.1.19
EthicsThe Ethics Committee of the School of Medicine of Pontificia Universidad Católica de Chile approved this study. The authors declare that the procedures followed in this study were in accordance with the regulations of the Ethics Committee of the Pontificia Universidad Católica de Chile Medical School and in accordance with those of the World Medical Association and the Helsinki Declaration.
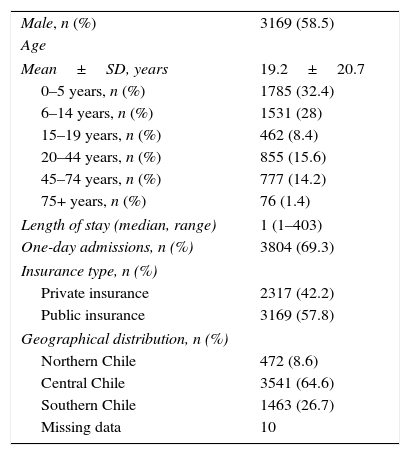
ResultsBetween 2001 and 2010 there were 5486 admissions with discharge diagnosis of PID, corresponding to 0.03% of overall hospitalisations. National PID admission rate was 3.38 per 100,000 inhabitants (95% CI 3.30–3.48), 58.5% of patients were male, mean age at admission was 19.2±20.7 years, and 66.3% were children under 18 years old. Median length of stay was one day (range 1–403 days). In-hospital lethality rate due to PID was 1%. According to the current IUIS classification,20 the PID groups with the highest and lowest in hospital lethality were the group of disorders of immune dysregulation (5.33%) and the group of predominantly antibody deficiencies (0.3%), respectively. No significant difference in lethality was observed between male and female patients (1.01 v/s 1.09%, P=0.8). Lethality due to PID during hospital admission was higher in patients 18 years or older compared to paediatric cases (1.6 v/s 0.7%, P<0.002); however, the age group with the highest lethality rate was that of patients younger than one year old (5.7%). Lethality due to PID during the first year of life was mainly explained by combined immunodeficiencies (66%) and disorders of immune dysregulation (13%) (Table 1).
Demographic characteristics of PID admissions in Chile, 2001–2010 (n=5486).
| Male, n (%) | 3169 (58.5) |
| Age | |
| Mean±SD, years | 19.2±20.7 |
| 0–5 years, n (%) | 1785 (32.4) |
| 6–14 years, n (%) | 1531 (28) |
| 15–19 years, n (%) | 462 (8.4) |
| 20–44 years, n (%) | 855 (15.6) |
| 45–74 years, n (%) | 777 (14.2) |
| 75+ years, n (%) | 76 (1.4) |
| Length of stay (median, range) | 1 (1–403) |
| One-day admissions, n (%) | 3804 (69.3) |
| Insurance type, n (%) | |
| Private insurance | 2317 (42.2) |
| Public insurance | 3169 (57.8) |
| Geographical distribution, n (%) | |
| Northern Chile | 472 (8.6) |
| Central Chile | 3541 (64.6) |
| Southern Chile | 1463 (26.7) |
| Missing data | 10 |
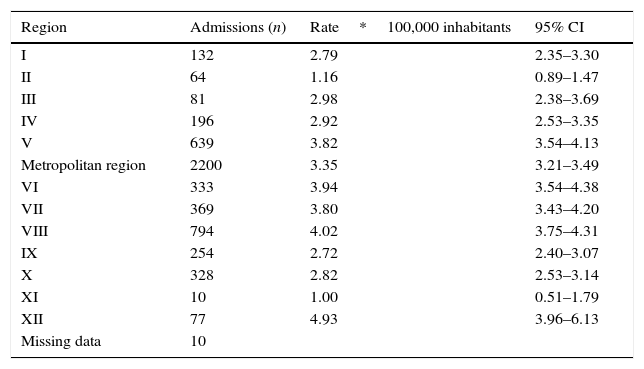
Sixty-four percent of all hospitalisations due to PID occurred in the central regions of Chile, with the Metropolitan region concentrating 40% of total PID admissions. A geographical analysis of PID admissions rates showed that both the central (rate 3.52 per 100,000 inhabitants; 95% CI 3.41–3.64) and southern (rate 3.38 per 100,000 inhabitants; 95% CI 3.21–3.56) regions of Chile showed a significantly higher rate of PID admissions when compared to northern Chile (rate 2.41 per 100,000 inhabitants; 95% CI 2.19–2.64) (P<0.001), but no significant difference was found between the admissions rate in central and southern Chile (P=0.19). The regions with the highest PID admission rates were the XII, VIII, VII, VI, V and the Metropolitan region (Table 2).
Regional PID admission rates in Chile, 2001–2010 (n=5486).
| Region | Admissions (n) | Rate*100,000 inhabitants | 95% CI |
|---|---|---|---|
| I | 132 | 2.79 | 2.35–3.30 |
| II | 64 | 1.16 | 0.89–1.47 |
| III | 81 | 2.98 | 2.38–3.69 |
| IV | 196 | 2.92 | 2.53–3.35 |
| V | 639 | 3.82 | 3.54–4.13 |
| Metropolitan region | 2200 | 3.35 | 3.21–3.49 |
| VI | 333 | 3.94 | 3.54–4.38 |
| VII | 369 | 3.80 | 3.43–4.20 |
| VIII | 794 | 4.02 | 3.75–4.31 |
| IX | 254 | 2.72 | 2.40–3.07 |
| X | 328 | 2.82 | 2.53–3.14 |
| XI | 10 | 1.00 | 0.51–1.79 |
| XII | 77 | 4.93 | 3.96–6.13 |
| Missing data | 10 |
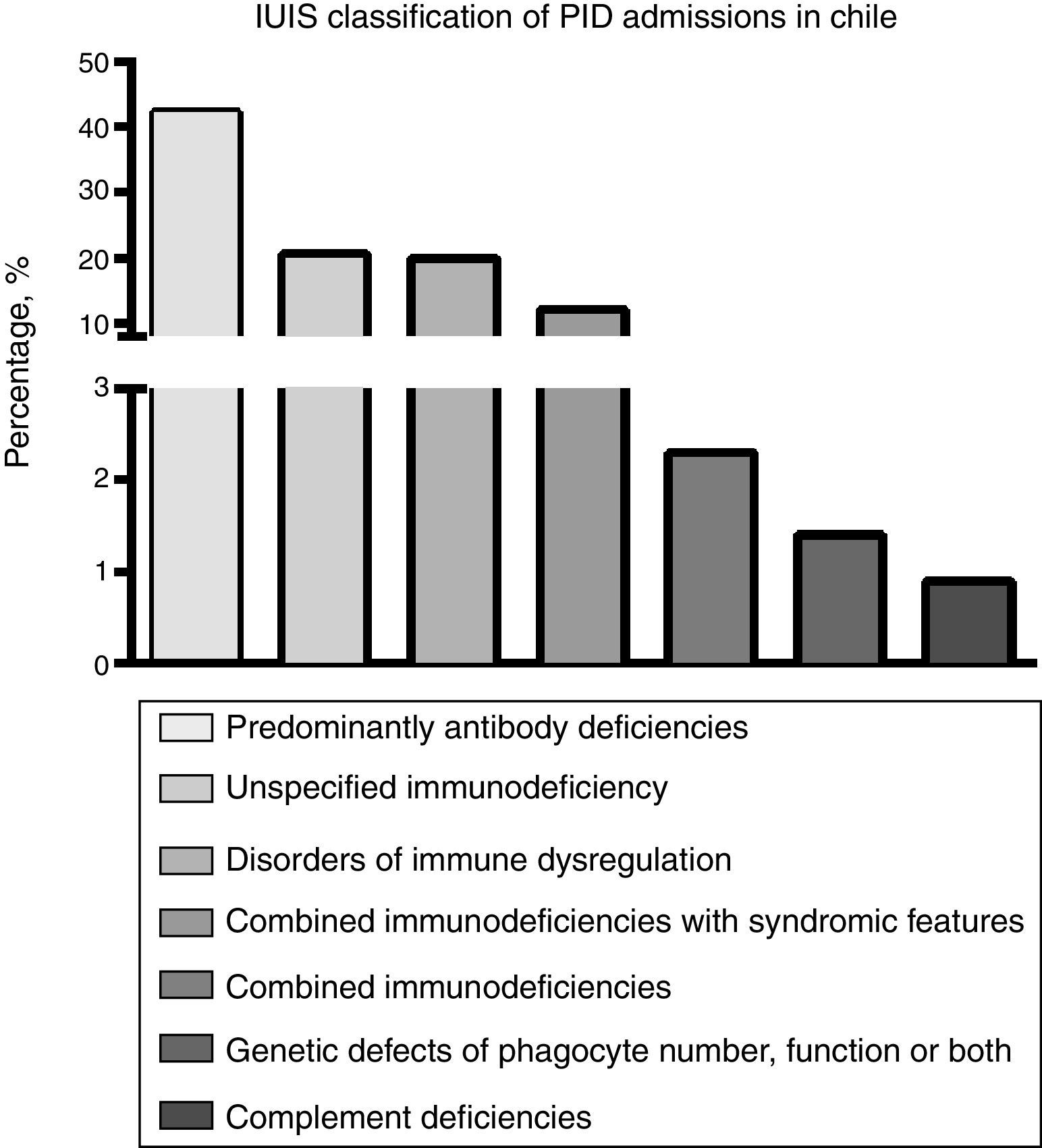
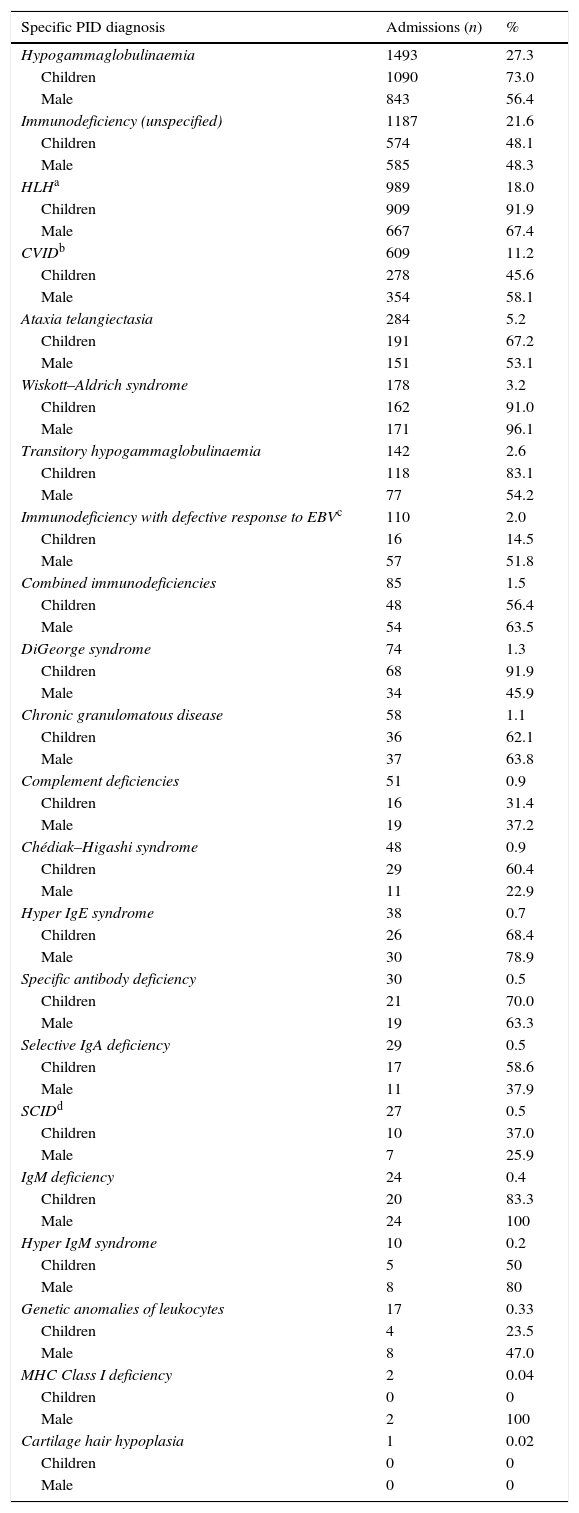
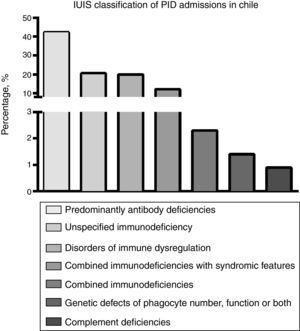
Using the current classification of PID,20 analysis of the hospital discharge database showed that antibody deficiency was the most frequent group of PID requiring hospitalisation in Chile (42.7%) (Fig. 1). The most frequently reported specific PID discharge diagnoses were: hypogammaglobulinaemia, unspecified immunodeficiency, haemophagocytic lymphohystiocytosis (HLH) and common variable immunodeficiency (CVID) (Table 3). Severe combined immunodeficiency (SCID) accounted for 0.5% of the PID-related admissions during the studied period; unexpectedly, only 37% of admissions with a diagnosis of SCID occurred in patients younger than 18 years old. While overall national admissions showed that 26.3% of patients had private insurance, a proxy of socioeconomic status in Chile, among PID admissions private insurance accounted for 42.2%.
Distribution of discharge diagnoses, age group and gender of patients admitted for PID in Chile, 2001–2010 (n=5486).
| Specific PID diagnosis | Admissions (n) | % |
|---|---|---|
| Hypogammaglobulinaemia | 1493 | 27.3 |
| Children | 1090 | 73.0 |
| Male | 843 | 56.4 |
| Immunodeficiency (unspecified) | 1187 | 21.6 |
| Children | 574 | 48.1 |
| Male | 585 | 48.3 |
| HLHa | 989 | 18.0 |
| Children | 909 | 91.9 |
| Male | 667 | 67.4 |
| CVIDb | 609 | 11.2 |
| Children | 278 | 45.6 |
| Male | 354 | 58.1 |
| Ataxia telangiectasia | 284 | 5.2 |
| Children | 191 | 67.2 |
| Male | 151 | 53.1 |
| Wiskott–Aldrich syndrome | 178 | 3.2 |
| Children | 162 | 91.0 |
| Male | 171 | 96.1 |
| Transitory hypogammaglobulinaemia | 142 | 2.6 |
| Children | 118 | 83.1 |
| Male | 77 | 54.2 |
| Immunodeficiency with defective response to EBVc | 110 | 2.0 |
| Children | 16 | 14.5 |
| Male | 57 | 51.8 |
| Combined immunodeficiencies | 85 | 1.5 |
| Children | 48 | 56.4 |
| Male | 54 | 63.5 |
| DiGeorge syndrome | 74 | 1.3 |
| Children | 68 | 91.9 |
| Male | 34 | 45.9 |
| Chronic granulomatous disease | 58 | 1.1 |
| Children | 36 | 62.1 |
| Male | 37 | 63.8 |
| Complement deficiencies | 51 | 0.9 |
| Children | 16 | 31.4 |
| Male | 19 | 37.2 |
| Chédiak–Higashi syndrome | 48 | 0.9 |
| Children | 29 | 60.4 |
| Male | 11 | 22.9 |
| Hyper IgE syndrome | 38 | 0.7 |
| Children | 26 | 68.4 |
| Male | 30 | 78.9 |
| Specific antibody deficiency | 30 | 0.5 |
| Children | 21 | 70.0 |
| Male | 19 | 63.3 |
| Selective IgA deficiency | 29 | 0.5 |
| Children | 17 | 58.6 |
| Male | 11 | 37.9 |
| SCIDd | 27 | 0.5 |
| Children | 10 | 37.0 |
| Male | 7 | 25.9 |
| IgM deficiency | 24 | 0.4 |
| Children | 20 | 83.3 |
| Male | 24 | 100 |
| Hyper IgM syndrome | 10 | 0.2 |
| Children | 5 | 50 |
| Male | 8 | 80 |
| Genetic anomalies of leukocytes | 17 | 0.33 |
| Children | 4 | 23.5 |
| Male | 8 | 47.0 |
| MHC Class I deficiency | 2 | 0.04 |
| Children | 0 | 0 |
| Male | 2 | 100 |
| Cartilage hair hypoplasia | 1 | 0.02 |
| Children | 0 | 0 |
| Male | 0 | 0 |
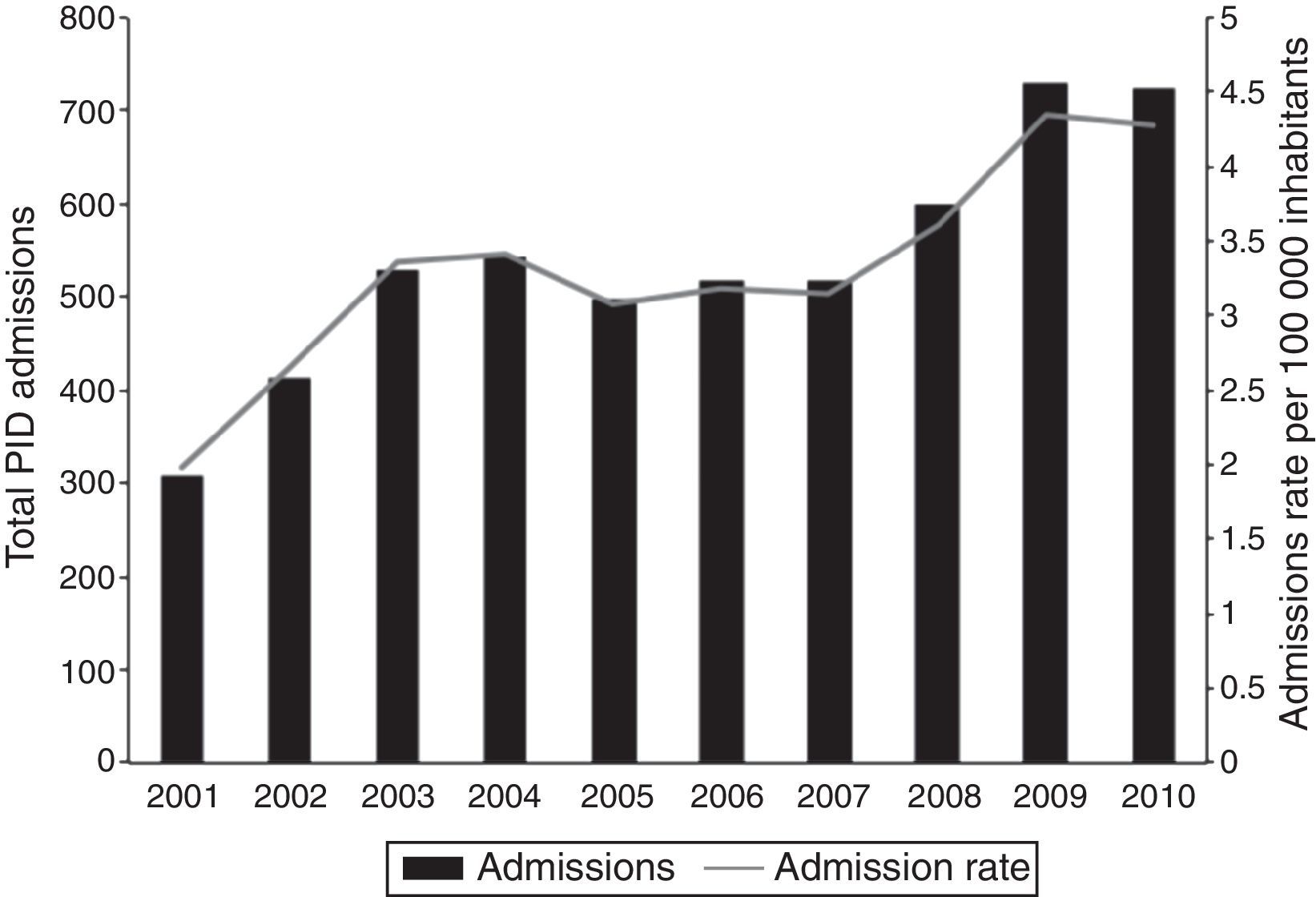
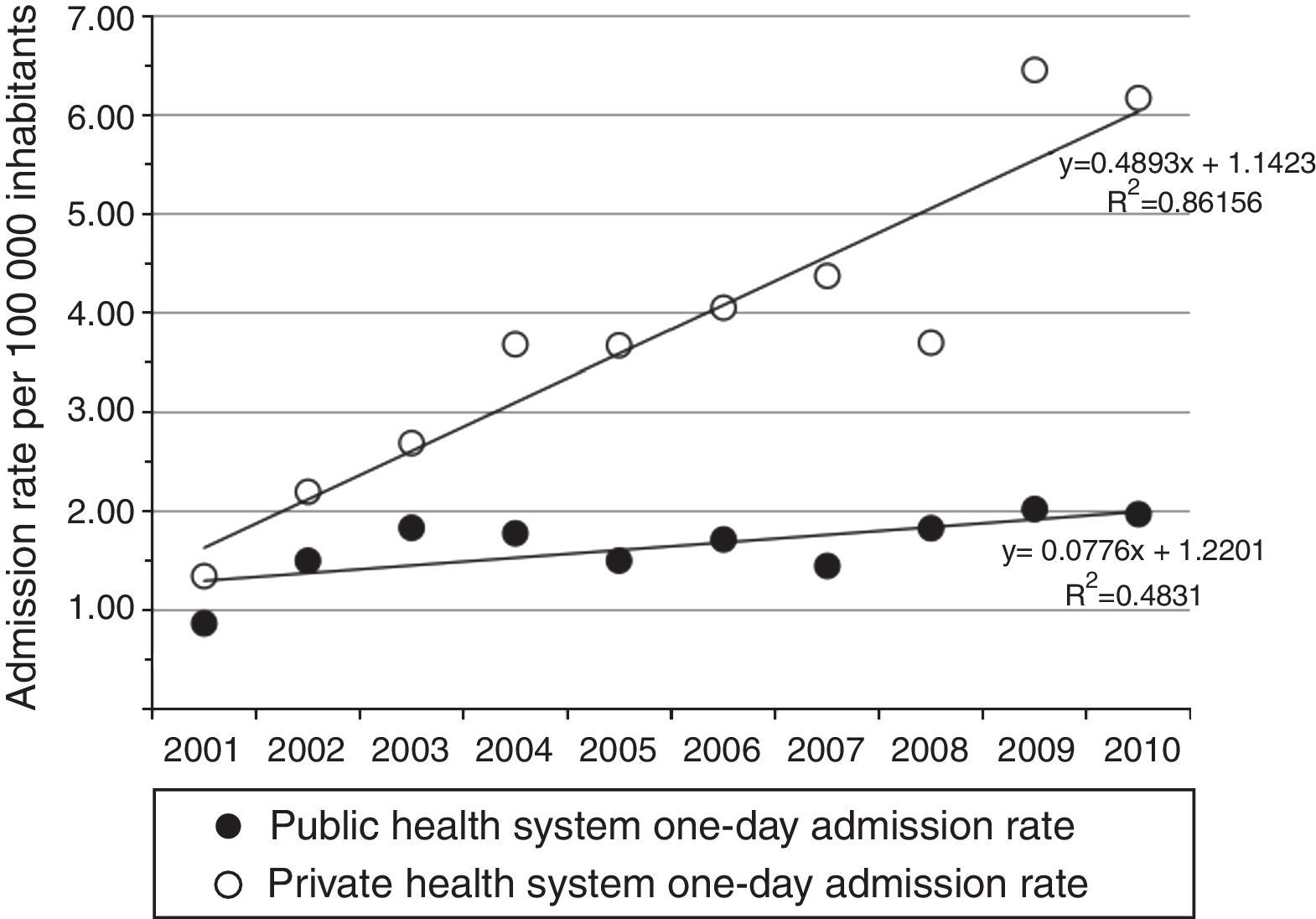
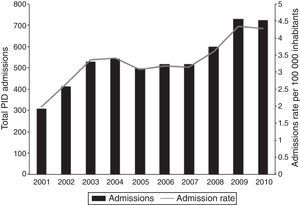
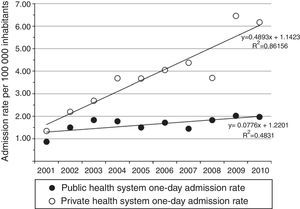
The total number of hospitalisations due to PID and the national PID admission rate significantly increased during the 2001–2010 period (β=37.9; P≤0.001 and β=0.2; P=0.001, respectively) (Fig. 2). Interestingly, almost 70% of the registered admissions due to PID lasted only one day. The observed increase in PID admissions was significant for one-day admissions (β=33; P≤0.001), whereas no significant yearly variation for longer hospitalisations was observed (β=4.8; P=0.175). The increasing trend in one-day admission rate was significant in patients with private, but not public, insurance (β=0.53; P≤0.001 vs. β=0.08; P=0.079, respectively) (Fig. 3). The increase in PID admission rates was significant in the adult and the paediatric (<18 years old) population (β=20.3; P=0.002 and β=17.8; P=0.014, respectively). The total monthly number of one-day admissions due to PID did not have significant variations throughout each year (P=0.84).
We report on a large nationwide decade-long study assessing the sociodemographic characteristics and diagnoses of admissions due to PID in Chile. Analysis of Chilean hospital discharge databases from 2001 to 2010 revealed an increasing trend of PID admission rate, mainly due to a significant increase in one-day admissions. The standard treatment for the most frequent PID is monthly immunoglobulin supplementation,12,15 which during the study period was exclusively administered as IVIG requiring all patients to be admitted to a hospital in order to obtain coverage from health insurance companies. Therefore, the sustained increase observed in one-day admissions suggests an improvement in IVIG access in Chile. This is also supported by the fact that there were no significant variations in monthly hospitalisations within each year.
In our study, the national PID admission rate was 3.38 per 100,000; which is higher than the expected 1:58,000 prevalence estimated from the LASID registry.4 The lack of unique patient identifiers and information on outpatient encounters makes it difficult to accurately estimate the prevalence of PID in Chile from this data; however, similar discrepancies between PID prevalence rates extrapolated from registries and from clinical encounters have been previously described in the literature.7 The observed PID admission rate may overestimate the real prevalence of PID due to the limitations of the information contained in the hospital discharge databases and the possibility of miscoding; however, it provides useful information for the development of health policies directed towards the Chilean population living with PID.
Using the current classification of PID,20 antibody deficiency was the PID group that accounted for almost half of the hospital admissions due to PID, being hypogammaglobulinaemia and CVID the most frequent diagnoses in this group, in agreement with international reports evaluating PID epidemiology.3,5,8,21 The second most frequent group of PID in this study was, surprisingly, that of diseases of immune dysregulation (20.9%), which has been reported to account for only 1% of PID diagnosis in the Latin American population.21 The overall frequency of admissions due to SCID was 0.5%, with an unexpected high rate of adult patients. Due to the lack of unique patient identifiers, the high frequency of adults with SCID may represent a few patients, diagnosed and treated during childhood, who have developed complications of their underlying diseases and require recurrent hospital admissions for management. However, this finding also raises the possibility of miscoding in the discharge diagnosis (e.g. patients with acquired immunodeficiency syndrome). However, the age and gender distribution for almost all other reported PIDs matches the epidemiological profile reported in the literature.
The unusually high frequency of rare PID in our study, like haemophagocytic lymphohistiocytosis (HLH), ataxia telangiectasia and Wiskott–Aldrich syndrome and the low frequency of more common PID like IgA deficiency and specific antibody deficiencies may be partially explained by the nature of the database used in this study. This database provide data only on patients requiring hospital admission; therefore, mild immunodeficiencies that can be managed in the outpatient setting are under-represented, while severe PID requiring admission for diagnostic or treatment purposes may be over-represented. Furthermore, the diagnosis of complex immunodeficiencies, like HLH, is often challenging in severely ill patients because current criteria are unspecific, there is no confirmatory gold standard and there are currently no centres carrying out genetic testing in Chile.22 The lack of national laboratories where a complete characterisation of the patient's immune system can be done, further contributes to the overestimation of severe PID with miscellaneous presentations. Thus, the surprisingly high frequency of HLH is unlikely to be explained by a high incidence or frequent readmissions of patients with familial haemophagocytic lymphohistiocytosis, but more likely due to secondary causes of severe immune dysregulation like macrophage activation syndrome in rheumatologic diseases or to a surplus in diagnosis due to the difficulties in obtaining laboratory tests to confirm the clinician's suspicion. The shortage of trained clinical immunologists and molecular diagnosis techniques in Chile may explain the fact that 21.6% of hospital discharges due to PID were listed as ‘unspecified immunodeficiency’ during the studied period.
Physician training and awareness of PID have contributed to advancing the care of patients with PID in Chile,4,21,23 where after many efforts, health authorities have begun to recognise PID as a special group of diseases and have started to facilitate access to treatment. However, important socioeconomic disparities remain. Having private health insurance in Chile is a proxy of high socioeconomic status. In this study, the observed increase in one-day admissions due to PID was only significant in the population with private health insurance suggesting that even though access to IVIG treatment has overall improved in Chile over the last decade, there are still significant disparities in access to appropriate treatment for Chilean PID patients between the public and the private healthcare systems. The results of this study raise awareness about important health disparities to be addressed by academic institutions training immunologists and health authorities in charge of developing treatment policies for PID.
The average potential use of IVIG for patients with antibody deficiency syndromes was recently evaluated by Stonebraker et al.24 They estimated that the potential need for IVIG usage was 72±40g per 1000 inhabitants, depending on the prevalence of PID in a given population and the treatment regimens adopted by physicians. According to an immunoglobulin market report, between 2007 and 2010, the Chilean IVIG market increased sales by 23% reaching 10.2g per 1000 inhabitants for all IVIG indications.25 The use of IVIG for the treatment of antibody deficiencies has been estimated to account for 20–30% of the overall IVIG usage,24 which makes the use of IVIG for antibody deficiency replacement in Chile to be far below Stonebraker's estimate. This suggests that PID patients requiring IVIG in Chile are probably underdiagnosed and/or undertreated; however, no data on the indications of IVIG in Chile is available and the hospital discharge databases do not provide information about treatments received during hospital stay.
The exact incidence of PID in Chile is unknown because new-born screening or other screening strategies have not yet been established and the current PID registry is incomplete. In-hospital lethality due to PID during the first year of life was the highest rate during the studied period and a 66% of the deaths were explained by combined immunodeficiencies. The implementation of screening programmes for severe immunodeficiencies has been described as an effective way to provide timely access to diagnosis and life-saving treatment for new-borns with severe immunodeficiencies.26 In light of this data and considering the disparities in access to clinical immunologist through Chile, further measures need to be taken to introduce PID screening tests into the currently existing Chilean new-born screening programme.
The prevalence of PIDs in Chile has been estimated to be 1:58,000 and the expected number of PID patients to be approximately 1600 as of 2006.4 The online Latin American (LASID) registry of PID was officially launched on 2009 and currently has less than a hundred Chilean patients registered. The available data from this registry does not allow us to establish time trends in PID diagnosis or analyse socioeconomic status of patients. Chilean patients included in the registry were entered almost exclusively by one tertiary centre, which impedes an accurate assessment of the epidemiology of PID in the country using the registry data. The lack of an accurate evaluation of PID epidemiology in Chile makes it difficult to estimate the real need for IVIG supplementation in PID patients and compare it to what is actually being administered in the distinct socioeconomic segments.
The geographical distribution of PID admissions in Chile showed a significantly higher admission rate in the central and southern areas of the country when compared to the northern regions. Geographical distribution differences in diagnosis requiring evaluation of paediatric subspecialists have been previously described in Chile.27 Besides the Metropolitan Region, in which Santiago is located, almost all the regions with high PID admission rates had large urban areas with academic institutions homing medical schools. A directed effort towards training immunologists and increasing awareness of PID should be made to improve diagnosis and treatment of underserved regions.
The strength of this study is that it includes all hospital admissions due to PID in Chile, both in the private and public sector, during the studied period, therefore allowing us to obtain information from Chilean patients living with PID regardless of their socioeconomic status and geographical location. The limitations of our study arise from the nature of the information obtained from the hospital discharge databases and include the fact that it is not possible to estimate the incidence or prevalence of PID due to the lack of unique patient identifiers and that not all PID patients are hospitalised. Nevertheless, we think this ICD-10 based approach is a very useful tool as it provides a valuable initial approach to PID epidemiology in Chile, and may be applicable in other countries worldwide where PID registry is not mandatory or has low coverage. Another caveat is the possibility of bias due to miscoding and misclassification of PID patients, as well as other diseases miscoded as PID. We attempted to reduce this bias by not including ICD-10 codes that are used for other common diseases such as neutropenia of different aetiology and by analysing the distribution of PIDs using the IUIS classification groups rather than single diagnoses. In addition, the database provides only one diagnostic code per admission, so patients with PID may have been missed in this study due to coding for the cause of admission (e.g. pneumonia) instead of the underlying PID.
In conclusion, we report a large nationwide study analysing sociodemographic characteristics, trends and healthcare disparities of PID admissions in Chile. A sustained increase in PID admissions has been experienced in Chile during the last decade, which might reflect an improvement in diagnosis and possibly reflect an increase in access to IVIG treatment in this population. High geographical segregation was also observed for PID admissions, most of them occurring in regions homing academic centres in large urban areas and probably reflecting a better access to care by trained clinical immunologists. Even though several organisations have been working on raising PID awareness, there is still an unmet need to train more physicians in the diagnosis and treatment of PID and to improve access to molecular diagnostic techniques in Chile. The recent opening of a Jeffrey Modell Foundation centre for PID research in Chile and the opening of the first paediatric immunology fellowship in the country should eventually lead to an extensive network of specialists throughout the country, improving patient care and diagnosis. Further efforts must be taken to improve the national registry of PID and to include PID screening in the national neonatal screening programme.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article. Authors declare that no patient data appear in this article.
Funding sourcesMillennium Institute on Immunology and Immunotherapy (Chile) grant P09/016-F.
Conflict of interestDr. Borzutzky has received an educational travel grant from Grifols.