We aimed to determine adverse reactions and influencing factors, within the scope of the number of patients and total infusions, in patients with primary immunodeficiencies receiving intravenous immunoglobulin (IVIG) replacement.
Materials and methodsChildren with primary immunodeficiencies receiving IVIG replacement in Izmir Dr Behcet Uz Children’s Hospital, between June 2014 and June 2016, were included in our study.
ResultsThe total number of the patients receiving IVIG replacement was 145 (37 female, 108 male). The number of total IVIG infusions was 1214. Adverse reactions were observed in 44.8% of the patients and 14.2% of the infusions. Common variable immunodeficiency was the most common diagnosis of the patients and adverse reactions most commonly developed in this group (24.2%). In all infusions the most frequent adverse reaction was headache (7.8%); fever was the most frequent immediate side effect (3.9%), whereas headache was the most common delayed adverse effect (5.1%). By logistic regression analyses, history of adverse reaction to IVIG in previous infusions, existence of concomitant infectious disease, past or family history of atopic disease, to receive IVIG infusion at the first time, or being under 10 years old were found associated with adverse reactions. There was no correlation between the concentration of IVIG preparations and the rate of side-effect development.
ConclusionsIn our study no severe adverse reaction to IVIG was observed, but many mild or moderate side effects occurred. Therefore, IVIG indications must be well identified. Patients, family of the patients and health care workers must be informed for adverse reactions.
Intravenous immunoglobulin (IVIG) was firstly licenced in 1981 for the treatment of primary antibody deficiencies. During the past two decades, human IVIG therapy has become the mainstay of various diseases in which the immune system is disrupted.1,2 Standard IVIG preparations are obtained from 5000 to 10000 donor plasma. Since they are prepared from a lot of donor plasma, they include various antibodies of the donors, arisen from infections and immunizations.3,4 IVIG therapy is used especially in patients with primary immunodeficiencies including primary antibody deficiencies such as X-linked agammaglobulinemia, common variable immunodeficiency (CVID), some types of Hyper-IgM syndrome and combined B and T cell deficiencies. In those types of patients, with IVIG replacement, immunoglobulins are passively transferred.1,4 The main purpose of the IVIG therapy is to obtain achieve functional IgG levels, to neutralise infection agents, and to provide sufficient passive antibodies for opsonization. In fact, IVIG therapy is more successful than the replacement of immunoglobulins, by affecting development and functions of the immune system cells.4
In the short term IVIG therapy decreases the frequency and severity of infections and improves quality of life. In the long term it prevents the patient from organ damage because of recurrent infections.5,6
In most of the practice guidelines a starting dosage of IgG between 400 and 600 mg/kg/monthly is recommended to achieve a serum trough IgG level of 600–800 mg/dL for immunodeficiencies.7,8
In generally, the adverse effects of IVIG infusions are mild and are related to the infusion rate. Formation of immunoglobulin aggregates leading to complement system activation can be prevented by decelerating infusion rates. In patients with primary antibody production defects, systemic adverse reactions are frequently seen in first infusions, especially in patients with very low immunoglobulin levels and having infusions after long untreated periods.3,4 Temporary slowdown of infusions or having pauses for 15−30 min may result in a disappearance of the side effects. The frequency of side effects decreases if infusions are given in four hours. Cases that require completely suspending the infusions are extremely rare. IgE types of antibodies to IgA may rarely cause anaphylactic reactions. In these rare cases, infusions must be promptly stopped, and anaphylactic reactions must be treated.3,4 Aseptic meningitis, renal failure, thromboembolism and hemolytic reactions are other rare but severe adverse effects. Transfusion related acute lung injury, enteritis and skin reactions are rarely encountered as late side effects.9
Although IVIG preparations seem to be similar to each other, they differ in immunoglobulin fractions, refining and mixture stages and the plasma used for the preparations. Thus, different preparations may be preferred in some different conditions. Every preparation may be tolerated differently from others and may lead to various adverse reactions.
In this study, in children with primary immunodeficiencies receiving IVIG replacement in Ambulatory Treatment Department of Izmir Dr Behcet Uz Children’s Hospital, between June 2014 and June 2016, we aimed to determine the features of the patients, frequency, type and severity of adverse reactions related to IVIG, risk factors and possible precautions for adverse reactions.
Materials and methodsThe Local Research Ethics Committee granted approval and the study was designed as a single institution retrospective cross-sectional study.
The study was performed in the Pediatric Immunology Department of Dr. Behcet Uz Children’s Research and Teaching Hospital between June 2014 and June 2016. All children with primary immunodeficiency receiving IVIG replacement in the ambulatory treatment department of the hospital were evaluated, retrospectively. From the patient files, data about age, gender, indication of IVIG replacement, concomitant diseases, other drugs used, past and family history of atopy were obtained. Dates of all infusions, concentrations and stabilising agent content of the IVIG solutions, administration of premedication, time and type of the side effects, existence of concomitant infectious diseases were recorded. Side effects were classified as immediate adverse reactions developed in 0−6 h, and delayed adverse reactions developed in 6 h to 1 week.10
We used SPSS for Windows 21.0 for all calculations. Descriptive statistical analyses of the dataset were performed. Categorical variables were compared using Fischer’s exact chi-square and Pearson chi-square tests. Significance was set at P < 0.05. The existence of an adverse reaction was accepted as independent variable and logistic regression analysis was applied.
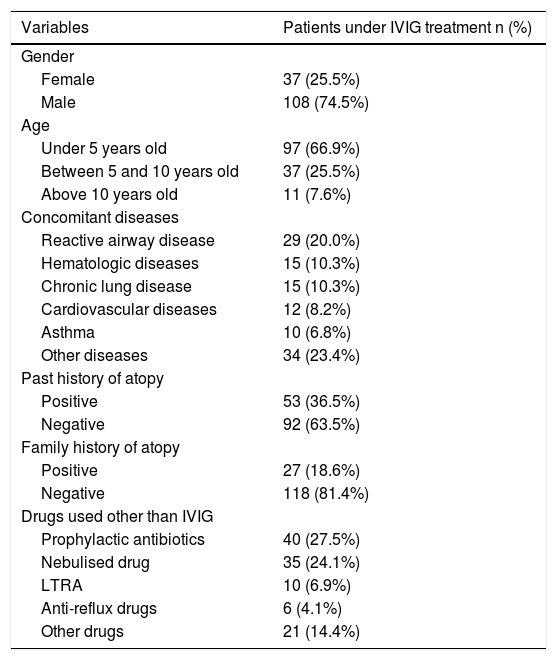
ResultsOne hundred and forty-five patients (37 female, 108 male) with primary immunodeficiency, under IVIG replacement and regular follow-up, not passed to subcutaneous administration in the course of the disease, were included in the study. Median age was 28 months in males and 41 months in females and 97 of the patients (66.9%) were under five years old. The most common concomitant disease was reactive airway disease (20%). Fifteen of the patients had a concomitant disease such as hematologic diseases (anaemia and thrombocytopenia) and chronic lung diseases (bronchopulmonary dysplasia and bronchiectasis). 53 of the patients had past history of atopy and 27 of them had family history of atopy in first degree relatives. The sociodemographic properties of the 145 patients are given in Table 1.
Sociodemographic properties of the patients.
| Variables | Patients under IVIG treatment n (%) |
|---|---|
| Gender | |
| Female | 37 (25.5%) |
| Male | 108 (74.5%) |
| Age | |
| Under 5 years old | 97 (66.9%) |
| Between 5 and 10 years old | 37 (25.5%) |
| Above 10 years old | 11 (7.6%) |
| Concomitant diseases | |
| Reactive airway disease | 29 (20.0%) |
| Hematologic diseases | 15 (10.3%) |
| Chronic lung disease | 15 (10.3%) |
| Cardiovascular diseases | 12 (8.2%) |
| Asthma | 10 (6.8%) |
| Other diseases | 34 (23.4%) |
| Past history of atopy | |
| Positive | 53 (36.5%) |
| Negative | 92 (63.5%) |
| Family history of atopy | |
| Positive | 27 (18.6%) |
| Negative | 118 (81.4%) |
| Drugs used other than IVIG | |
| Prophylactic antibiotics | 40 (27.5%) |
| Nebulised drug | 35 (24.1%) |
| LTRA | 10 (6.9%) |
| Anti-reflux drugs | 6 (4.1%) |
| Other drugs | 21 (14.4%) |
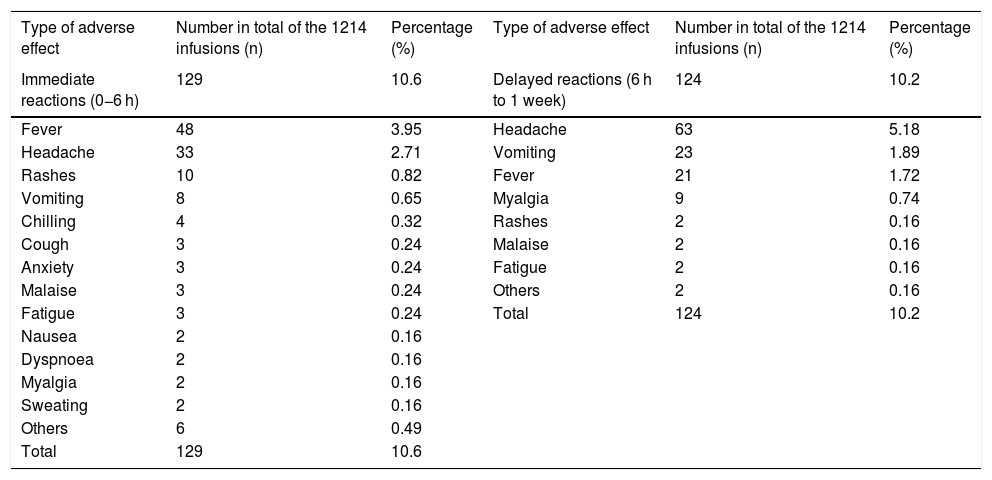
In 145 patients 1214 infusions were performed; 93 of the 145 patients had their first administration of IVIG in their lives. Adverse reactions occurred in 65 of the 145 (44.8%) patients. For the patients with their first administration of IVIG, adverse reactions occurred in 23 (24.7%) infusions. Recurrent infusions adverse reactions developed in 149 of 1121 (13.2%). Fifty-three percent of the adverse reactions were immediate and 47% of them were delayed reactions. The most common immediate adverse reactions were fever (3.9%) and headache (2.7%). The most common delayed adverse reactions were headache (5.1%), vomiting (1.8%) and fever (1.7%) (Table 2).
Immediate and delayed adverse effects in total of the infusions.
| Type of adverse effect | Number in total of the 1214 infusions (n) | Percentage (%) | Type of adverse effect | Number in total of the 1214 infusions (n) | Percentage (%) |
|---|---|---|---|---|---|
| Immediate reactions (0−6 h) | 129 | 10.6 | Delayed reactions (6 h to 1 week) | 124 | 10.2 |
| Fever | 48 | 3.95 | Headache | 63 | 5.18 |
| Headache | 33 | 2.71 | Vomiting | 23 | 1.89 |
| Rashes | 10 | 0.82 | Fever | 21 | 1.72 |
| Vomiting | 8 | 0.65 | Myalgia | 9 | 0.74 |
| Chilling | 4 | 0.32 | Rashes | 2 | 0.16 |
| Cough | 3 | 0.24 | Malaise | 2 | 0.16 |
| Anxiety | 3 | 0.24 | Fatigue | 2 | 0.16 |
| Malaise | 3 | 0.24 | Others | 2 | 0.16 |
| Fatigue | 3 | 0.24 | Total | 124 | 10.2 |
| Nausea | 2 | 0.16 | |||
| Dyspnoea | 2 | 0.16 | |||
| Myalgia | 2 | 0.16 | |||
| Sweating | 2 | 0.16 | |||
| Others | 6 | 0.49 | |||
| Total | 129 | 10.6 |
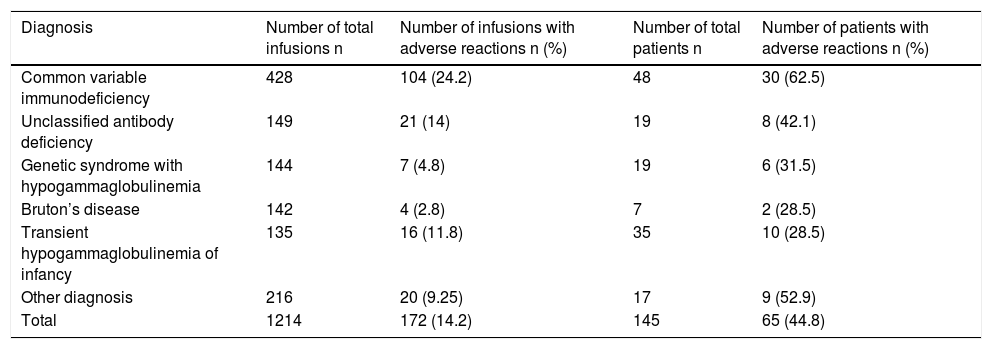
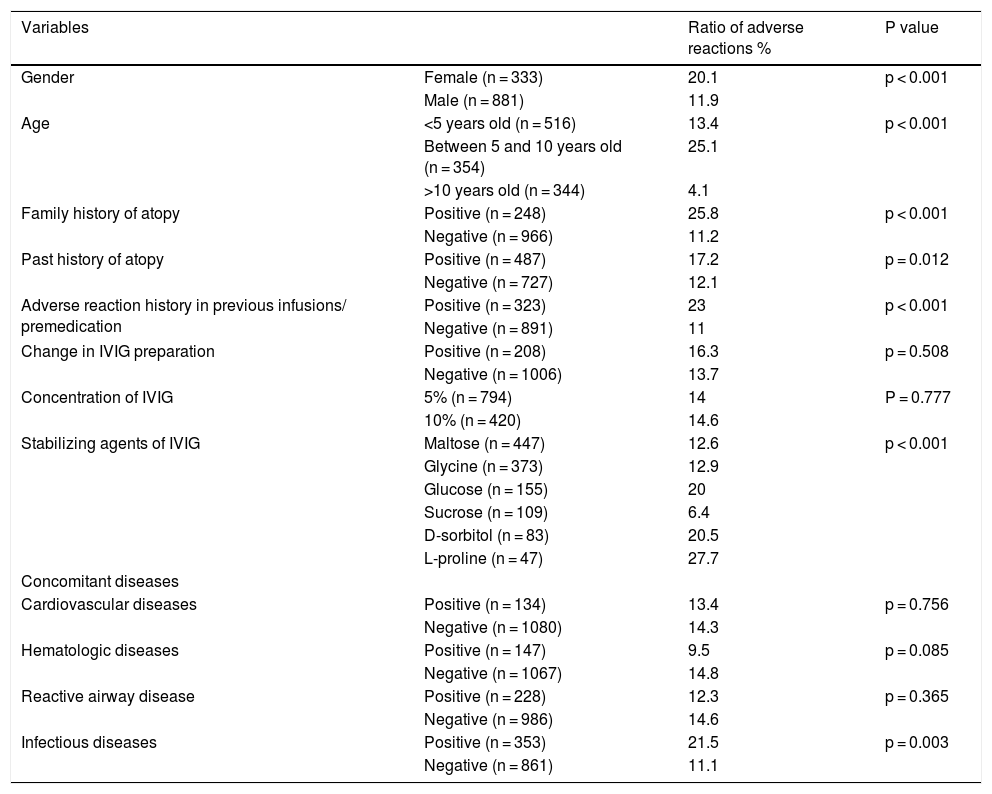
In total, headache was the most common adverse reaction (7.8%). In two of these patients, headache was so severe that it required hospitalization, intravenous hydration, dexamethasone, antihistaminic, analgesic and antiemetic drugs. 3.37% of the total infusions were interrupted, 0.25% were slowed and 0.91% were stopped completely. 428 of the infusions (35.2%) were performed for common variable immunodeficiency (CVID) and 149 of the infusions (12.2%) were administered for unclassified antibody deficiencies (UAD). In infusions performed in patients with CVID, adverse reactions occurred in 104 (24.2%) of them. For UAD, this proportion was found to be 14% (Table 3). Adverse reactions developed in 11.9% of the infusions in males and 20.1% of the infusions in females (p < 0.001). Adverse reactions were evaluated in terms of age groups: under five years old in 13.4%, between five and 10 years in 25.1%, above 10 years in 4.1% of the infusions adverse reactions occurred. Adverse reactions were significantly higher in children between five and 10 years old (p < 0.001). In patients with a family history of atopy, adverse reactions were encountered more frequently, in 25.8% of the infusions (p < 0.001). Adverse reactions in patients with a history of atopy were again encountered more frequently, in 17.2% of the infusions (p = 0.012). No significant association was found between adverse reactions and concomitant cardiovascular diseases, hematologic diseases or reactive airway disease. Premedication was used in patients with adverse reaction history in previous infusions. In 26.6% of the total infusions, premedication was found to be administered. The most common premedication was antihistamines (13.67%), then ibuprofen (6.5%) and these drugs together (5.18%). In infusions without premedication, adverse reactions appeared in 11% of the infusions, but in infusions with premedication adverse reactions arose in 23% of the infusions (p < 0.001) These results indicated that a previous history of adverse reactions to IVIG infusion increases the risk of adverse reactions and premedication may prevent these reactions in 77% of the infusions. In patients with concomitant infectious disease, adverse reactions developed in 21.5% of the infusions, without concomitant infections developed in 11.1% of the infusions. In the administration of 5% IVIG preparations, adverse reactions occurred in 14% of the infusions, and in 10% IVIG preparations adverse reactions occurred in 14.6% of the infusions (p = 0.777). In 34 (19.7%) of the 172 infusions with adverse effects changing of the IVIG preparation was present. Changing the IVIG preparation did not affect the adverse reaction rate. During the follow-up period no severe reactions such as aseptic meningitis, thromboembolism or haemolytic reactions appeared. The distribution of adverse reaction rates in total of the infusions is given in Table 4.
Adverse effects according to the diagnosis of the patients.
| Diagnosis | Number of total infusions n | Number of infusions with adverse reactions n (%) | Number of total patients n | Number of patients with adverse reactions n (%) |
|---|---|---|---|---|
| Common variable immunodeficiency | 428 | 104 (24.2) | 48 | 30 (62.5) |
| Unclassified antibody deficiency | 149 | 21 (14) | 19 | 8 (42.1) |
| Genetic syndrome with hypogammaglobulinemia | 144 | 7 (4.8) | 19 | 6 (31.5) |
| Bruton’s disease | 142 | 4 (2.8) | 7 | 2 (28.5) |
| Transient hypogammaglobulinemia of infancy | 135 | 16 (11.8) | 35 | 10 (28.5) |
| Other diagnosis | 216 | 20 (9.25) | 17 | 9 (52.9) |
| Total | 1214 | 172 (14.2) | 145 | 65 (44.8) |
Distribution of adverse effects in terms of total infusions.
| Variables | Ratio of adverse reactions % | P value | |
|---|---|---|---|
| Gender | Female (n = 333) | 20.1 | p < 0.001 |
| Male (n = 881) | 11.9 | ||
| Age | <5 years old (n = 516) | 13.4 | p < 0.001 |
| Between 5 and 10 years old (n = 354) | 25.1 | ||
| >10 years old (n = 344) | 4.1 | ||
| Family history of atopy | Positive (n = 248) | 25.8 | p < 0.001 |
| Negative (n = 966) | 11.2 | ||
| Past history of atopy | Positive (n = 487) | 17.2 | p = 0.012 |
| Negative (n = 727) | 12.1 | ||
| Adverse reaction history in previous infusions/ premedication | Positive (n = 323) | 23 | p < 0.001 |
| Negative (n = 891) | 11 | ||
| Change in IVIG preparation | Positive (n = 208) | 16.3 | p = 0.508 |
| Negative (n = 1006) | 13.7 | ||
| Concentration of IVIG | 5% (n = 794) | 14 | P = 0.777 |
| 10% (n = 420) | 14.6 | ||
| Stabilizing agents of IVIG | Maltose (n = 447) | 12.6 | p < 0.001 |
| Glycine (n = 373) | 12.9 | ||
| Glucose (n = 155) | 20 | ||
| Sucrose (n = 109) | 6.4 | ||
| D-sorbitol (n = 83) | 20.5 | ||
| L-proline (n = 47) | 27.7 | ||
| Concomitant diseases | |||
| Cardiovascular diseases | Positive (n = 134) | 13.4 | p = 0.756 |
| Negative (n = 1080) | 14.3 | ||
| Hematologic diseases | Positive (n = 147) | 9.5 | p = 0.085 |
| Negative (n = 1067) | 14.8 | ||
| Reactive airway disease | Positive (n = 228) | 12.3 | p = 0.365 |
| Negative (n = 986) | 14.6 | ||
| Infectious diseases | Positive (n = 353) | 21.5 | p = 0.003 |
| Negative (n = 861) | 11.1 | ||
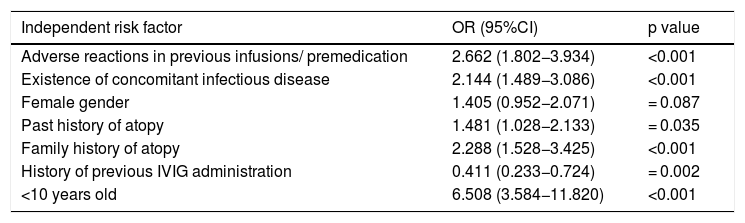
The existence of adverse reaction was accepted as independent variable and logistic regression analysis was applied. History of adverse reactions in previous infusions, concomitant infectious disease, being under 10 years old, past or family history of atopy, no history of receiving previous IVIG infusion were found to increase the risk of adverse reactions (Table 5).
Logistic regression analysis of the risk factors for adverse reaction development related to IVIG.
| Independent risk factor | OR (95%CI) | p value |
|---|---|---|
| Adverse reactions in previous infusions/ premedication | 2.662 (1.802−3.934) | <0.001 |
| Existence of concomitant infectious disease | 2.144 (1.489−3.086) | <0.001 |
| Female gender | 1.405 (0.952−2.071) | = 0.087 |
| Past history of atopy | 1.481 (1.028−2.133) | = 0.035 |
| Family history of atopy | 2.288 (1.528−3.425) | <0.001 |
| History of previous IVIG administration | 0.411 (0.233−0.724) | = 0.002 |
| <10 years old | 6.508 (3.584−11.820) | <0.001 |
Adverse effects associated with IVIG therapy vary with the type of present disease and IVIG preparation. In patients with immunodeficiency, administration of IVIG in constant intervals, using the same IVIG preparation that the patient previously tolerated and using the same dosages decreases the rate of systemic side effects. Subcutaneous immunoglobulin therapy can be applied in our clinic but in our study we analysed only intravenous immunoglobulin administration, only one patient was passed subcutaneous immunoglobulin therapy because of adverse effects and no adverse effects were seen after subcutaneous immunoglobulin therapy. In patients without immunodeficiency with neurologic and hematologic diseases, and in patients receiving high dose IVIG (1–2 gr/kg) systemic adverse reactions occur more frequently.9 Donofrio et al. demonstrated that, in patients with chronic inflammatory polyneuropathy, systemic adverse effects occurred after high dose IVIG administration in 55% of 113 patients and in 18% of the infusions.11
In the study including 72 centres, 1705 patients with primary and secondary immunodeficiency and 15,548 IVIG infusions with the same preparation, Struff et al. showed that systemic adverse reactions developed in only 0.6% of the patients and 0.064% of the infusions.12 In the review of nine different clinical trials, Schroder et al. found that in patients with primary antibody deficiency, systemic side effects occurred in 26–44% of the patients and 5–27% of the infusions.13 Brennan et al. disclosed that systemic adverse reactions occurred in 59 of 459 (13%) of the patients with primary antibody deficiency and occurred in 111 of 13,508 (0.8%) of the infusions,14 in their study including four centres. In 58 patients under IVIG therapy (33 patients for primary immunodeficiency, 25 patients for immune modulation), Singh-Grewal et al. demonstrated that systemic adverse effects developed in 44.8% of the patients and 24.3% of the infusions.15 In our study, side effects related to IVIG occurred in 44.8% of the 145 patients and 14.2% of the infusions. Common variable immunodeficiency was the most common diagnosis of the patients and the highest side effect ratio (24.2%) was found in that group.
Adverse reactions related to IVIG are classified as immediate and delayed reactions. Systemic reactions usually develop in the early period (in the first six hours). Early reactions may be related with the activation of complement system due to aggregation of immunoglobulin molecules (immunoglobulin G complexes bind to Fc receptors on monocytes and phagocytes, activate complement system and promote releasing of inflammatory mediators) and stabilising agents in the preparations. Immediate side effects are generally mild and limited. Fever, trembling, headache, rashes, nausea and vomiting, anxiety, generalized body pain, heart rate and blood pressure changes are observed in early periods. These side effects can be prevented by slowing down the infusion rate and using premedication (antihistamines, corticosteroids, paracetamol). Anaphylactic reactions due to IVIG are extremely rare. Delayed adverse reactions are generally systemic and more complicated reactions but seem rare. Persistent headache, aseptic meningitidis, renal failure, thromboembolism, hematologic reactions are examples of delayed reactions.10 In a prospective study, Singh-Grewal et al. evaluated 58 patients and 345 IVIG infusions. Immediate reactions occurred in 10.3% of the patients and 3.5% of the infusions and delayed reactions occurred in 41.4% of the patients and 20.9% of the infusions. The most common immediate side effects were found as headache (0.9%), pain in infusion area (0.9%) and vertigo (0.9%). Headache was the most common (12.8%) delayed side effect.15 In our study, when all infusions were evaluated, immediate side effects were more common (53%). Fever (3.9%) and headache (2.7%) were the most common of these immediate reactions. Headache (5.1%), vomiting (1.8%) and fever (1.7%) were the most common delayed reactions.
IVIG-related headache may occur as an immediate or delayed side effect. Singh-Grewal et al. demonstrated that headache developed in 26 of 58 patients and 24.3% of the infusions mostly as a delayed adverse effect.15 Headache is more frequent in patients under IVIG treatment for neurologic or autoimmune diseases. However, it also may develop in patients with primary immunodeficiency even in standard doses. In a cohort study including 117 patients with primary immunodeficiency under IVIG therapy (1765 infusions) with the dosage of 600 mg/kg in 3–4 weeks interval, headache was found as the most common delayed adverse effect.16 In our study, we also found headache to be the most common side effect, in 7.8% of all infusions. Headache required hospitalization and medical treatment in two of these patients.
If there is a history of side effect in previous infusions, premedication and hydration should be performed and infusion rate should be slowed down. Changing of the preparation may rarely be required. Premedication with antihistamines and non-steroid anti-inflammatory drugs decreases the rate of adverse effects. Hydrocortisone has also been demonstrated to decrease IVIG-infusion related adverse reactions.17 In our clinic, patients with previous history of adverse effects were routinely administered premedication. In our study, we found that premedication was required in 26.6% of the infusions. Antihistamines (13.7%), ibuprofen (6.5%), and these two drugs together (5.18%) were the most common drugs used for premedication. In patients with history of previous adverse reactions, premedication prevented 77% of these reactions in the following infusions.
Renal failure after IVIG administration has rarely been reported previously. This condition has been considered to be the consequence of osmotic load.18 Between 1985 and 1998, a total of 88 renal failure cases had been reported to the Federal Drug Association (FDA). Renal failure had been reported to develop after the IVIG preparations which include sucrose as a stabilizing agent instead of d-sorbitol.18 In these patients hematuria, mild or moderate proteinuria may occur. Before the first IVIG infusion and in the follow-up, renal function tests should be monitored. In patients with an underlying renal disease, preparations without sucrose should be preferred. In our study, none of the patients developed renal failure, hematuria or proteinuria.
Related to IVIG, cardiovascular adverse effects may rarely occur, such as blood pressure and heart rate changes, heart failure and sudden death. None of these complications was observed in our patients.
Since IVIG preparations include various donor plasma derivates, viral pathogens may be capable of being transmitted and may cause hepatitis.19 In our patients, viral hepatitis did not develop due to IVIG treatment, because IVIG preparations have been routinely screened for frequently encountered viral agents such as hepatitis B and C and HIV.
Hematologic manifestations generally occur as delayed side effects. Neutropenia and hemolytic anaemia are usually mild and transient.20–22 Lymphopenia may occur with up to 33% decrease of pre-treatment values, but generally recovers in a period of 30 days.23 Thrombocyte activation and thromboembolic complications such as cerebral infarct, deep vein thrombosis and pulmonary embolism, may develop in 1–3% of the patients, due to the increase in blood viscosity caused by stabilising agents in the IVIG preparations. To prevent from thromboembolic complications, we have to avoid from high doses, high infusion rates and preparations with high concentrations.24
Severe nephrologic, cardiac and thromboembolic complications in our patient group did not occur. This condition is thought to be the consequence of administering IVIG in replacement doses to our patients but not in immunomodulatory doses and absence of underlying severe systemic disease.
Dashti-Khavidaki et al. demonstrated similar adverse effect ratios between children (7.4%) and adults (6.4%) in a study including 99 patients and 3004 infusions. In this study, when children were compared in two groups as under 10 years old and between 10–17 years old, ratio of the adverse effects did not differ.25 Kaba et al. found similar adverse event ratios between male (22.6%) and female (28.3%) patients.26 In our study, adverse effects were encountered significantly more in children between 5–10 years old (25.1%) than other age groups (under 5 years 13.4%, above 10 years 4.1%). Adverse reactions were more frequent in female children (20.1%) than males (11.9%) (p < 0.001).
Family history of atopy has been reported as a risk factor for adverse reactions to IVIG infusions. Palabrica et al. demonstrated that adverse effects of IVIG infusions were more frequent in patients with a family history of atopic diseases.27 In our study, adverse effects were significantly more frequent in patients with family history of atopy (25.8%) and past history of atopy (17.2%), than the patients without family history of atopy (11.2%) and past history of atopy (12.1%). Concomitant cardiovascular and hematologic diseases and reactive airway disease were not found associated with increased adverse events.
The presence of an active infectious disease concurrently with the IVIG infusion has been reported as a risk factor for IVIG adverse effects. Increased adverse effects are considered to be the result of antigen-antibody complexes and the rapid release of bacterial components such as lipopolysaccharides.28 Similar to the literature, we found significantly more frequent adverse effects in the patients with an active infectious disease on the IVIG infusion day.
Side effects to IVIG develop more frequently in the first infusions, without a previous history of IVIG administration. Sing-Grewal et al. found that adverse reactions occurred in 16.2% of the patients receiving IVIG for the first time but 6.9% of the patients who had IVIG infusions previously, in their study including 3004 infusions.15 The Immune Deficiency Foundation reported that 34% of the IVIG infusions with adverse reactions were associated with the first infusion. Similar to the previous literature we found the adverse reaction ratio significantly more in the first infusions (24.7%) than in the consequent infusions (13.2%).
In the literature, there are studies demonstrating increased incidence of adverse effects with the change of IVIG preparation.25,29 Dashti-Khavidaki et al. found that adverse effects occurred after the change of IVIG preparation in 15.7% of the patients.25 In our study this ratio was 19.7%, similar to that previous study.
The concentration of the IVIG preparation (5% or 10%) has not been found to affect the incidence of side effects.30 In our study, consistent with the literature, no significant difference in the frequency of side effects was found between 5% concentration IVIG (14.1%) and 10% concentration IVIG (14.6%) administrations (p = 0.0777).
In our study, we accepted that the existence of adverse reaction was an independent variable and we applied logistic regression analysis. History of adverse reactions in previous infusions, concomitant infectious disease, being under 10 years old, past or family history of atopy, no history of receiving previous IVIG infusion were found to increase the risk of adverse reactions.
ConclusionsIn conclusion, IVIG is a safe and well tolerable treatment. No serious reaction to IVIG developed in our study. Headache was the most common adverse reaction and it rarely required hospitalization and medical treatment. Other adverse reactions were all mild systemic reactions. In the stage of planning IVIG therapy, the characteristics of the patients and risk factors should be taken into consideration. Dosage, treatment interval, type of the preparation should be individual for each patient. Patients, their families and health care workers should be informed about potential adverse effects.
Conflict of interestThe authors have no conflict of interest to declare.