Chronic spontaneous urticaria (CSU) affects approximately 1% of the population, affecting both children and adults. Omalizumab (Oma) is a therapeutic option for patients with refractory forms of CSU.
ObjectivesTo determine the effectiveness and safety of Oma in the treatment of CSU.
MethodsSystematic review (Cochrane Collaboration methodology) of randomized clinical trials comparing Oma to placebo in refractory CSU treatment. The search is based on MEDLINE; EMBASE, Central Cochrane Library, and LILACS. The outcomes evaluated were: control of the illness, adverse events, and quality of life.
ResultsOf the 848 identified studies 13 were selected for further review and six were included in the meta-analysis. For all outcomes, high-quality evidence has confirmed that Oma is effective in the treatment of CSU. The dosage of 300mg/month achieved better results; namely a significant reduction in pruritus, papules, and urticaria activity, as well as an increase in the number of patients with a controlled condition, improvement in the quality of life and no differences in adverse events compared to the placebo.
ConclusionsHigh-quality evidence demonstrates that Oma is effective and safe in the treatment of CSU refractory to therapy with H1 antihistamines.
Urticaria is a common disease that affects one in five people at some point in life.1 Its characteristics are the appearance of urticaria, angioedema or both.2 When symptoms persist daily or almost daily for more than six weeks, urticaria is classified as chronic, and has a prevalence ranging from 0.5% to 1%; it is moreover more frequent in females.3
In most chronic urticaria there is no specific trigger (chronic spontaneous urticaria, CSU), whereas in some individuals the symptoms may be induced by well-defined stimuli such as heat, cold, pressure, ultraviolet light, or increased body temperature (induced chronic urticaria). The diagnosis of CSU is exclusively clinical, without the need for routine tests. The diagnostic confirmation of induced urticaria uses specific provocative tests for each of the different types.2
Although the pathophysiology of CSU has not yet been fully elucidated, there is a great deal of evidence pointing to mast cell activation by autoimmune mechanisms. Either IgE class antibodies against its proteins (auto-allergens) or antibodies of the IgG class against the high-affinity receptor for IgE or against IgE itself, once activated, the release of pre- and neo-formed mediator mast cells occurs, including histamine, which is primarily responsible for the symptoms of urticaria.
CSU has a high impact on the patients’ quality of life, significantly interfering with daily professional, school, and social activities. Studies using a specific questionnaire to assess the quality of life in urticaria sufferers indicate that the disease negatively affects aspects such as anxiety, depression, sleep, and self-esteem.4 Thus, symptom control becomes the primary goal of treatment.
Current guidelines recommend second-generation non-sedating antihistamines at usual doses, or up to two to four times as high as first and second lines of treatment, respectively. The response to treatment is evaluated by objective tools such as the Urticaria activity score in seven days (UAS7) and the Urticaria control test (UCT).2 However, up to 40% of patients may not achieve disease control (UAS7≤6 or UCT≥12) and are considered refractory to treatment.5
Omalizumab (Oma) is an anti-IgE monoclonal antibody, whose clinical and real-life data demonstrate safety and efficacy for the treatment of urticaria refractory to antihistamines. It is recommended as the third line of treatment for CSU.
The objectives of this systematic review, complemented by meta-analysis, were to evaluate the effectiveness and safety of Oma in the treatment of CSU refractory to H1 antihistamine treatment.
Materials and methodsA systematic review was carried out of randomized, placebo-controlled trials (comparator treatment) of patients (12- to 75-year-olds, regardless of sex) with CSU refractory to treatment with antihistamine and treated with Oma and assessing the outcomes at 12 weeks of following, using the Cochrane Collaboration method.6
OutcomesEvaluated outcomes: response to treatment using the Urticaria Activity Score (evaluates papules and pruritus, 0–3 scale) for seven days (UAS7≤6, maximum value 42),7 complete response (UAS7=0)2; Weekly itch severity score for seven days (WISS; scale from 0 [absent] to 21 [intense])8; Weekly wheal score (scale – none=0; 1–6=1; 7– 12=2 and more than 13=3)9; at least one adverse event and quality of life (Dermatology Life Quality Index).10,11
Search strategyThe search strategy for the relevant studies was carried out with the basis of MEDLINE, EMBASE, CENTRAL Cochrane Library, and LILACS. The search included studies published until April 15, 2018. The uniterms used in the search were: urticaria, chronic urticaria, hives, omalizumab, Xolair, monoclonal antibody, rhuMAb-E25, anti-immunoglobulin E, randomized controlled trial, and antihistamines.
Each of the articles was reviewed, simultaneously and independently by two reviewers (EMKS, MJB), who extracted all data related to population, demographics, and treatment periods. The quality of each trial was determined independently by the two reviewers using The Cochrane Collaboration’s tool for assessing the risk of bias.6
Statistical analysisFor the quantitative analysis the principle of "intention to treat" was used. When possible, the data were summarized in meta-analyses using Review Manager 5.2 software, developed by the Cochrane Collaboration.12 For dichotomous data, the Relative Risk (RR, the proportion of events in the treatment group about the proportion of events in the control group) and its respective Confidence Interval (CI) of 95% (95% CI) were calculated. For the analysis of continuous variables, the means difference with 95% CI was calculated.
The studies’ heterogeneity was evaluated by the Chi-square test (p<0.1 indicates heterogeneity) and the I2 test (>50% represents heterogeneity). Possible causes of heterogeneity are differences in the population, interventions, and evaluations of outcomes.
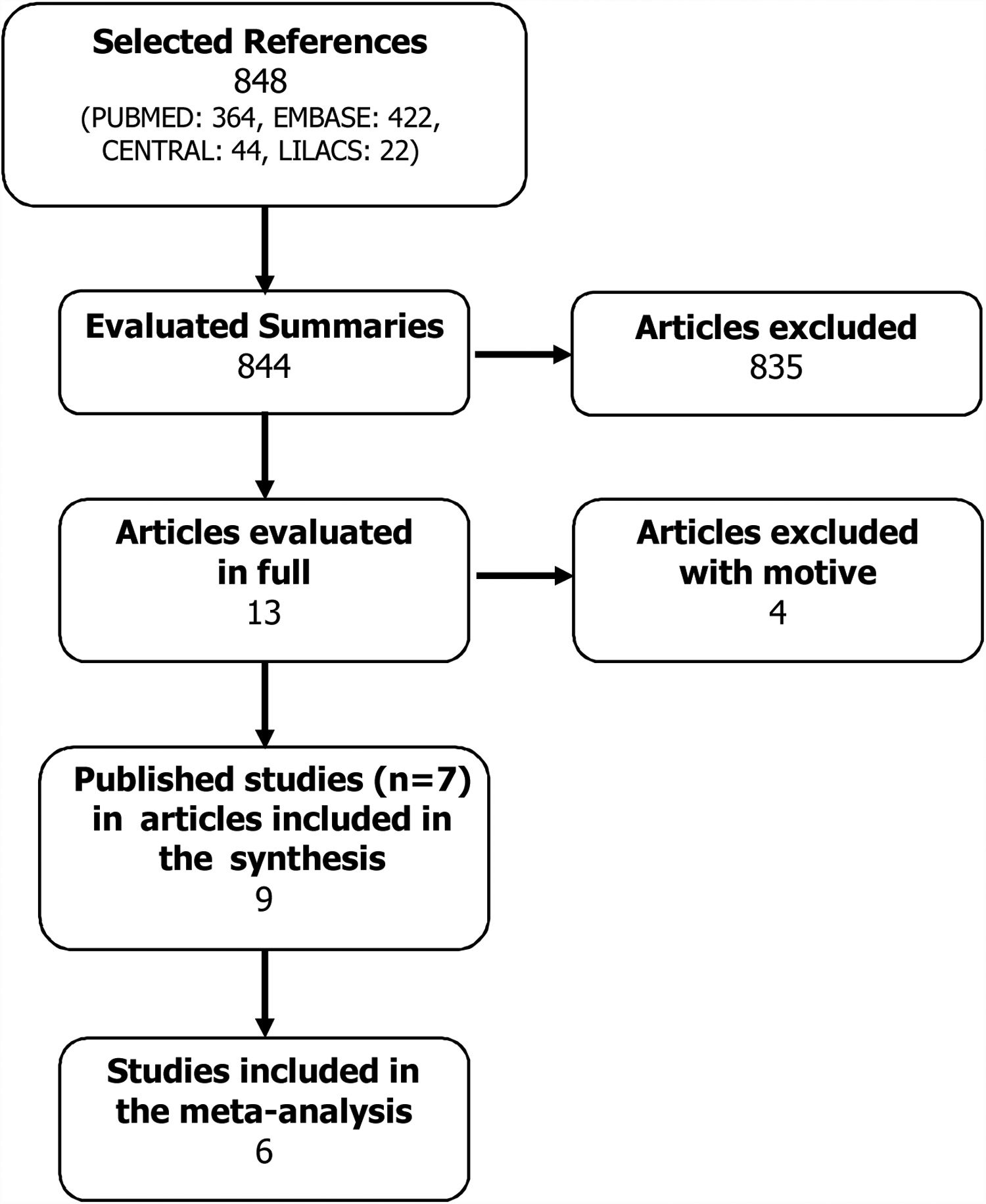
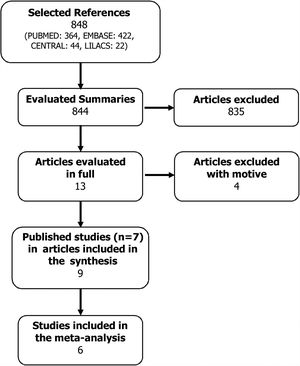
ResultsSearch strategyA total of 848 possible references were identified. All abstracts of the articles found were evaluated by the two authors, and of these, 13 articles were selected because they presented the potential to be included in the systematic review.
These articles were obtained in their entirety and evaluated according to the inclusion criterion. In this stage, four articles were excluded with a motive: use of Oma doses equal to those of asthma,13,14 subgroup analysis of the POLARIS study15 and no report of outcomes16 (Fig. 1). Thus, seven studies were included in this systematic review (Table 1), whose results were published in nine articles.8,17–24
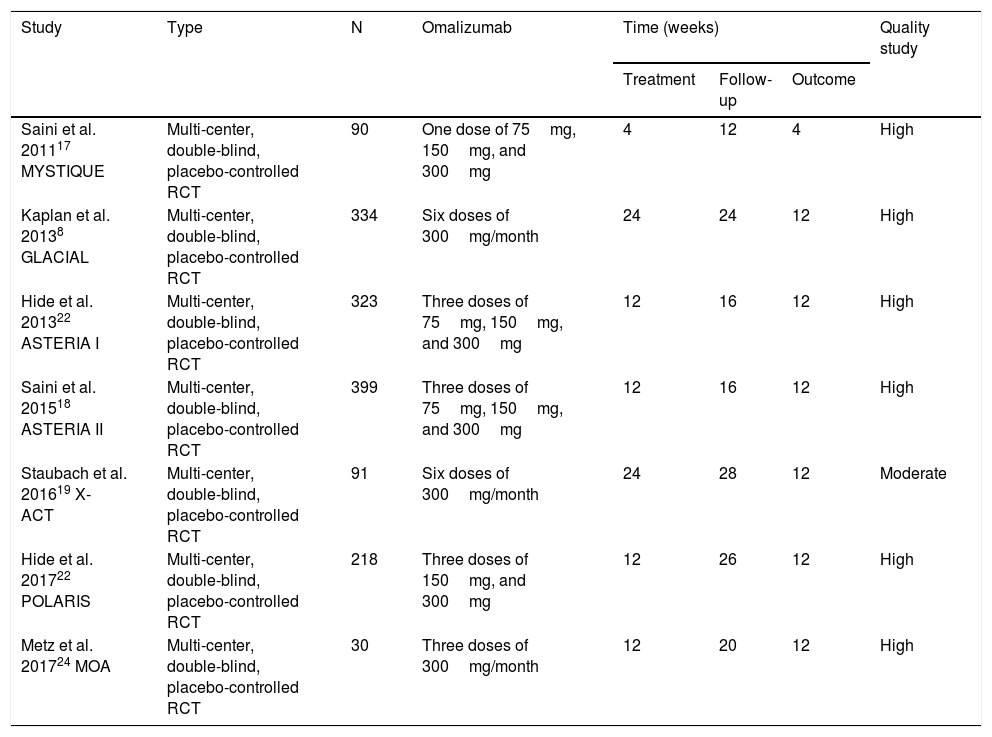
Characteristics of the studies included in the meta-analysis.
| Study | Type | N | Omalizumab | Time (weeks) | Quality study | ||
|---|---|---|---|---|---|---|---|
| Treatment | Follow-up | Outcome | |||||
| Saini et al. 201117 MYSTIQUE | Multi-center, double-blind, placebo-controlled RCT | 90 | One dose of 75mg, 150mg, and 300mg | 4 | 12 | 4 | High |
| Kaplan et al. 20138 GLACIAL | Multi-center, double-blind, placebo-controlled RCT | 334 | Six doses of 300mg/month | 24 | 24 | 12 | High |
| Hide et al. 201322 ASTERIA I | Multi-center, double-blind, placebo-controlled RCT | 323 | Three doses of 75mg, 150mg, and 300mg | 12 | 16 | 12 | High |
| Saini et al. 201518 ASTERIA II | Multi-center, double-blind, placebo-controlled RCT | 399 | Three doses of 75mg, 150mg, and 300mg | 12 | 16 | 12 | High |
| Staubach et al. 201619 X-ACT | Multi-center, double-blind, placebo-controlled RCT | 91 | Six doses of 300mg/month | 24 | 28 | 12 | Moderate |
| Hide et al. 201722 POLARIS | Multi-center, double-blind, placebo-controlled RCT | 218 | Three doses of 150mg, and 300mg | 12 | 26 | 12 | High |
| Metz et al. 201724 MOA | Multi-center, double-blind, placebo-controlled RCT | 30 | Three doses of 300mg/month | 12 | 20 | 12 | High |
RCT: randomized clinical trial.
The seven included studies totaled 1405 participants with at least a six-month history of CSU, with activity for at least eight consecutive weeks before the study started, refractory to previous use of antihistamines, and with a UAS7 score equal to or greater than 16. Just one study included only patients with at least four episodes of angioedema in the last six months.19,20
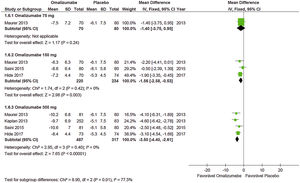
Effects of interventions (outcome at 12 weeks)Weekly itch severity score (WISS)The analysis of Oma’s action in WISS control compared to placebo showed that the 75mg dose was effective and determined a minimal difference (MD)=−1.86 (95% CI: −3.15 to −0.57; p=0.005), data obtained from two studies with 318 participants. A dose of 150mg was also effective: MD=−2.73 (95% CI: −3.74 to −1.73, p<0.0010), data obtained from three studies with 465 participants. A dose of 300mg determined MD=−4.82 (95% CI: −5.66 to −3.98, p<0.001), data obtained from six studies with 917 participants. The dose of 300mg showed better performance. Only the meta-analysis with the 75mg dose presented some heterogeneity, so the fixed model of analysis was used.
Weekly wheal score (WWS)Oma was effective in controlling the mean number of weekly papules compared to a placebo at doses of 150mg and 300mg, which did not occur with 75mg (MD=−2.00 [95% CI: −4.10 to 0.10; p=0.06] taken from a study with 161 participants). With 150mg MD=−3.60 (95% CI: −5.11 to −2.09; p<0.001) was significant and data was obtained from two studies with 305 participants. Also, with 300mg MD=−5.90 (95% CI: −7.06 to −4.75; p<0.001), obtained from five studies with 756 participants. No significant heterogeneity was observed in the analyses, so the fixed model was used.
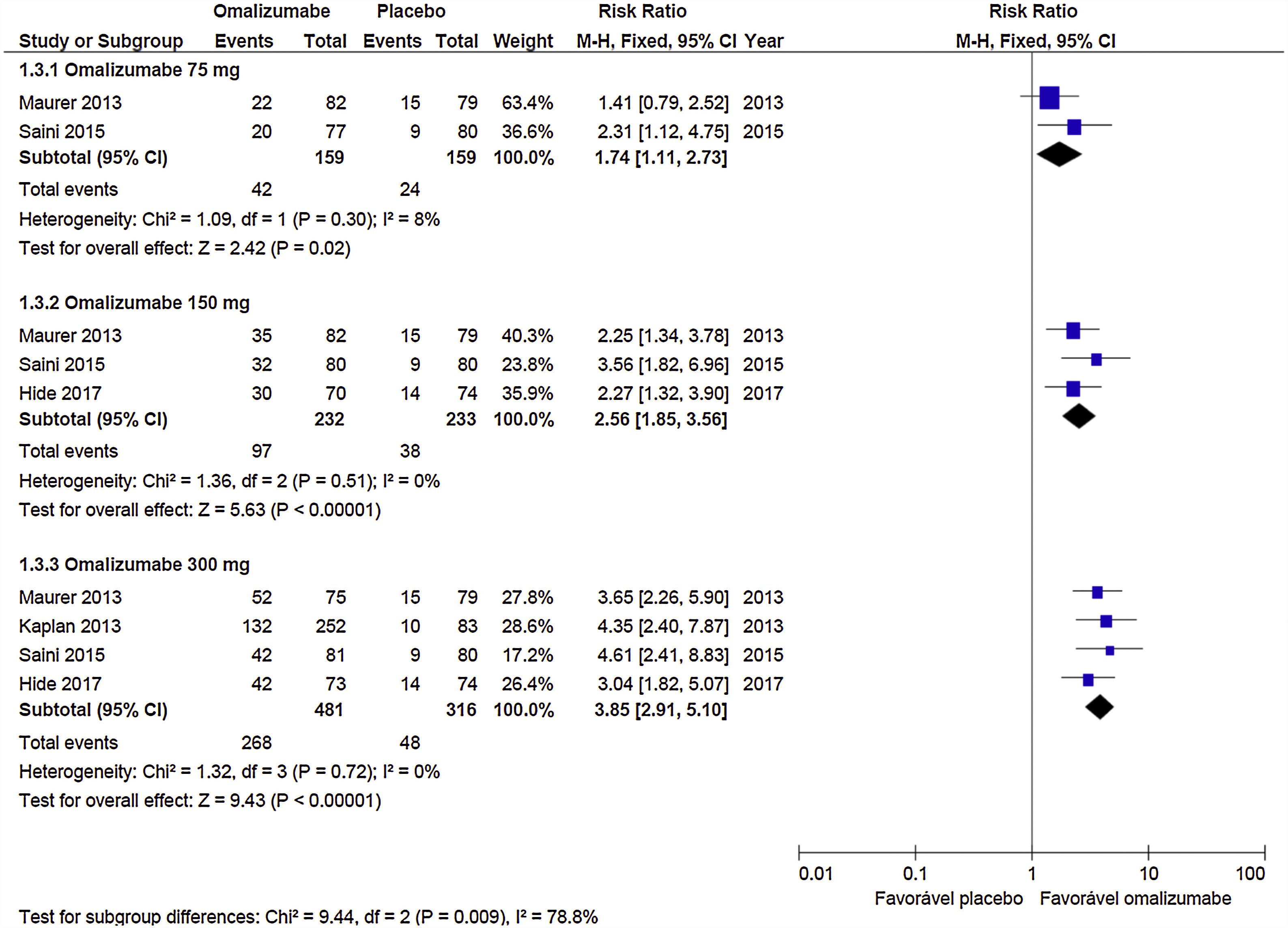
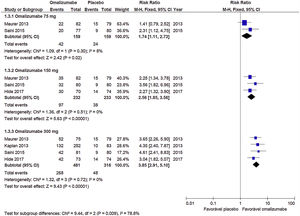
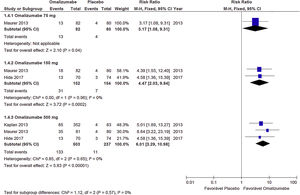
Response to treatment (UAS7≤6)Oma was shown to be effective in CSU control in the three doses used. A dose of 75mg determined RR=1.74 (95% CI: 1.11–2.73, p=0.02), referring to data obtained from two studies with 318 participants. The 150mg dose documented RR=2.56 (95% CI: 1.85–3.56; p<0.001) from three studies with 465 participants. The 300mg dose indicated RR=3.85 (95% CI: 3.85– 5.10; p<0.001) obtained from four studies with 797 participants (Graph 1). No significant heterogeneity was observed in the analyses, so the fixed model was used.
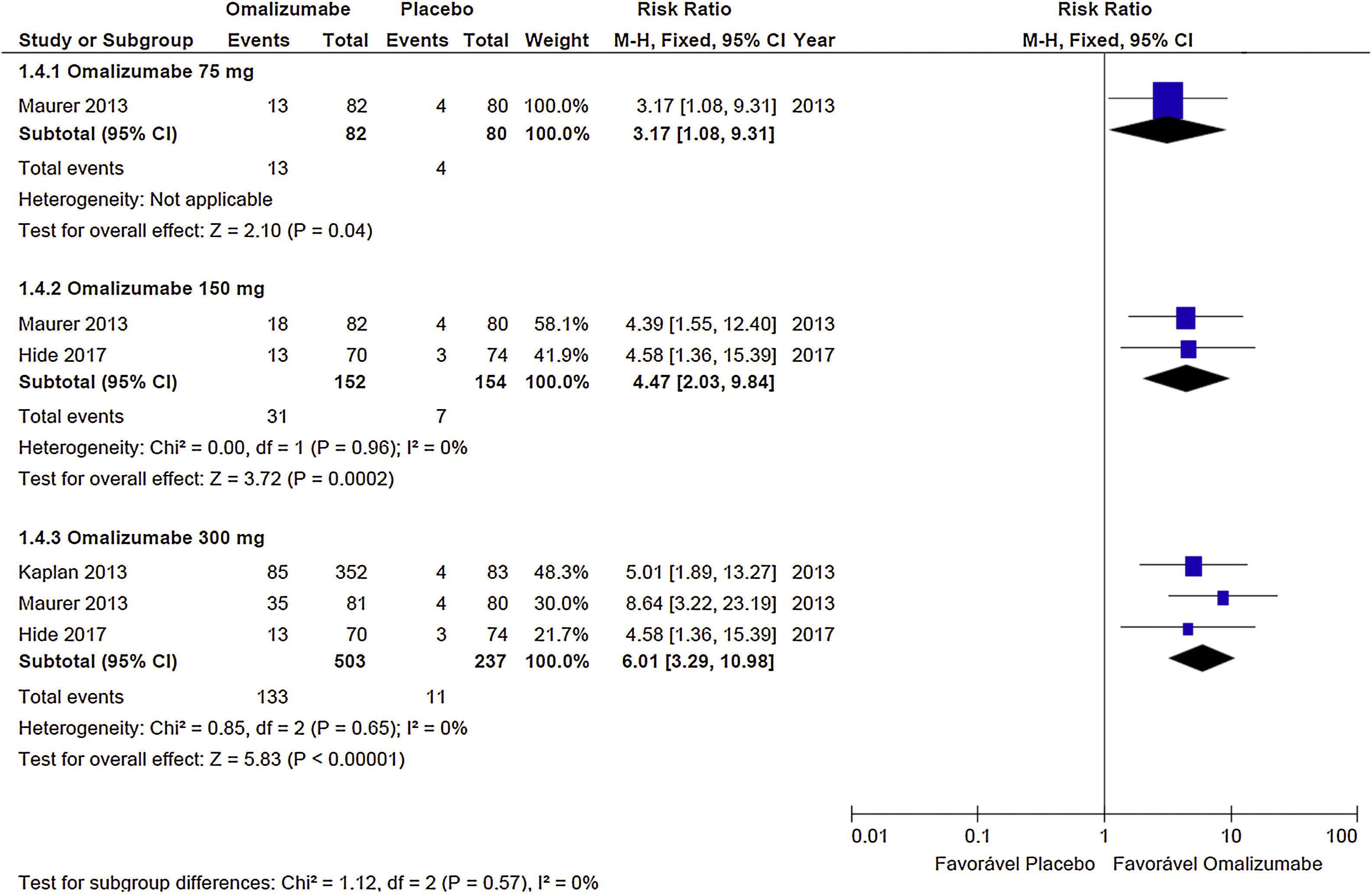
Complete response (UAS7=0)The Oma showed evidence of effectiveness in obtaining complete CSU response, in all doses evaluated, although the 300mg dose showed a greater proportion of participants. With 75mg the RR=3.17 (95% CI: 1.08– 9.31; p=0.04), obtained from a study with 17 participants. The 150mg dose showed RR=4.47 (95% CI: 2.03–9.84; p<0.001) derived from two studies and 306 participants. The 300mg dose documented RR=6.01 (95% CI: 3.29–10.98; p<0.0010) obtained from three studies with 740 participants (Graph 2). No significant heterogeneity was observed in the analyses, so the fixed model was used.
Adverse events – at least oneThe three Oma dosages evaluated were equal to the placebo for the frequency of adverse events. The most reported events were gastrointestinal manifestations, headache, infection at the site of administration, and skin changes. With the 75mg dose, we observed RR=1.07 (95% CI: 0.91–1.26; p=0.34) derived from two studies with 305 patients. The 150mg dose revealed RR=1.14 (95% CI: 1.00–1.29, p=0.05) obtained from three studies with 473 patients. The 300mg dose had RR=1.05 (95% CI: 0.96–1.14, p=0.34) obtained from six studies with 922 patients. No significant heterogeneity was observed in the analyses, so the fixed model was used.
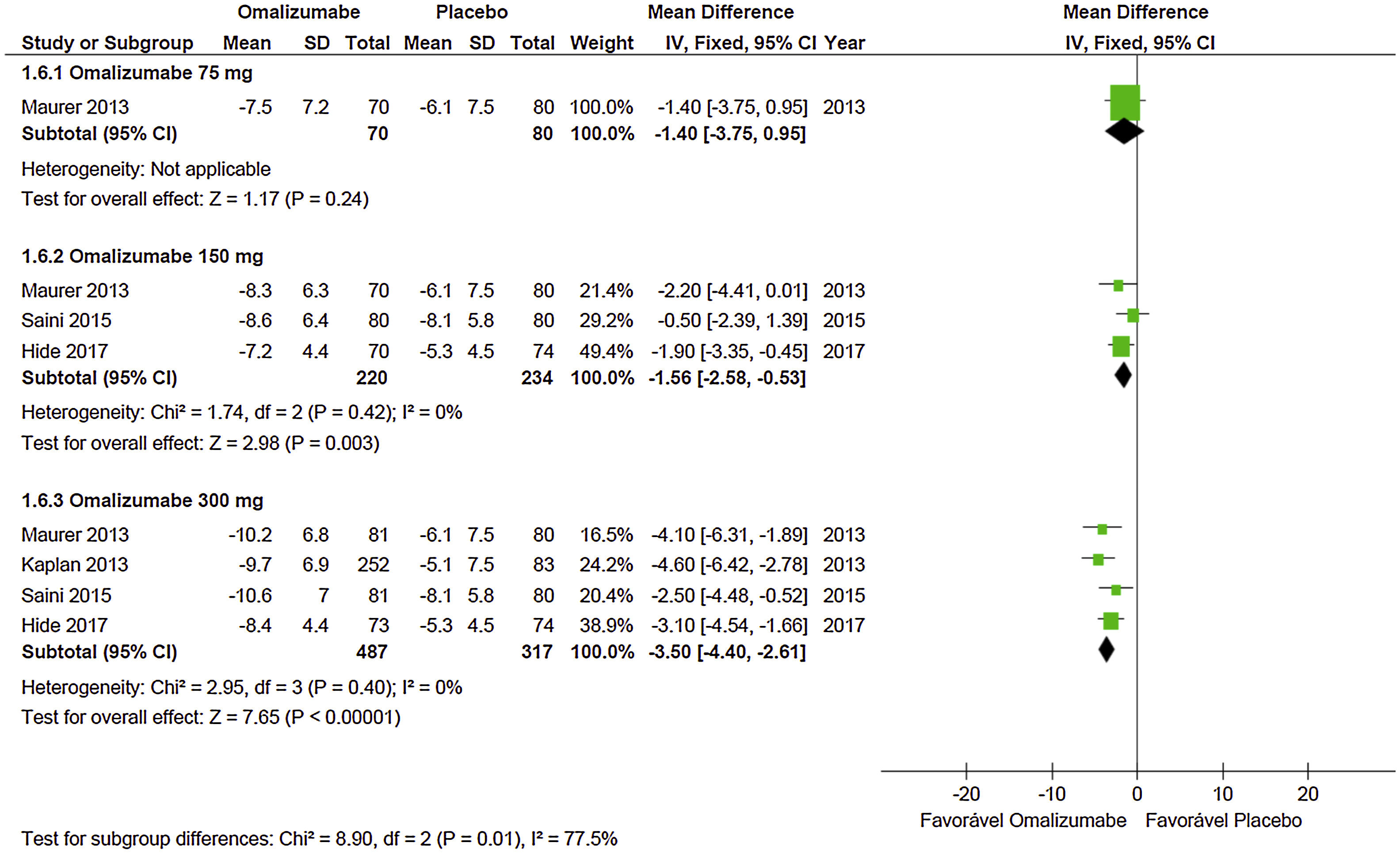
Quality of lifeEvidence shows that Oma acts effectively on the participants’ quality of life when used in doses of 150mg and 300mg. With a dose of 75mg MD=−1.40 (95% CI: −3.75 to 0.95, p=0.24) was not significant – data extracted from a study with 150 participants. At the dose of 150mg MD=−1.56 (95% CI: −2.58 to −0.53; p=0.003) it was significant and obtained from three studies with 454 participants. In the case of the dose of 300mg, MD=−3.50 (95% CI: −4.40 to −2.61; p<0.001) it was significant and obtained from four studies with 804 participants (Graph 3). No significant heterogeneity was observed in the analyses, so the fixed model was used.
DiscussionOf the seven studies included in this meta-analysis, six showed a low risk of bias and only one was considered to be at moderate risk.19 This fact alone assures us of having high quality evidence in favor of Oma. Furthermore, the consistency of the results obtained by the various included studies, with little heterogeneity, and the analysis of a large number of participants (N=1405) reinforce this statement.
This study revealed, with high-quality evidence, that Oma is effective in the treatment of CSU in those patients refractory to antihistamine treatment when compared to a placebo. Although the antihistamines’ refractoriness was variably defined in the different evaluated studies, the results obtained were all significant. This is an important finding because the consensus recommendation is that Oma is indicated only in patients who do not respond to high doses of antihistamines, and some of the studies used in this systematic review and meta-analysis did not include this patient profile type. On the other hand, several real-life studies corroborate the efficacy data observed in clinical studies, even in refractory patients at high doses.
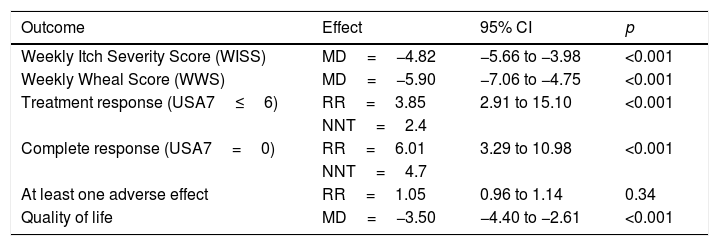
Different dosages were evaluated in pivotal studies, the most efficient being 300mg every four weeks (Table 2). For this reason, in part of the studies there is no comparison between different doses, only between the 300mg dose and the placebo. It is also interesting to note that in real-life studies this too was the dose with the best-observed response.25
Summary of the meta-analyses of Omalizumab 300mg in the treatment of Chronic Spontaneous Urticaria.
| Outcome | Effect | 95% CI | p |
|---|---|---|---|
| Weekly Itch Severity Score (WISS) | MD=−4.82 | −5.66 to −3.98 | <0.001 |
| Weekly Wheal Score (WWS) | MD=−5.90 | −7.06 to −4.75 | <0.001 |
| Treatment response (USA7≤6) | RR=3.85 | 2.91 to 15.10 | <0.001 |
| NNT=2.4 | |||
| Complete response (USA7=0) | RR=6.01 | 3.29 to 10.98 | <0.001 |
| NNT=4.7 | |||
| At least one adverse effect | RR=1.05 | 0.96 to 1.14 | 0.34 |
| Quality of life | MD=−3.50 | −4.40 to −2.61 | <0.001 |
MD: minimum difference; 95% CI: 95% confidence interval; RR: relative risk; NNT: number needed to treat.
The outcomes to assess the effectiveness and safety of Oma treatment used here have been used by most studies, which in a way, facilitated the assessments. It is important to use standardized tools to assess disease activity and control, as well as to evaluate quality of life. The consensus-recommended tools for use not only in clinical studies, but also in clinical practice are UAS7, UCT, and DLQI (Dermatology Quality of Life Index), or CU-QoL (Chronic Urticaria Quality of Life).
The selected outcomes chosen for the identification of studies we took into account were systematically elected by other authors, i.e. disease activity (UAS7, UAS7≤6, UAS7=0, WISS, WWSS), quality of life and adverse events. Similar to other researchers, we have selected, by definition, the manifestation of at least one adverse event.
The adverse effect profile and a frequency similar to that observed in the placebo group confirm Oma’s safety in the recommended dosing regimen for CSU treatment. The primary adverse events were mild and included gastrointestinal manifestations, headache, the reaction at the application site, and skin changes.
The quality of life of patients with urticaria or dermatological disease has been evaluated by several instruments: Dermatology Quality of Life Index (DQLI) (1011), Children’s Dermatology Life Quality Index (CDLQI),26 Dermatology Quality Of Life Scales (DQOLS),27 Dermatology-Specific Quality of Life (DSQL),28 Skindex-29,29 Skindex-16,30 Chronic Urticaria and Quality of Life Questionnaire (CU-Q2oL),31 Urticaria Severity Score (USS),32 among others.33 This fact makes it difficult to compare results obtained by studies using different instruments in the evaluation of the quality of life. Most of the studies included in this one employed DLQI20–23 except for Staubach et al.,19 who used the (CU-Q2oL) and Angioedema Quality of Life (AE-QoL)34 and therefore, were not included in the analysis.
Our results are compatible with those of other published systematic reviews, although they used different methods.35–37 The main difference of this review is the update, with the inclusion of recent studies.15,19
The mean duration of CSU is estimated to be between six months and five years and may be longer in more severe cases, especially in patients with concomitant angioedema, association with induced urticaria, or with a positive autologous serum test.38–40 This entails the substantial commitment of time from professional, social, leisurely activities, and family life, besides the direct and indirect costs, due to the type of treatment and remote nature of the work. These data indicate the importance of a medication that controls symptoms, improves quality of life and does not lead to serious adverse effects. Anti-IgE therapy in CSU is an effective and safe option for cases refractory to treatment with high doses of antihistamines. The only present limitation is its high cost. Efforts are needed for careful incorporation into the public and supplementary health networks.
Conflict of interestThe authors have no conflict of interest to declare.