Diagnosing primary triple-negative breast cancer (TNBC) with lung metastases or primary TTF1negative lung carcinoma can be difficult due to lack of a TNBC standard immunoprofile.
We report a rare case of a woman with an age range between 50 and 55 years, who presented with a mass of the left breast evolving for 2 months. The core needle biopsy concluded to a TNBC. Complete staging with a thoraco-abdomino-pelvic and cerebral computed tomography revealed a huge lung mass associated with laterotracheal and subcarineal adenomegaly next to spinal lytic lesions and bilateral adrenal masses consisting with metastasis. The lung biopsy revealed a poorly differentiated squamous cell carcinoma. The patient was then scheduled to chemotherapy.
Pathological and immunohistochemistry studies are limited to establish the formal diagnosis between primary metaplastic carcinoma that metastasize to the lung and primary lung poorly differentiated squamous cell carcinoma. Confrontation with radiology and clinical presentation is highly important to resolve the problem.
Diagnosticar el cáncer de mama triple negativo (TNBC) primario con metástasis de pulmón o el cáncer de pulmón TTF1negativo primario puede resultar difícil, debido a la falta de inmunoperfil TNBC estándar.
Reportamos un caso raro de una mujer, con rango de edad comprendido entre 50 y 55 años, que acudió con una masa en el seno izquierdo con evolución de dos meses. La biopsia por punción concluyó TNBC. La estadificación completa con tomografía computarizada toraco-abdomino-pélvica y cerebral reveló una gran masa pulmonar asociada a adenomegalia latero-traqueal y subcarineal próxima a lesiones líticas espinales y masas adrenales bilaterales consistentes con metástasis. La biopsia de pulmón reveló carcinoma de células escamosas débilmente diferenciado, programándose quimioterapia a la paciente.
Los estudios patológicos e inmunohistoquímicos son limitados para establecer el diagnóstico formal entre cáncer metaplásico primario que metastatiza al pulmón, y el carcinoma de células escamosas de pulmón primario débilmente diferenciado. La confrontación con radiología y presentación clínica es altamente importante para resolver el problema.
Breast and lung cancers are considered to be systemic disease even at early stages and patient mortality is due to metastatic disease.
Metastatic breast involvement is rare, with an incidence of 0.4–3% reported in clinical trials.1,2 Metastatic breast localization of lung cancer is not rare.3 The challenge for clinicians and pathologists is to determine the primary from the metastatic cancer.
In this paper, we will present a case of a women with synchronous poorly differentiated malignancies of the breast and the lung, in order to present a diagnostic strategy for this challenging clinical situation.
Case reportWe report the case of a woman, with age range between 50 and 55 years, non-smoker, with no history who complained of an enormous palpable mass of the left breast.
Physical examination revealed a firm, poorly defined, and ulcerated mass of the left breast measured 10 cm.
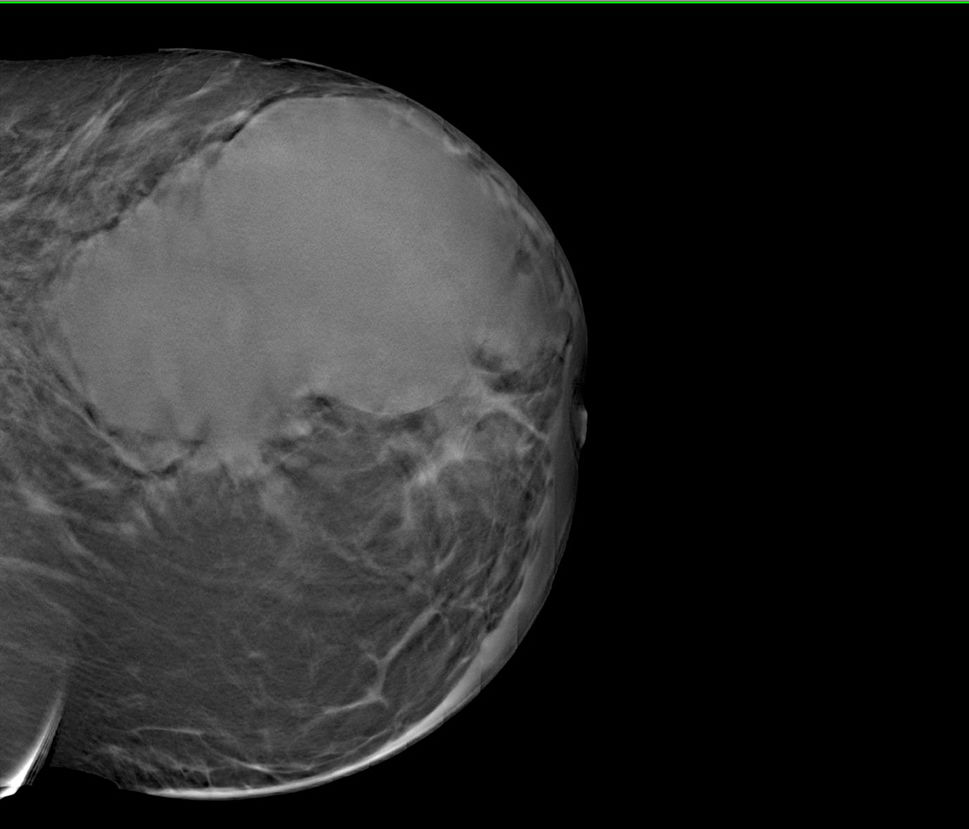
Ultrasonography and mammography showed a solitary bulky mass of the outer upper quadrant of the left breast, irregular shaped, dense, and highly suspicious of malignancy (Fig. 1).
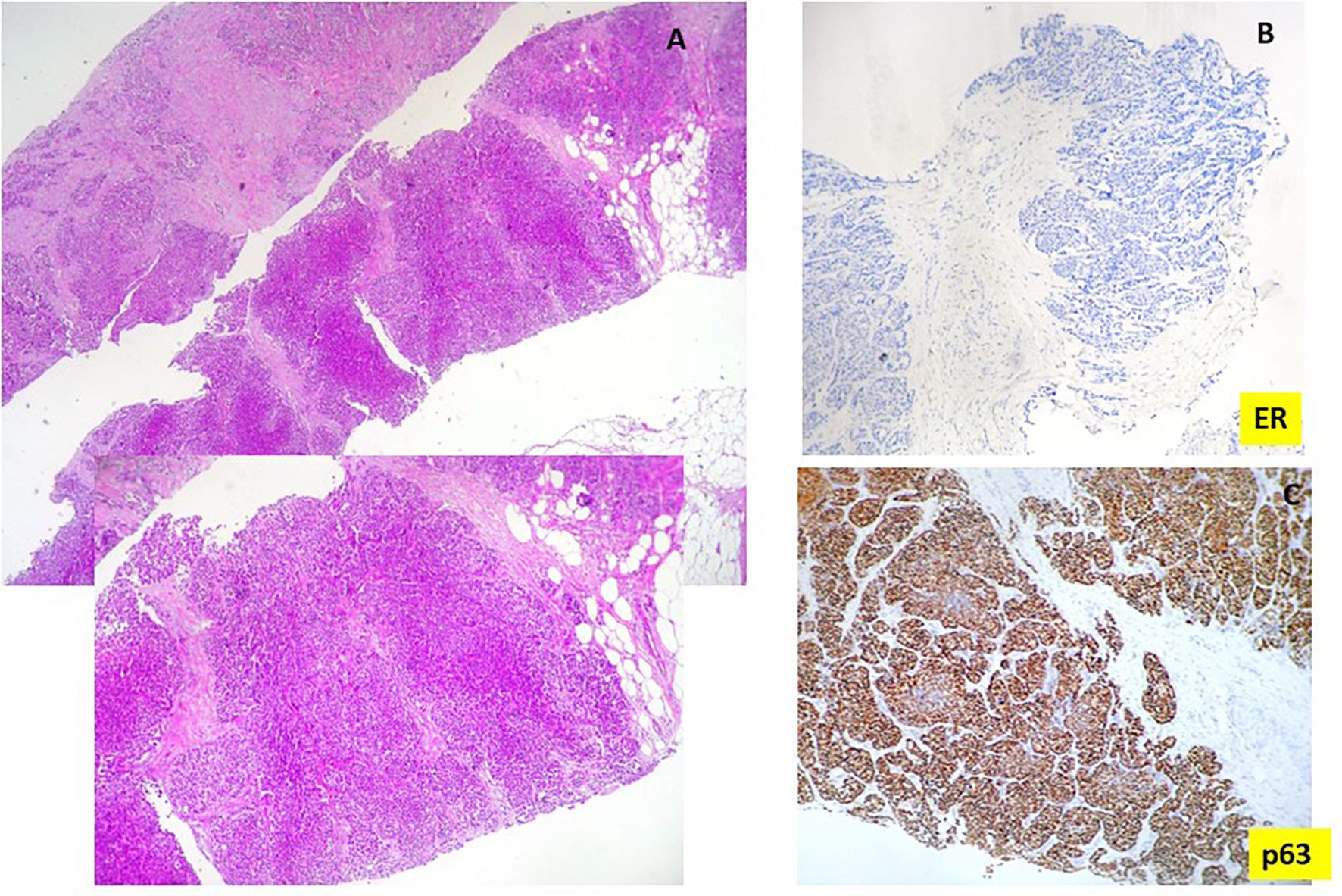
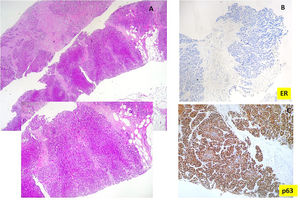
The biopsy revealed the presence of an undifferentiated carcinoma with extensive tumor necrosis (Fig. 2A). Tumor cells were negative for estrogen (Fig. 2B) and progesterone receptor (ER, PR) as well as for the human epidermal growth factor receptor-2 (HER2neu).
A computed tomography (CT) showed proximal parenchymal mass that partially obstructs the right lung with endobronchial bud, associated to laterotracheal and subcarinal necrotic adenomegaly. Axillary and supraclavicular region were free of the disease.
Bilateral adrenal metastatic masses were observed associated to multiple spinal lytic lesions.
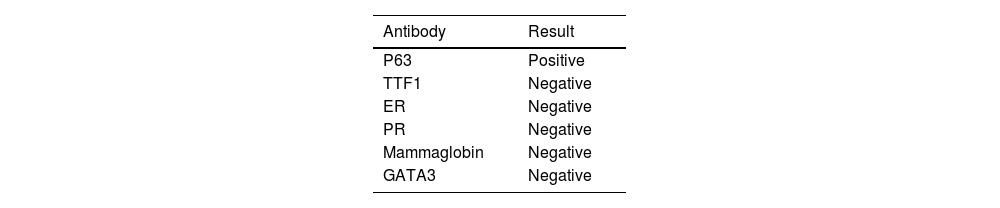
Fibroscopy showed the presence of an endobronchial mass that was biopsied. Histologic examination showed the presence of poorly differentiated carcinoma with marked nuclear atypia. Tumor cells expressed p63 and were negative for TTF1, ER, and PR. Based on these new data, an additional immunohistochemical study was performed on the breast biopsy. It showed that tumor cells were positive for p63 (Fig. 2C), and negative for mammaglobin and GATA3.
The immunohistochemical study via trichorhinophalangeal syndrome type 1 (TRPS1) was not performed as we didn't have the antibody (Table 1).
Three diagnostic hypotheses were raised:
1) A primary metaplastic triple negative breast carcinoma (TNBC) with distant metastases involved the lung: This hypothesis was evoked because of the size of the breast tumor, the sex of the patient, and the incidence of breast cancer (BC), the triple negative character on immunohistochemical study.
2) A primary lung poorly differentiated squamous cell carcinoma revealed by a breast metastasis: This hypothesis is supported by the frequency of squamous cell carcinoma of the lung attested by the positivity of p63 on immunohistochemical study, the size of the lung lesion, the presence of mediastinal lymph node metastases, and the model of distant metastases which in accordance with a primary lung cancer.
3) Synchronous lung and BCs: As patients with BC have an increased risk of developing another primary non-BC, particularly lung cancer, compared with the general population.
The patient was referred for chemotherapy but unfortunately died 1 month after diagnosis.
DiscussionBreast and lung cancer are currently the most commonly diagnosed cancers and the leading cause of cancer death worldwide. Despite advances in the diagnosis and treatment of these 2 malignancies, tumors can be diagnostically and therapeutically challenging.4–5
Pathologic confirmation of metastatic or progressive disease is an important clinical step in disease monitoring and treatment planning. However, the differential diagnosis of primary lung cancer and metastatic BC can be challenging. Among BC subtypes, TNBC is the most aggressive subtype with no significant tumor markers for its breast origin.6
Metastatic disease to the breast accounts for about 2% in relevant series. The most common metastatic lesion affecting the breast is the contralateral mammary carcinoma.4,5 However, a great number of primary non-mammary cancer may involve the breast such as the lung cancer.7–8
In our case, there were no axillary adenomegaly, which favor the diagnosis of primary poor differentiated squamous cell carcinoma of the lung rather than the primary breast carcinoma. The involvement of axillary lymph nodes is uncommon in metastases than in primary BCs.
Besides, the presence of mediastinal adenomegalies associated with a unique enormous mass support the diagnosis of a primary lung cancer. Moreover, adrenal metastasis and the presence of endobronchial tumor, on fibroscopy, often occur in the lung cancer.
Lung metastases from BC are rare and occur most commonly in TNBC.5
TNBC usually metastasizes to other organs through blood vessels.9–11
The distant organs to which BC preferentially metastasizes, of which bone, liver, lung, and brain are among the most common sites, are associated with the patients' survival outcome.12–14 Based on the fact that TNBC presents a predictive metastatic site to the lung via hematogenous pathway, a primary TNBC cannot be rule out.
Pathology and even immunohistochemistry couldn't sometimes diagnose the primary from the metastatic disease.
Morphologic features of metaplastic TNBCs and poorly differentiated squamous cell carcinoma can be quite similar, especially if carcinoma in situ, which favors the primary BC, is lacking on the breast biopsy.
Routinely employed markers of breast differentiation such as mammaglobin and GATA3 tend to be specific but fail to be useful in many cases due to suboptimal sensitivity.15 Besides, tumor cells in lung cancer can express ER, mammaglobin, and GATA3. In BC, the tumor cells may be positive for TTF1 and p63. That‘s why, metastatic breast carcinoma can often pose a diagnostic challenge for the pathologist due to non-specific cytomorphologic features and a paucity of sensitive immunomarkers of breast differentiation.
None of the immunohistochemical markers is specific for breast or lung origin. The combination of p63, ER, GATA3, mammaglobin, SOX10, and trichorhinophalangeal syndrome type 1 (TRPS1) may be helpful in this setting, with high-level expression for mammaglobin and GATA3 associated to intense expression of ER favor breast origin. Unfortunately, approximately 17% of cases are negative with all markers, and these cancers have a roughly equal chance of being either breast or lung in origin.
While ER and PR IHC are prognostically important, they are often not helpful in establishing a diagnosis either, especially in metastases from triple-negative or high Nottingham grade carcinomas.
In surgical pathology, GATA3 is sensitive but not entirely specific in this setting. Although GATA3 labeling is highest in estrogen receptor–positive carcinomas, it also labels estrogen receptor–negative carcinomas and thus has particular diagnostic utility in the setting of TNBCs, which are typically negative for other mammary-specific markers. However, due to the lack of specificity, it must be used in a panel of antibodies to be helpful for the diagnosis of primary breast carcinoma.
Trichorhinophalangeal syndrome type 1 (TRPS1) is a newly identified breast marker.16
TRPS1 detects almost all GATA3 negative and TNBC, while GATA3 can detect invasive breast carcinoma with apocrine features, a special type TNBC which is negative for TRPS1. Ai et al. have demonstrated in their study that TRPS1 maintained high sensitivity in both metaplastic and non-metaplastic TNBC that was significantly higher than that of GATA (86% versus 51% in non-metaplastic TNBC and 86% versus 21% in metaplastic TNBC). Also, high/strong TRPS1 expression was observed in most high-grade metaplastic carcinomas including high-grade spindle/sarcomatous, squamous, polymorphic/giant carcinoma cells, and carcinoma with heterologous mesenchymal differentiation.16
While TRPS1 is a newly identified marker with clinical usage of only 1 year, it has the potential to be considered one of the best markers for differentiating between triple-negative breast carcinoma and metastatic carcinoma.
Mammaglobin has moderate sensitivities and good specificities for breast carcinoma. However, its utility is very limited in metastatic metaplastic breast carcinoma.17–18
SOX10: SOX10 is a nuclear transcription factor that plays an important role in the neural crest development and differentiation.19 Laurent et al demonstrated in a series of 177 cases of primary and metastatic TNBC that 100% of the cases present a positive expression for SOX10. The staining was present in the tumor cell nuclei and was easy to read.4
Gross cystic disease fluid protein 15 (GCDFP15) is another antibody that can be useful in this situation with 98% specificity.4
Laurent et al presented the best sequential immunohistochemical analysis to differentiate TNBC from TTF1-negative lung carcinoma which was first SOX10 followed by GATA3, and finally GCDFP15. This order is important in the diagnostic workup of small biopsies from lung nodules in women with a previous history of TNBC.
Immunohistochemical study is limited to retain the formal diagnoses due to the absence of a specific immunoprofile for TNBC.
The diagnosis should be performed by a combination of clinical presentation and evaluation of the pathology and specific antibodies.
ConclusionDistinguishing between primary metaplastic carcinoma that metastasize to the lung and primary lung poorly differentiated squamous cell carcinoma can be challenging for clinicians and pathologists. This distinction can be made by comparing the clinical and radiological presentation, morphology of tumor lesion to that of the primary breast tumor or by identifying an in-situ component and an immunohistochemical study. Novel markers are still required to determine the true nature of these lesions.
FundingThis work has not received any funding.
Ethics approval statementThis case has been followed all protocols on the publication of patient data of Salah Azeiz Institute. We also declare that privacy has been respected, and the consent publication was obtained from the husband's patient.
Patient consentThe authors declare that they have obtained the patient's consent.