The fungi present in the decaying remains enable a better understanding of the processes of decomposition after death. There are not many studies about fungi on decaying bodies and it is not known which fungal sampling methods are effective.
AimsThe main objective of this study was to find the best method for sampling fungi in carcasses, prove the effectiveness of this method and identify the fungal colonies in animal carcasses from experimental burials.
MethodsSamples from 13 carcasses of Sus scrofa domestica, from the experimental project Taphos-m, were taken with different materials: spatula, sterile swabs and RODAC contact plates.
ResultsRODAC contact plates with the RBA culture medium showed higher proliferation of fungal colonies. Thirty genera of fungi were isolated from different substrates (bone, tissue, lime). Most of the fungi genera or groups identified have been described before in the literature, but the substrates they came from were different in some cases.
ConclusionsSampling with RODAC contact plates was found to be the most effective method, as it provides a nutritional culture medium that may allow growth since the moment of sampling. Fungi colonies grew better in RBA culture medium because bacterial growth is inhibited. Most of the observed fungi are related to the environment but some others have been found related to decomposing bodies for the first time.
Los hongos presentes en los cuerpos sin vida permiten entender mejor el proceso de la descomposición. Sin embargo, no existen muchos trabajos que estudien su presencia en los cuerpos en descomposición, probablemente porque se desconocen los métodos de muestreo más efectivos.
ObjetivosEl objetivo principal de este estudio es conocer el método de muestreo óptimo, probar su eficacia e identificar las colonias de hongos presentes en las carcasas de cerdos enterrados de manera experimental.
MétodosSe tomaron muestras de las carcasas de 13 cerdos domésticos (Sus scrofa domestica) enterrados en las instalaciones Taphos-m, mediante distintos materiales: espátula, hisopos estériles y placas de contacto RODAC.
ResultadosEl mayor crecimiento de colonias se observó en las muestras recogidas con las placas de contacto RODAC con medio de cultivo RBA. En total se identificaron 30 géneros de hongos en diferentes sustratos (hueso, tejido y cal). La mayoría de ellos ya habían sido vinculados a cuerpos en descomposición, pero no siempre se encontraron en el mismo sustrato.
ConclusionesEl muestreo con placas de contacto RODAC resultó ser el más efectivo al proporcionar un medio de cultivo para que continúe el crecimiento fúngico desde el muestreo. En el medio de cultivo RBA crecieron el mayor número de colonias al inhibirse el crecimiento bacteriano. La mayoría de los hongos identificados están relacionados con el ambiente en el cual se muestrearon, pero algunos otros son asociados con cuerpos en descomposición por primera vez.
Taphonomy is the study of the processes on decaying corpses that take place from death to the recovery of the corpse. The different agents that cause those processes can be used to reconstruct the taphonomic history of the body.7 Numerous anthropological studies include some types of taphonomic analyses; since only a few manage to interpret the events that the organisms have gone through after death, the original state and deposition of the body cannot be inferred most of the times. More experimentation is probably needed to understand which taphonomic agents cause the effects observed in cadaveric remains and which taphonomic processes occur in different contexts. For this reason, in Catalonia (Spain), the experimental project Taphos-m (official number S25902001) was created to study the taphonomic agents and processes responsible for the effects observed in bodies from different kinds of burials (plain graves and graves featuring other materials). Domestic pigs (Sus scrofa domestica L. 1758) were used as the animal model.1,9,10
Some of the observed taphonomical effects on corpses are produced by fungi. However, there are not many researches on Forensic Mycology focused on burials or related to other post mortem situations.21,24–26 The most usual mycological studies in Forensic Science are related to intoxications due to poisonous mushrooms or hallucinogenic fungi.5,13 The evolution of sampling and laboratory methodological processes has increased the applications of Forensic Mycology. The quality and quantity of the mycological information that can be obtained from different contexts has also increased. Nowadays it is possible to know the cause of death, estimate the post mortem interval and locate a burial site or even the location where a murder took place by studying the spores on the cadaver, among other things.13,26 However, even with those advances, fungal evidence is not always collected, maybe due to unfamiliarity with the information that the analysis of fungi may provide, and/or the inexperience of how to perform it.
There are several methods for obtaining mycological samples from ancient remains: tweezers,27 adhesive tape strips,18,25,28 sterile swabs,2,4,17,18,25,27 spatulas14,17 or contact plates.17,19 In order to avoid damaging the remains, sterile swab is the most commonly used sampling method. However, RODAC (Replicate Organism Detection and Counting) contact plates are a non-destructive means that have also been used for sampling delicate substrates, such as mummies.2,4,17,18,25 Therefore, we hypothesize that the different sampling methods may be more or less effective depending on the type of samples (specifically on buried corpses), sampling location and time interval between sampling and inoculation.
The aim of this study was: (i) to evaluate the effectiveness of different sampling methods in cadaveric remains and determine which culture medium is the best for sampling fungi and (ii) to identify the fungal colonies growing on the animal remains from experimental burials of the Taphos-m project.
Materials and methodsField methodsFor this study, 13 experimental burials of Taphos-m were selected; four of them (carcasses 5–8) corresponded to burials with lime (Table 1). Samples were collected either from bones or tissue, depending on the cadaveric state of the pig carcasses (Fig. 1): skeletonization, mummification, saponification or any intermediate state.16 In the burials with lime, the samples were also collected from lime plates.
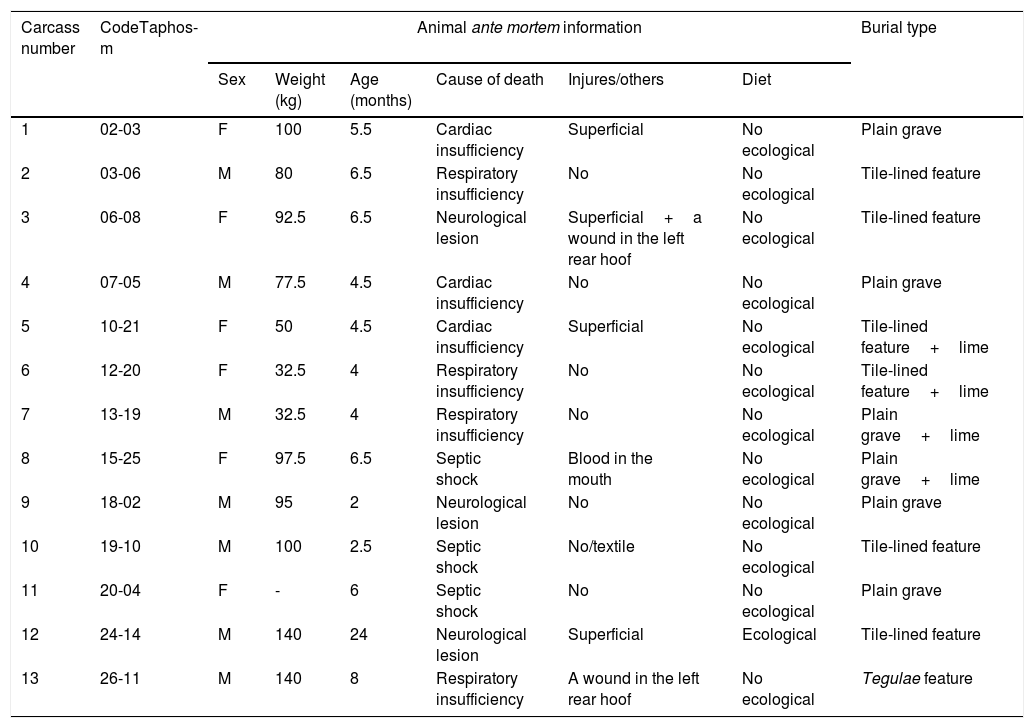
Biological information of the pigs sampled in the study (ante mortem information) and information about the burial type.
| Carcass number | CodeTaphos-m | Animal ante mortem information | Burial type | |||||
|---|---|---|---|---|---|---|---|---|
| Sex | Weight (kg) | Age (months) | Cause of death | Injures/others | Diet | |||
| 1 | 02-03 | F | 100 | 5.5 | Cardiac insufficiency | Superficial | No ecological | Plain grave |
| 2 | 03-06 | M | 80 | 6.5 | Respiratory insufficiency | No | No ecological | Tile-lined feature |
| 3 | 06-08 | F | 92.5 | 6.5 | Neurological lesion | Superficial+a wound in the left rear hoof | No ecological | Tile-lined feature |
| 4 | 07-05 | M | 77.5 | 4.5 | Cardiac insufficiency | No | No ecological | Plain grave |
| 5 | 10-21 | F | 50 | 4.5 | Cardiac insufficiency | Superficial | No ecological | Tile-lined feature+lime |
| 6 | 12-20 | F | 32.5 | 4 | Respiratory insufficiency | No | No ecological | Tile-lined feature+lime |
| 7 | 13-19 | M | 32.5 | 4 | Respiratory insufficiency | No | No ecological | Plain grave+lime |
| 8 | 15-25 | F | 97.5 | 6.5 | Septic shock | Blood in the mouth | No ecological | Plain grave+lime |
| 9 | 18-02 | M | 95 | 2 | Neurological lesion | No | No ecological | Plain grave |
| 10 | 19-10 | M | 100 | 2.5 | Septic shock | No/textile | No ecological | Tile-lined feature |
| 11 | 20-04 | F | - | 6 | Septic shock | No | No ecological | Plain grave |
| 12 | 24-14 | M | 140 | 24 | Neurological lesion | Superficial | Ecological | Tile-lined feature |
| 13 | 26-11 | M | 140 | 8 | Respiratory insufficiency | A wound in the left rear hoof | No ecological | Tegulae feature |
Code Taphos-m: reference number of the burial; M: male; F: female.
Sampling was carried out in the excavation campaigns between 2015 and 2018, 3–6 years after the corps were buried.8,20,22 Samples were collected during the excavation work, and once the taphonomical study was finished the pig carcasses were discarded following the governmental law for animal residues. Four sampling strategies and materials were used in the field (Table 2): (a) previously disinfected spatula (2015–2016); (b) sterile swabs (2017); (c) MEA and RBA RODAC contact plates (2017); and (d) BHI, two MEA and RBA RODAC contact plates (2018). When sampling with spatula only visible fungal colonies were collected in sterile Falcon tubes8,20; for the rest of techniques sampling was systematically performed, even when no apparent fungal growth was observed, taking samples from the skull, thorax and hind limbs. All samples were kept between 3 and 5°C prior to the laboratory procedure.22 In addition, soil samples from the graves and the area sorrounding the facilities were collected in the different campaigns as control samples.20,22
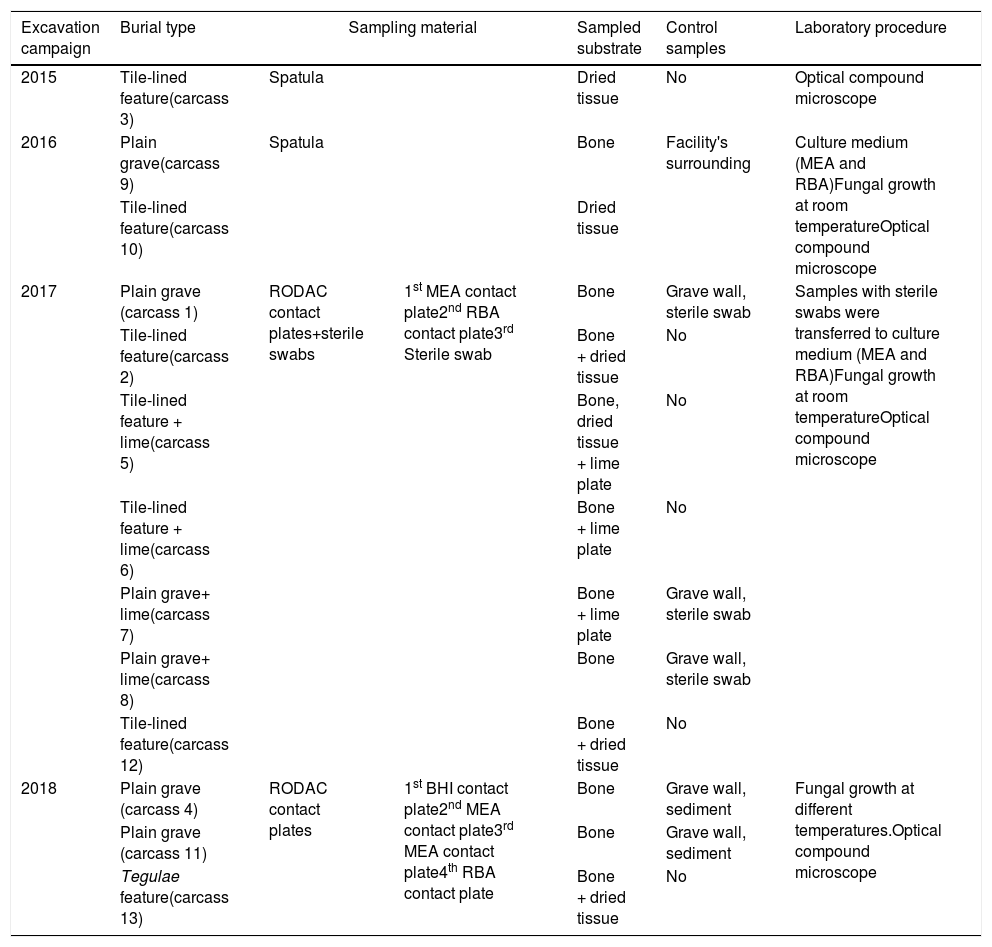
Sampling strategies used in Taphos-m burial sites for the mycological study. All contact plates used are RODAC contact plates.
| Excavation campaign | Burial type | Sampling material | Sampled substrate | Control samples | Laboratory procedure | |
|---|---|---|---|---|---|---|
| 2015 | Tile-lined feature(carcass 3) | Spatula | Dried tissue | No | Optical compound microscope | |
| 2016 | Plain grave(carcass 9) | Spatula | Bone | Facility's surrounding | Culture medium (MEA and RBA)Fungal growth at room temperatureOptical compound microscope | |
| Tile-lined feature(carcass 10) | Dried tissue | |||||
| 2017 | Plain grave (carcass 1) | RODAC contact plates+sterile swabs | 1st MEA contact plate2nd RBA contact plate3rd Sterile swab | Bone | Grave wall, sterile swab | Samples with sterile swabs were transferred to culture medium (MEA and RBA)Fungal growth at room temperatureOptical compound microscope |
| Tile-lined feature(carcass 2) | Bone + dried tissue | No | ||||
| Tile-lined feature + lime(carcass 5) | Bone, dried tissue + lime plate | No | ||||
| Tile-lined feature + lime(carcass 6) | Bone + lime plate | No | ||||
| Plain grave+ lime(carcass 7) | Bone + lime plate | Grave wall, sterile swab | ||||
| Plain grave+ lime(carcass 8) | Bone | Grave wall, sterile swab | ||||
| Tile-lined feature(carcass 12) | Bone + dried tissue | No | ||||
| 2018 | Plain grave (carcass 4) | RODAC contact plates | 1st BHI contact plate2nd MEA contact plate3rd MEA contact plate4th RBA contact plate | Bone | Grave wall, sediment | Fungal growth at different temperatures.Optical compound microscope |
| Plain grave (carcass 11) | Bone | Grave wall, sediment | ||||
| Tegulae feature(carcass 13) | Bone + dried tissue | No | ||||
The samples were studied at the Mycology Laboratory in the Unit of Botany of the Universitat Autònoma de Barcelona. The nutritional media (Liofilchem, Italy) used in this study were Malt Extract Agar (MEA), Rose Bengal Agar with chloramphenicol (RBA) and Brain-Heart Infusion Agar (BHI). The samples obtained with contact plates were kept at room temperature (22–24°C) and the samples obtained with spatulas and sterile swabs were transferred in a biosafety cabinet into Petri dishes with nutritive culture medium (MEA or RBA) and kept under laboratory conditions to allow the development of fungal colonies. In addition, some MEA culture media from carcasses sampled in 2018 were incubated at 35°C to enhance the growth of potential thermophilic fungi. In total, 134 culture plates (MEA, RBA or BHI) were used with the samples obtained in 2015–2016 (28 plates), in 2017 (70 plates) and in 2018 (36 plates). Control samples of soils were inoculated or transferred diluted on MEA and RBA plates. The procedure performed to identify the fungi isolated from soil samples was the same as that applied for the ones isolated from the carcasses. In total, 16 control plates were used.
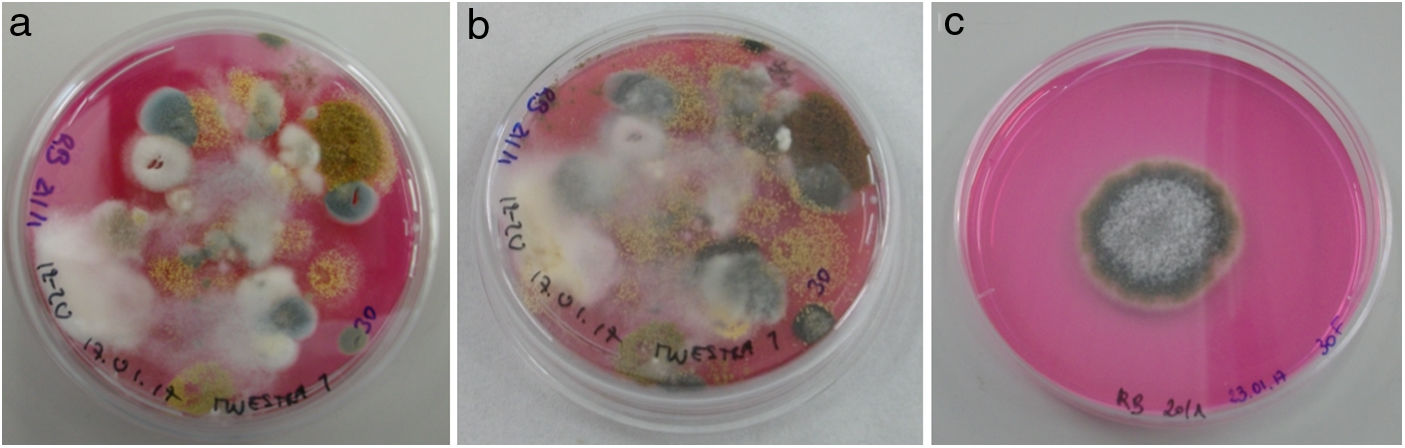
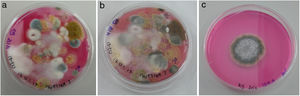
All culture media with fungal colonies were photographed every week for 3 weeks (Fig. 2). The growing colonies were transferred to individual plates to isolate each fungal species, and microscope preparations were made for the morphological identification of the fungal genera using different dichotomous identification keys.6,23 The material for the microscope preparations was previously disinfected with alcohol. The observations were made by optical microscopy ZEISS Axio Scope.A1, and digital photographs were taken with Jenoptik ProgRes C3 Captur software.
The morphological similarities between the genera Acremonium and Fusarium, and Geotrichum and Chrysonilia, led us to create two groups for this study: Acremonium – Fusarium and Geotrichum – Chrysonilia.
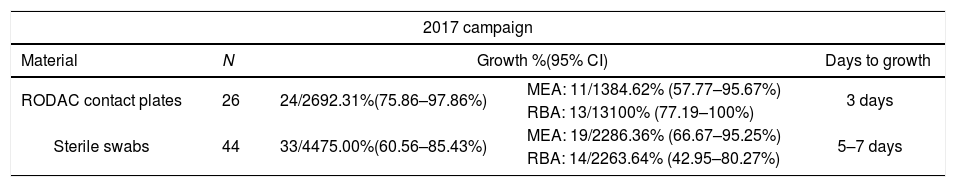
ResultsThe optimal sampling methodBeing sampling with a spatula an invasive method it was rejected as the best one. Therefore, the optimal sampling method would be sterile swabs or RODAC contact plates. In 2017, a total of 70 samples were obtained: 26 with RODAC contact plates and 44 with sterile swabs. Fungal colonies grew on 24 of the 26 contact plates (92.3%): 84.62% in MEA and 100% in RBA. Colonies grew in 33 of the 44 samples obtained with a sterile swab (75%): 86.36% in MEA and 63.64% in RBA. Fungi growth in culture media from RODAC contact plates occurred in 3 days, while samples obtained with sterile swabs did not grow until 5–7 days after inoculation (Table 3).
Growth of fungal colonies from the samples obtained in 2017 and 2018 with different methodologies and incubated at room temperature (22–24°C).
| 2017 campaign | ||||
|---|---|---|---|---|
| Material | N | Growth %(95% CI) | Days to growth | |
| RODAC contact plates | 26 | 24/2692.31%(75.86–97.86%) | MEA: 11/1384.62% (57.77–95.67%) | 3 days |
| RBA: 13/13100% (77.19–100%) | ||||
| Sterile swabs | 44 | 33/4475.00%(60.56–85.43%) | MEA: 19/2286.36% (66.67–95.25%) | 5–7 days |
| RBA: 14/2263.64% (42.95–80.27%) | ||||
| 2018 campaign | ||||
|---|---|---|---|---|
| Material | N | Growth %(95% CI) | Days to growth | |
| RODAC contact plates | 27 | 23/2785.19%(67.52–94.08%) | MEA: 9/9100% (70.09–100%) | 3 days |
| RBA: 9/9100% (70.09–100%) | ||||
| BHI: 5/955.55% (26.67–81.12%) | ||||
In 2018 sampling was only performed with RODAC contact plates. A total of 36 culture plates (27 of them incubated at 22–24°C and the remaining 9 at 35°C) were used for sampling different anatomical parts of the carcasses. Fungal colonies at 22–24°C grew in 23 of the 27 plates (85.19%): 100% in MEA and RBA, and 55.55% in BHI (Table 3). Fungi growth took place also in 3 days. There was fungal growth in all MEA plates (9) incubated at 35°C. The fastest growth rate (2 days) was registered on MEA plates with samples from carcass 13. Fungal samples from sterile swabs grew better in MEA and fungal samples from RODAC contact plates grew well in both culture media. RBA culture medium was the best because there was fungal growth in all the plates. In BHI culture medium, on the contrary, fungal growth was observed in only 55.55% of the cultures (Table 3).
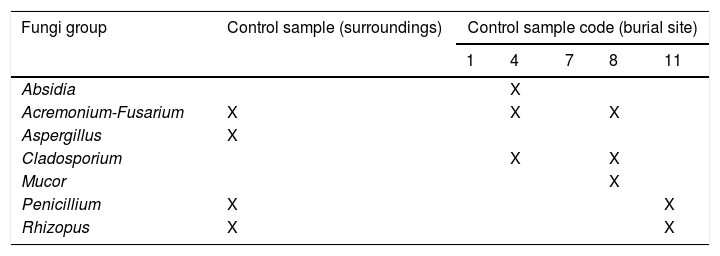
Fungi representationControl samples from soil were inoculated on 16 plates, and 12 fungal isolates in total were recovered. The genera isolated from these samples were Absidia, Acremonium-Fusarium, Aspergillus, Cladosporium, Mucor, Penicillium and Rhizopus (Table 4A).
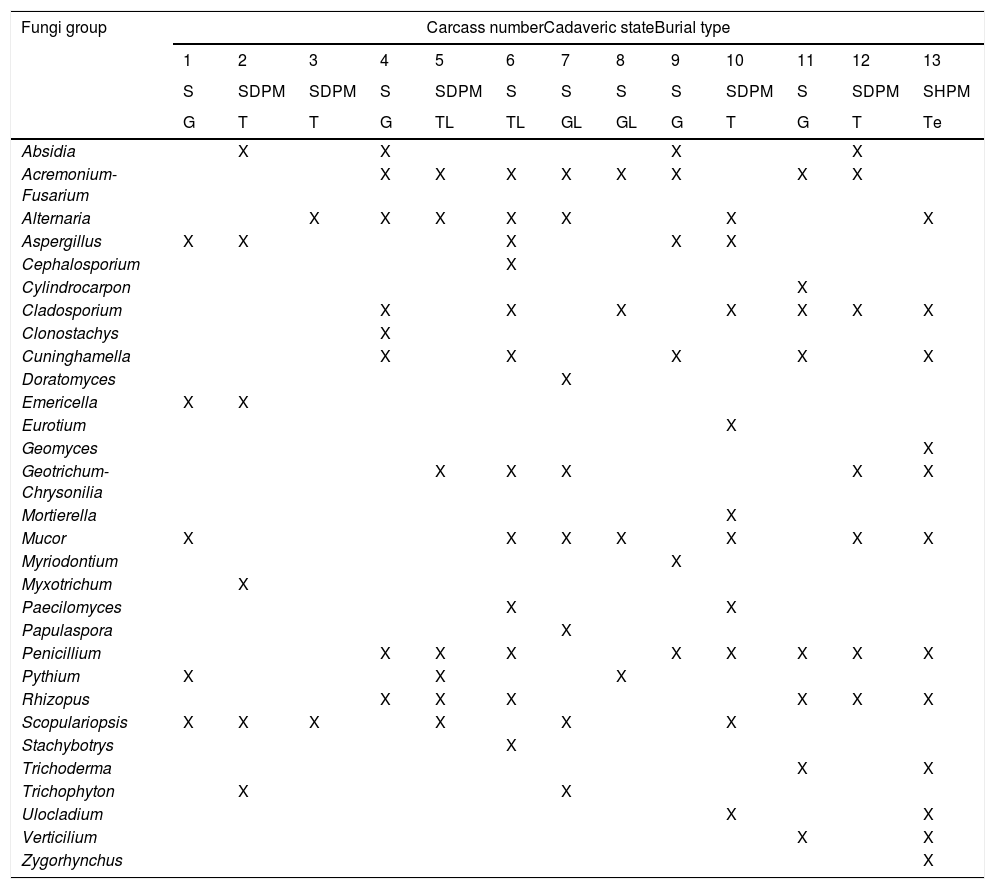
The pig carcasses presented three different cadaveric states (Table 4B): 7 carcasses were skeletonized, 5 were skeletonized with dry putrid matter, and 1 carcass was skeletonized with humid putrid matter. Therefore, samples were collected from bones as well as mummified and humid tissue. All samples were incubated in 134 plates, and 30 fungal genera were identified in 112 plates. Although more than one genus was isolated in all the cases, eight carcasses were characterized by the isolation of a fungal genus that was unique in each of them. Acremonium-Fusarium (found in 9 carcasses), Penicillium (8 carcasses) and Alternaria, Cladosporium and Mucor (7 carcasses) were the most represented groups or genera (Table 4B). However, Rhizopus was the only genus identified in those plates incubated at a higher temperature (35°C).
Fungi isolated in Taphos-m carcasses according to their cadaveric states and the type of burial (“X” means presence).
| Fungi group | Carcass numberCadaveric stateBurial type | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| S | SDPM | SDPM | S | SDPM | S | S | S | S | SDPM | S | SDPM | SHPM | |
| G | T | T | G | TL | TL | GL | GL | G | T | G | T | Te | |
| Absidia | X | X | X | X | |||||||||
| Acremonium-Fusarium | X | X | X | X | X | X | X | X | |||||
| Alternaria | X | X | X | X | X | X | X | ||||||
| Aspergillus | X | X | X | X | X | ||||||||
| Cephalosporium | X | ||||||||||||
| Cylindrocarpon | X | ||||||||||||
| Cladosporium | X | X | X | X | X | X | X | ||||||
| Clonostachys | X | ||||||||||||
| Cuninghamella | X | X | X | X | X | ||||||||
| Doratomyces | X | ||||||||||||
| Emericella | X | X | |||||||||||
| Eurotium | X | ||||||||||||
| Geomyces | X | ||||||||||||
| Geotrichum-Chrysonilia | X | X | X | X | X | ||||||||
| Mortierella | X | ||||||||||||
| Mucor | X | X | X | X | X | X | X | ||||||
| Myriodontium | X | ||||||||||||
| Myxotrichum | X | ||||||||||||
| Paecilomyces | X | X | |||||||||||
| Papulaspora | X | ||||||||||||
| Penicillium | X | X | X | X | X | X | X | X | |||||
| Pythium | X | X | X | ||||||||||
| Rhizopus | X | X | X | X | X | X | |||||||
| Scopulariopsis | X | X | X | X | X | X | |||||||
| Stachybotrys | X | ||||||||||||
| Trichoderma | X | X | |||||||||||
| Trichophyton | X | X | |||||||||||
| Ulocladium | X | X | |||||||||||
| Verticilium | X | X | |||||||||||
| Zygorhynchus | X | ||||||||||||
S: skeletonization; SDPM: skeletonization with dry putrid matter; SHPM: skeletonization with humid putrid matter; T: tile-lined feature; G: plain grave; Te: tegulae feature; TL: tile-lined feature with lime; GL: plain grave with lime.
Although different substrates were sampled, the same fungi, genera or groups, were found in the different substrates. From 30 fungi genera or groups, 26 were identified from bone samples: Acremonium-Fusarium, Alternaria, Aspergillus, Cephalosporium, Clonostachys, Cunninghamella, Cylindrocarpon, Doratomyces, Emericella, Eurotium, Fusarium, Geomyces, Geotrichum-Chrysonilia, Mucor, Myriodontium, Paecilomyces, Papulaspora, Penicillium, Pythium, Scopulariopsis, Stachybotrys, Trichoderma, Trichophyton, Ulocladium, Verticillium and Zygorhynchus. The following 16 fungi genera or groups were identified from mummified and humid tissue samples: Absidia, Acremonium-Fusarium, Aspergillus, Cladosporium, Eurotium, Geomyces, Geotrichum-Chrysonilia, Mortiriella, Mucor, Myxotrichum, Rhizopus, Scopulariopsis, Trichophyton, Ulocladium, Verticillium and Zygorhynchus. Finally, only 10 fungi genera or groups were isolated from the lime plates: Acremonium-Fusarium, Alternaria, Aspergillus, Cladosporium, Geotrichum-Chrysonilia, Paecilomyces, Penicillium, Rhizopus, Scopulariopsis and Trichophyton.
In relation to the typology of the burial site, greater fungal representation was observed in the carcasses in graves lined with tiles and in the tegulae feature than in plain graves. The highest fungal representation (12 identified genera) was found in the tile-lined feature (with lime) of carcass 6, and in the tegulae feature of carcass 13 (Table 4B).
DiscussionAlthough several procedures were considered effective for sampling at Taphos-m, our results show that some methods yield better results than others. Sampling methods were evaluated throughout the study to establish the optimal one for collecting fungi from the decomposing pig carcass at the Taphos-m experimental project. Sampling with a spatula was rejected because it was considered an invasive method. Sampling with sterile swab was considered the easiest and the most convenient sampling method,2,4,17,18,25 especially when the area to sample from is difficult to access (some control areas in the present study). However, sampling with RODAC contact plates offers a basic culture medium to continue the fungal growth just from the moment of sampling. The best results in fungal growth were observed in the samples collected with RODAC contact plates because there was not a break in the nutritional demand that could delay the growth, and so quick proliferation took place in 2–3 days. Due to the aforementioned results, and also considering the minimal presence of colonies in the control samples, it seems that sampling with RODAC contact plates is the best method to find fungi in bodies under different cadaveric states.
RBA was the culture medium with the best performance (100% growth) among the culture media used in the present study since there was no bacteria competition as in the MEA. The fungal colonies in BHI culture medium did not show the expected growth. This could be due to a quick bacterial spread, usual in bodies in decomposition, which could inhibit the fungi proliferation in MEA and BHI media. It must be taken into consideration that not all fungal colonies that grew in the laboratory could be classified: in cases of slow or null growth reproductive structures were not present and, therefore, it was impossible to identify the fungi.
The fungi identified in the control samples are globally distributed and they are related to soils, plants, fertilizers, indoor environments and animals.5,6,23 In addition, they are described in many studies related to corpses in different cadaveric states buried inside plain graves.4,15,19,24 In our study, the most repeated genera in control and carcass samples were Acremonium-Fusarium, Penicillium, Cladosporium and Rhizopus. Considering that the Acremonium-Fusarium group consists of two genera of saprophytic fungi, globally distributed and identified in different habitats such as mucous membranes, human hair, clothes, plant remains, cultivated soils and corpses15,19,24 it is not surprising that it is the most isolated fungi group in Taphos-m carcasses. Penicillium and Rhizopus species have been identified in corpses in different cadaveric states (mummies and skeletons), in mucous membranes and organs, in clothing and in soil under corpses,2,4,12,15,18,19,24–26 and Cladosporium is a saprophytic genus described in soils under corpses.26 Therefore, their presence in carcasses was related to the decomposition process.
There were not many differences between the fungi genera or groups found on the skeletons and those on the carcasses with tissue. The fungal genera identified in the different pig carcasses have also been identified on decaying bodies from other burial contexts.2,12,15,18,19,24–27Cephalosporium and Clonostachys are related to humid environments and to different types of soil,27 and although they have not been found in the control samples, their presence in the carcasses could be related to the environment of the facilities. Other fungi present in decaying carcasses such as Trichoderma, Geomyces and Ulocladium are related to the dermis and have keratinophilic activity.11 Only Rhizopus was identified in all culture media incubated at high temperatures. Therefore, there were probably two strains of Rhizopus in Taphos-m burials: a thermophilic strain (isolated in carcass 13) and a thermotolerant strain (isolated in carcasses 4 and 11). The last one could be the same as the one identified in other culture media incubated at room temperature.
The fungal genera or groups identified in the lime plates were the same as those in the rest of the burial sites and those described in other works.3,4,12,15,18,19,24–27 Those samples came from lime plates in contact with the body, therefore fungi grow on lime plates because of the contact with organic matter (carcass) in decomposition.
Finally, the rest of the genera, Cephalosporium, Clonostachys, Cunninghamella, Cylindrocarpon, Doratomyces, Emericella, Fusarium, Geomyces, Paecilomyces, Papulaspora, Pythium, Stachybotrys, Ulocladium and Zygorhynchus have been found for the first time in bone samples, and Absidia, Geomyces, Myxotrichum, Ulocladium and Zygorhynchus have been found for the first time in decayed tissue samples.
Although the sample size is quite small, an interesting number of fungal genera were identified. The results of this study provide new data about the fungi that may be recovered from bodies buried for more than 3 years in different types of burials and under different cadaveric states. These results show that fungi have no preference for a certain cadaveric state or type of burial. The presence of fungi on the pig carcasses depends on environmental conditions and, if these are favourable, the fungi grow on the bodies because these are an excellent source of organic matter for them.2,6,23,24 A larger study could provide more information about the relationship between the fungi and the different types of burials. Increasing the sample size would also make it possible to analyze the influence of other variables (sex, age, etc.) on the decomposition of bodies, and, consequently, on the fungi found. Similarly, by improving the culture media, greater fungal growth could be achieved in the laboratory. In the present study the presence of bacteria inhibited the growth of fungi in some plates.
ConclusionsThe search for the fungi that may be present on the pig carcasses buried experimentally in different burial sites have revealed many fungi genera on the cadaveric remains even though the colonies were not visible. Sampling with RODAC contact plates gives better results than sterile swabs because the media allows any potential growth from the time of sampling. In addition, the fact that there was no break in the fungi cycle could explain the great fungal diversity of the burials of Taphos-m. The genera found on the bodies were similar regardless the state of decomposition. Some genera isolated in Taphos-m had already been identified before in the same kind of substrate (7) or in a different one (16), but 15 fungi genera identified in this study had never been related to corpses in decomposition before. However, it is necessary to further study whether their presence has more to do with the bodies themselves or with the environment in which they were buried.
Conflict of interestsThe authors declare that they have no conflict of interest.
We would like to thank Dr. Josep Girbal for his dedication in this study and for the help in the design of sampling strategies, and Zoè Rabionet and Rubén Salazar for their contribution to the field and laboratory work. We would also like to express our gratitude to the Central Unit of Crime Scene-Forensic Division of the Mossos d’Esquadra (Catalonian Police) for the photographic record and the material provided for the exhumations. The project has the support of the Departament d’Agricultura, Ramaderia, Pesca, Alimentació i Medi Natural of the Generalitat de Catalunya, the research group Grup de Recerques de les Terres de Ponent, the Institut d’Estudis Ilerdencs in Catalonia (Spain) and the AGAUR of Generalitat de Catalunya (2017-SRG-1630).