The basidiomycetous yeast Cryptococcus gattii is an emerging and primary pathogen. There is a lack of information about its environmental spread outside outbreak regions in Mediterranean Europe, North and South America. Environmental sampling for C. gattii and molecular characterization of the obtained isolates will provide an insight into the global spread of the various genotypes.
MethodsWoody debris of native divi-divi (Caesalpinia coriaria) trees were sampled across Bonaire, Dutch Caribbean. Colonies suspected for Cryptococcus species were subjected to standard mycology investigations and identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Isolates identified as C. gattii were subjected to amplified fragment length polymorphism genotyping, mating-type analysis and multi-locus sequence typing.
ResultsTen colonies of C. gattii were cultured from different trunk hollows of the same divi-divi tree. Molecular characterization showed that all isolates were genotype AFLP6/VGII and mating-type α. Multi-locus sequence typing revealed that all isolates were genetically indistinguishable from each other.
ConclusionsC. gattii is present in the environment of Bonaire, which suggests that this yeast is likely to be present in the environment of other Caribbean islands.
La levadura Cryptococcus gattii es un basidiomiceto emergente y patógeno primario. Existe poca información acerca de su dispersión en medio ambiente fuera de las regiones con brotes descritos por esta levadura en la Europa mediterránea, Norte y Sur de América. Los muestreos del medio ambiente para la búsqueda de C. gattii y la caracterización molecular de los aislamientos obtenidos puede proveer de una visión global sobre la dispersión de varios de sus genotipos.
MétodosSe tomaron muestras de residuos de madera de árboles nativos divi-divi (Caesalpinia coriaria) en Bonaire, Caribe Holandés. Las colonias susceptibles de pertenecer a las especies de Cryptococcus se sometieron a un estudio micológico estándar e identificación por espectrometría de masas MALDI-TOF (Matrix-Assisted Laser Desorption Ionization-Time of Flight). Los aislamientos identificados como C. gattii se sometieron a genotipado mediante AFLP (Amplified Fragment Length Polymorphism), obtención del tipo sexual y MLST (Multi-locus Sequence Typing).
ResultadosSe obtuvieron diez colonias de C. gattii en el cultivo de nuestras de diferentes agujeros de un mismo árbol divi-divi. La caracterización molecular mostró que todos los aislamientos eran genotipo AFLP6/VGII y tipo sexual α. El tipado mediante MLST reveló que todos los aislamientos eran genéticamente indistinguibles unos de otros.
ConclusionesC. gattii está presente en el medio ambiente de Bonaire, lo que sugiere que esta levadura podría estar presente en el ambiente de otras islas del Caribe.
Cryptococcus gattii and its sibling Cryptococcus neoformans are the major basidiomycetous yeast pathogens within the genus Cryptococcus that comprises over one hundred recognized species.14 Annually, nearly an estimated one million individuals with HIV/AIDS develop cryptococcal meningitis with high estimated death rates of 6,25,000 subjects.19C. neoformans, with its two varieties grubii and neoformans, has a global distribution and affects mainly immunocompromised individuals, such as those with HIV/AIDS or those under immunosuppressive therapy. Although C. gattii is mainly restricted to subtropical and tropical climate zones and is known to be a major source of cryptococcosis among immunocompetent subjects, it has also emerged as a significant pathogen in Canada and the Pacific Northwest of the USA.1,2,9,10,20
Molecular techniques, such as amplified fragment length polymorphism (AFLP) fingerprinting, PCR-fingerprinting, restriction fragment length polymorphism fingerprinting and multi-locus sequence typing (MLST), have provided a detailed insight into the genetic diversity of the C. neoformans/C. gattii species complex.1,18Cryptococcus neoformans var. grubii (serotype A) can be divided into the three genotypes: AFLP1/VNI, AFLP1A/VNII/VNB and AFLP1B; C. neoformans var. neoformans (serotype D) is represented by genotype AFLP2/VNIV, and the intervarietal C. neoformans hybrid (serotype AD) corresponds with genotype AFLP3/VNIII.1,18C. gattii consists of five haploid genotypes, with genotypes AFLP4/VGI, AFLP6/VGII and AFLP10/VGIV representing the serotype B isolates, and genotypes AFLP5/VGIII and AFLP7/VGIV corresponding with isolates exhibiting the C serotype.9 Interspecies hybrids have been described and thus far have only been isolated from clinical sources.1
During the past decade ongoing and expanding outbreaks of C. gattii occurred in temperate climate zones affecting previously healthy humans and animals.6,10,20 Besides the changing distribution pattern, it has been observed that certain C. gattii genotypes may be found more often in immunocompromised individuals than in immunocompetent subjects.9 Notably, environmental isolation of C. gattii was found to be a valuable tool to enhance the current knowledge of the geographical spread, genotypic diversity and the environmental niche of this pathogenic species.3,4,6,16,20
Within the Caribbean, C. gattii has rarely been reported as clinical or veterinary infection.9–11,13 Despite large-scale environmental sampling of a plethora of environmental sources that includes many tree and cacti species9,10,12,15 the fungus has been, up until now, only recovered from the environment of Puerto Rico. Here we describe the environmental isolation and molecular characterization of C. gattii isolates from decayed woody debris of a native divi-divi tree (Caesalpinia coriaria) in Bonaire, Dutch Caribbean.
Woody debris, collected from inside trunk hollows of living divi-divi trees in April 2013, were cultured on simplified niger seed agar as described.4 The sampled divi-divi trees were located in Lagun Goto (N12° 14′ 3.1344″, E-68° 22′ 6.366″), Rincon village (N12° 14′ 24.1116″, E-68° 19′ 32.5662″) and neighboring surroundings of Hato village (N12° 10′ 11.8734″, E-68° 17′ 1.2366″). Plates were incubated at 28°C and periodically observed for chocolate brown colonies of C. gattii and C. neoformans up to 7 days. Suspected colonies of Cryptococcus spp. were purified by dilution plating and identified by their morphological and biochemical profiles using VITEK2 and API 20C AUX (bioMérieux, Marcy I’Etoile, France). Cryptococcus spp. colonies were also inoculated on l-canavanine-glycine bromothymol blue medium; a blue-color change suggestive of C. gattii was observed.1 Identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry was performed using the MALDI Biotyper, and spectra were compared to the reference spectra in the commercial database (Bruker Daltonics, Bremen, Germany).17
Ten colonies identified as C. gattii were obtained from decayed wood of different trunk hollows of the same divi-divi tree. The isolates have been deposited in the culture collection of the CBS-KNAW Fungal Biodiversity Centre under accession numbers CBS12864–CBS12873.
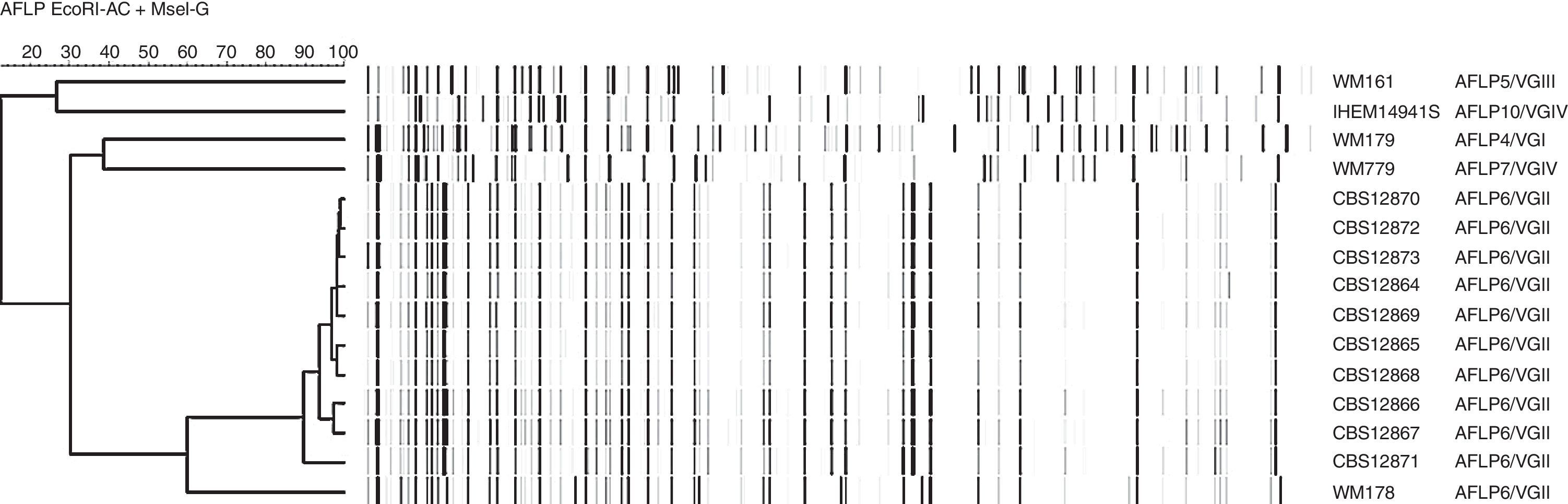
All ten C. gattii isolates were subsequently subjected to molecular characterization as described previously.4,6,9 Analysis of the AFLP fingerprints, using UPGMA clustering and the Pearson correlation coefficient in BioNumerics version 6.6.8 (Applied Maths, Sint-Martens-Latem, Belgium) showed that all ten isolates clustered together with the reference strain representing genotype AFLP6/VGII (Fig. 1). Mating-type analysis by conventional PCR revealed that all ten isolates exhibit the STE12-allele with the α mating-type.9
Amplified fragment length polymorphism fingerprint dendrogram of ten C. gattii isolates from Bonaire compared to the reference strains WM179 (AFLP4/VGI), WM161 (AFLP5/VGIII), WM178 (AFLP6/VGII), WM779 (AFLP7/VGIV) and IHEM14941 (AFLP10/VGIV) representing all known AFLP genotypes within C. gattii.
In-depth genetic analysis was initiated by amplification and sequencing of the ten nuclear loci CAP10, CAP59, GPD1, IGS1, LAC1, MPD1, PLB1, SOD1, TEF1 and URA5, as previously described.9 Multi-locus sequence typing links clinical isolates to their geographical origin and demonstrates their relatedness to the isolates obtained from environmental and veterinary sources.4,5,7,9,11–13 Sequences for each of the ten loci and for all ten C. gattii isolates revealed that they were genetically indistinguishable from each other. Genbank accession numbers for sequences identical to those obtained in the current study were DQ861598 (=CAP10), DQ096433 (=CAP59), DQ096382 (=GPD1), KF439871 (=IGS1), DQ096400 (=LAC1), DQ861595 (=MPD1), DQ198344 (=PLB1), HM207449 (=SOD1), DQ861593 (=TEF1) and HQ606091 (=URA5).
Phylogenetic analysis was performed as described before by using a bootstrapped maximum likelihood analysis (1000 bootstraps) in MEGA version 5.2 with the best fitting nucleotide substitution model being Hasegawa-Kishino-Yano plus gamma distributed with invariant sites and with C. gattii reference strains from the other genotypes as an outgroup.9 To compare the C. gattii isolates with those from other localities, the recently published MLST-dataset of C. gattii AFLP6/VGII isolates was included.9
As depicted in Fig. 2, all isolates from Bonaire clustered together with the genotype AFLP6/VGII isolate CBS1930 from Aruba. This isolate, obtained in July 1953, originated from clinical material of a child. This isolate was experimentally inoculated in a goat to see whether the atypical yeast isolate was the culprit of infection (original correspondence about this isolate in the CBS-KNAW archive). Other available isolates from Latin America were only distantly related, with Brazilian and French Guyana isolates as the closest siblings (Fig. 2).
Bootstrapped maximum likelihood phylogenetic analysis of the multi-locus sequence typing data from a global set of C. gattii genotype AFLP6/VGII isolates (data from 9) compared with those isolated from a divi-divi tree on Bonaire.
Interestingly, isolate CBS1930 was found to have mating-type a while the isolates from Bonaire were all mating-type α. Genetically, the isolates from Aruba and Bonaire differed slightly from each other suggesting that there is a clonal population of C. gattii AFLP6/VGII on these Caribbean islands. However, due to the presence of different mating-types within this set of isolates the conclusion of one clonal population needs to be taken with caution. Another explanation may be that although isolates from Dutch Caribbean islands are genetically closely related they may form different clonal populations. This scenario is identical to the one observed in Mediterranean population of C. gattii AFLP4/VGI, where Italian isolates with the mating-type a and Spanish isolates with the mating-type α were genetically indistinguishable from each other using the same set of ten sequenced nuclear loci.9
Unfortunately, the only C. gattii AFLP6/VGII isolates available from the Caribbean, isolated from cacti on the island of Puerto Rico, could not be compared with our data since these isolates are not available to the research community.8,15
To conclude, C. gattii AFLP6/VGII is present in the environment of the Dutch Caribbean and Puerto Rico, suggesting that C. gattii might be present in other Caribbean localities. Little is known about the epidemiology of C. gattii in Central America and the Caribbean; therefore environmental sampling at other Caribbean islands, as well as investigating the occurrence of this pathogenic yeast among clinical samples from this region, should be considered.
Conflict of interestThe authors declare that there are no competing interests.
The authors would like to thank Dr. Kika Colom (Alicante, Spain) for preparing the Spanish-language abstract.