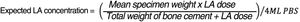Currently, we do not have a gold standard for pain management after total knee arthroplasty. We may use one of more drug delivery systems, none of which are ideal.
An ideal depot delivery system would provide therapeutic, nontoxic, doses of drug at the surgical side, especially during 72h postoperatively.
The bone cement used in arthroplasties has been used as a drug delivery system, especially antibiotics, since 1970. Based on this principle, we developed this study with the aim to characterize the elution profile of two local anaesthetics (lidocaine hydrochloride and bupivacaine hydrochloride) from PMMA (polymethilmethacrylate) bone cement.
Material and methodsPalacos® R+G bone cement and lidocaine hydrochloride or bupivacaine hydrochloride specimens were obtained depending on the study group. These specimens were immersed in PBS (phosphate buffered saline) and removed from the solution at different set times.
Subsequently, the concentration of local anaesthetic in the liquid was analyzed by liquid chromatography.
ResultsThe percentage of lidocaine eluted from PMMA bone cement in this study was 9.74% of the total lidocaine content per specimen at 72h and 18.73% at 336h (14 days). In case of bupivacaine, the elution percentage was 2.71% of the total bupivacaine content per specimen at 72h and 2.70% at 336h (14 days).
ConclusionsLocal anaesthetics elute in vitro from PMMA bone cement, reaching doses at 72h close to the doses used in anaesthetic blocks.
En la actualidad no se dispone de un gold standard para el manejo del dolor postoperatorio tras una artroplastia total de rodilla, dado que se pueden administrar analgésicos a través de diferentes vías y ninguna de ellas está exenta de riesgos. El sistema ideal de administración de analgésicos debería proporcionar dosis terapéuticas, no tóxicas, en el sitio quirúrgico, especialmente durante las primeras 72h.
El cemento óseo utilizado en las artroplastias se ha usado como un medio de liberación de fármacos, especialmente antibióticos, desde 1970. Con base en dicho principio, se desarrolló este estudio con el objetivo de conocer el perfil de elución de dos anestésicos locales (hidrocloruro de lidocaína e hidrocloruro de bupivacaína) desde el cemento óseo de polimetilmetacrilato (PMMA).
Material y métodosSe obtuvieron especímenes de cemento óseo Palacos® R+G e hidrocloruro de lidocaína o hidrocloruro de bupivacaína según el grupo de estudio. Estos especímenes se sumergieron en PBS (phosphate buffered saline) y se retiraron de la solución en diferentes cortes temporales establecidos. Posteriormente, se analizó la concentración de anestésico local en el líquido mediante cromatografía líquida.
ResultadosEl porcentaje de lidocaína eludida del cemento óseo PMMA de este estudio ha sido del 9,74% del contenido total de lidocaína por espécimen a las 72h y del 18,73% a las 336h (14 días). En el caso de la bupivacaína, el porcentaje de elución ha sido del 2,71% del contenido total de bupivacaína por espécimen a las 72h y del 2,70% a las 336h (14 días).
ConclusionesLos anestésicos locales eluyen in vitro desde el cemento óseo, alcanzando a las 72h dosis cercanas a las dosis utilizadas en bloqueos anestésicos.
Total knee arthroplasty (TKA) is one of the most widely used procedures performed in patients with chronic refractory knee pain in whom conservative treatment has failed.1
Postoperative pain after TKA implantation tends to be moderate to severe in intensity and can be difficult to control, especially during the first three postoperative days.2 Achieving optimal pain control improves function, facilitates rehabilitation, and reduces the progression from acute to chronic pain.3 Currently, there is no gold standard for postoperative pain management following TKA,4–7 inasmuch as analgesics can be administered by different routes, none of which is ideal, given that adverse reactions are frequent and complications can arise.8 The ideal analgesic delivery system for postoperative pain management following TKA is the one that is most effective for the patient. The optimal analgesic delivery system should be able to provide therapeutic, non-toxic doses at the surgical site, especially during the first 72h. In the context of post-TKA pain management, polymethylmethacrylate (PMMA) bone cement could be used as a local drug delivery system to achieve an analgesic effect.
Since 1970, studies have been conducted to establish the suitability of PMMA bone cement as a local drug delivery system, especially for antibiotics.9 The elution pattern of these drugs displays a high rate of initial drug release followed by declining drug release over the next few days.10
The elution of the drugs from the cement will be influenced by certain factors, such as the uptake of water by the cement, the porosity of the cement matrix, the composition of the cement and its surface, the size of the drug particles, and the drug content.11–13
Continuing on from these initial studies and taking into account that local anaesthetics (LAs) have an excellent safety and effectiveness profile, some authors have investigated the elution capacity of LAs from PMMA bone cement,8,14 arriving at elution figures that vary depending on the drug and the cement used.
The objective of this study is to analyze the elution profile of lidocaine hydrochloride and bupivacaine hydrochloride from PMMA bone cement with gentamicin.
Material and methodsThis study was conducted with the approval of the clinical research ethics committee (HCB 2020/0097) and in accordance with international standards ISO 5833:2022 Implants for surgery-Acrylic resin cements and the Standard Specification for Acrylic Bone Cement ASTM F451-16.
Two study groups were defined: the group in which cement with lidocaine hydrochloride (GL) and the group in which cement with bupivacaine hydrochloride (GB) were used.
The LA dose was defined by factoring in the elution percentages reported in previous studies8,14 and aiming for a total eluted dose of 0.20g lidocaine and 0.14g bupivacaine. These doses correspond to the standard doses used in anaesthetic blocks and the typical estimate of 3mg/kg and 2mg/kg for a patient with an average weight of 70kg for lidocaine15 and bupivacaine,16 respectively. The doses thereby calculated were 2.48g in the lidocaine group (GL) and 3.58g in the bupivacaine group (GB). Lidocaine hydrochloride and bupivacaine hydrochloride (Fagron®, Terrassa) were used.
Palacos® R+G (Heraeus Medical GMBH, Germany), a fast-setting, high-viscosity PMMA composite bone cement containing gentamicin, was used.
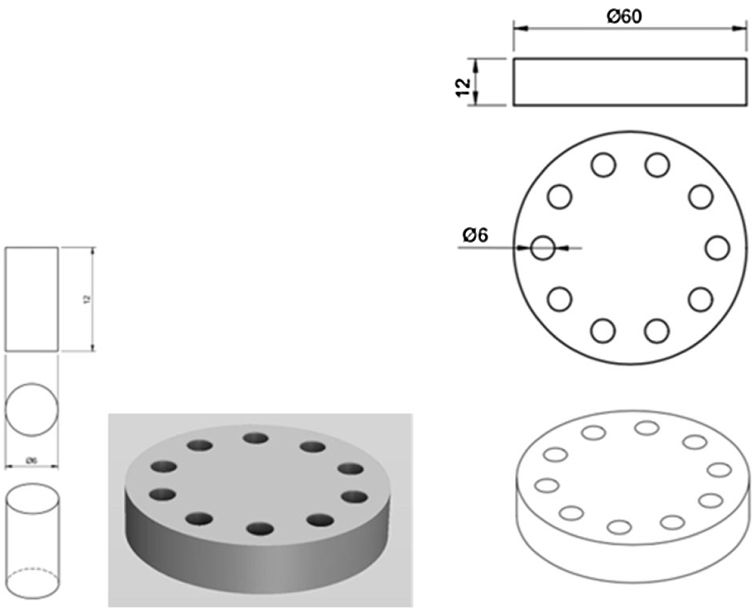
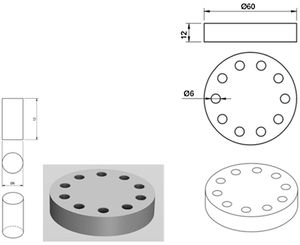
Moulds (Fig. 1) were manufactured with which to create cylindrical specimens of 6mm diameter×12mm height by machining Teflon. As the standard does not specify the dimensions of the specimens for elution analyses, the references for mechanical studies on this type of cement as specified in ISO 5833:2002, were used.
To prepare the specimens for the GL and GB groups, the powdered LAs (lidocaine hydrochloride or bupivacaine hydrochloride) were mixed with the powder component of the cement using the geometric dilution method. The liquid component of the cement was added to the mixture as per the manufacturer's recommendations. The mixture was allowed to stand for 30s and was introduced into the moulds, removing the excess. It was left to dry for 30min and then the specimens were removed.
A sample size of 3 specimens per study group and cut-off time were set arbitrarily, given that there are currently no regulations governing the study of elution.
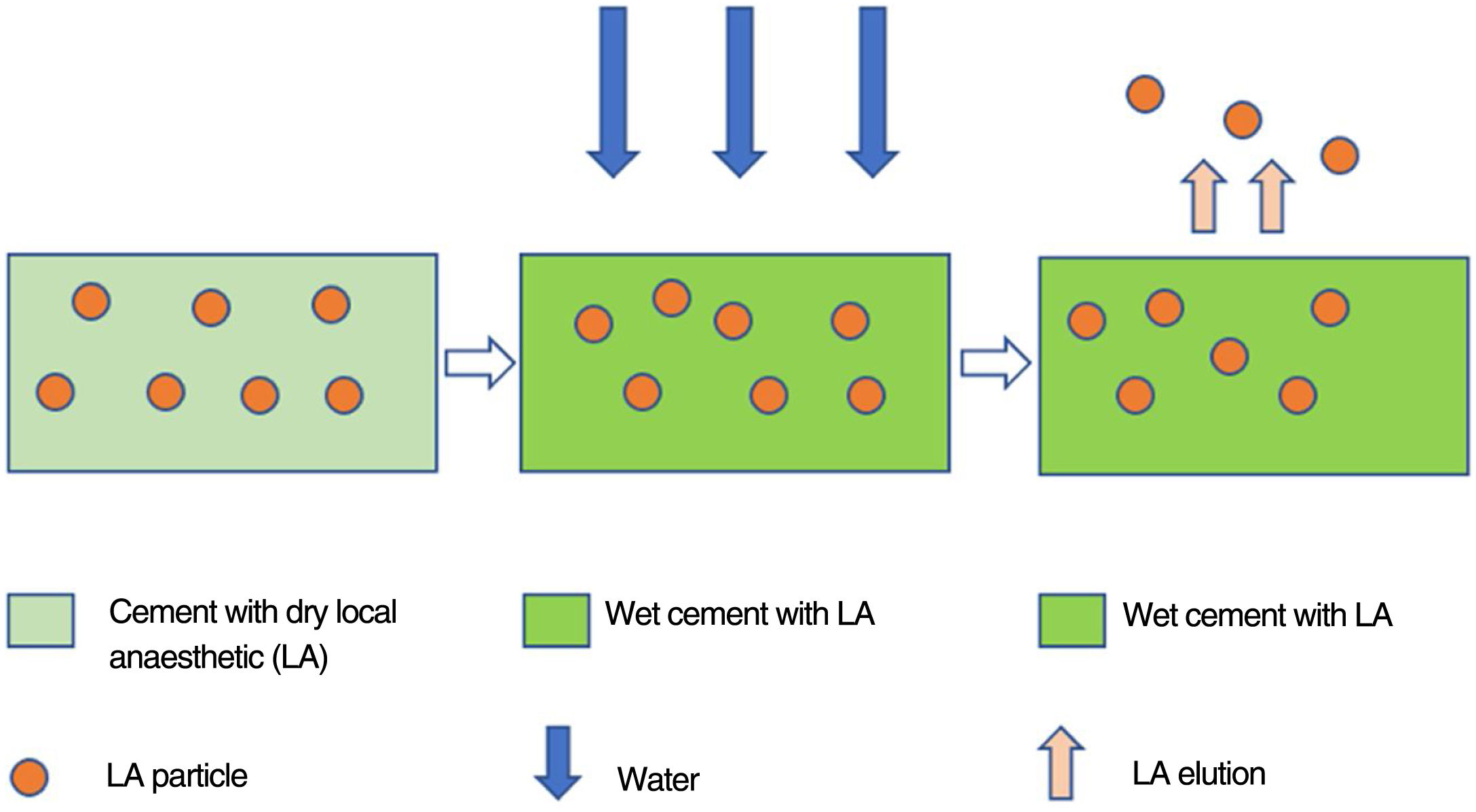
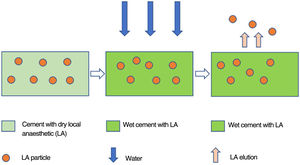
The elution process follows the law of diffusion (Fig. 2), takes place starting from the surface and correlates directly with cement's capacity to absorb water. In order to obtain specimens with similar contact surfaces, the three most homogeneous specimens (A, B, C) of each group in terms of size and weight were selected.
Specimens were placed in cryovials, immersed in 4ml of saline (PBS) at room temperature and placed on a reciprocating shaker. At the set cut-off points (1h, 3h, 6h, 24h, 48h, 72h, 168h, and 336h) the specimens were removed from the solution and the cryovials were stored at −80°C. In the samples thus obtained, the concentration of LA in the liquid was analyzed by liquid chromatography (HPLC).
In order to lend internal validity to the measurement method, the LA concentration analysis was repeated in triplicate, in each study group and time point.
ResultsTables 1 and 2 present the concentration of lidocaine and bupivacaine in μg/ml in each of the samples analyzed, as well as the mean concentration and standard deviation (SD) at each time point. In addition, the percentage of lidocaine and bupivacaine released with respect to the total LA present in the cement mixture is provided.
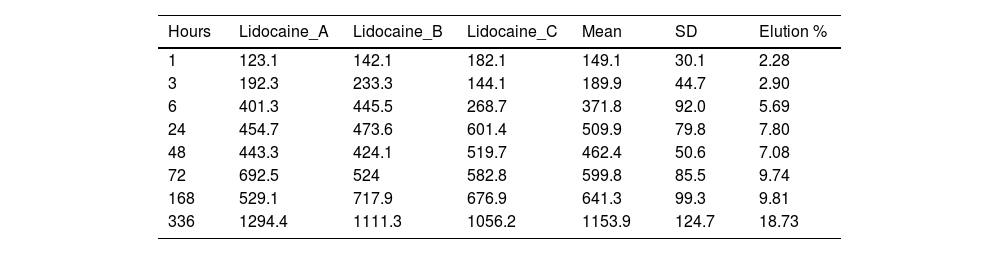
Concentration (μg/ml) and mean elution (%) of lidocaine.
| Hours | Lidocaine_A | Lidocaine_B | Lidocaine_C | Mean | SD | Elution % |
|---|---|---|---|---|---|---|
| 1 | 123.1 | 142.1 | 182.1 | 149.1 | 30.1 | 2.28 |
| 3 | 192.3 | 233.3 | 144.1 | 189.9 | 44.7 | 2.90 |
| 6 | 401.3 | 445.5 | 268.7 | 371.8 | 92.0 | 5.69 |
| 24 | 454.7 | 473.6 | 601.4 | 509.9 | 79.8 | 7.80 |
| 48 | 443.3 | 424.1 | 519.7 | 462.4 | 50.6 | 7.08 |
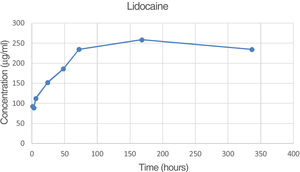
| 72 | 692.5 | 524 | 582.8 | 599.8 | 85.5 | 9.74 |
| 168 | 529.1 | 717.9 | 676.9 | 641.3 | 99.3 | 9.81 |
| 336 | 1294.4 | 1111.3 | 1056.2 | 1153.9 | 124.7 | 18.73 |
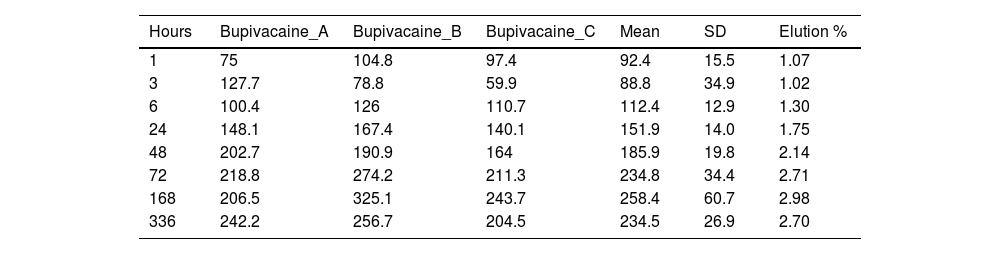
Concentration (μg/ml) and mean elution (%) of bupivacaine.
| Hours | Bupivacaine_A | Bupivacaine_B | Bupivacaine_C | Mean | SD | Elution % |
|---|---|---|---|---|---|---|
| 1 | 75 | 104.8 | 97.4 | 92.4 | 15.5 | 1.07 |
| 3 | 127.7 | 78.8 | 59.9 | 88.8 | 34.9 | 1.02 |
| 6 | 100.4 | 126 | 110.7 | 112.4 | 12.9 | 1.30 |
| 24 | 148.1 | 167.4 | 140.1 | 151.9 | 14.0 | 1.75 |
| 48 | 202.7 | 190.9 | 164 | 185.9 | 19.8 | 2.14 |
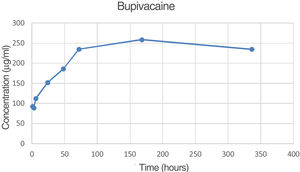
| 72 | 218.8 | 274.2 | 211.3 | 234.8 | 34.4 | 2.71 |
| 168 | 206.5 | 325.1 | 243.7 | 258.4 | 60.7 | 2.98 |
| 336 | 242.2 | 256.7 | 204.5 | 234.5 | 26.9 | 2.70 |
To calculate the percentage of lidocaine released with respect to the total lidocaine present in the specimen, the following estimation was made: in the GL group, 2.48g of lidocaine was added to 40.8g of powdered cement, representing a total powder weight of 43.28g. In the GB group, 3.58g of bupivacaine was added to 40.8g of powder cement representing a total powder weight of 44.38g. If the average weight of the specimens was 0.43g and they were immersed in 4ml of PBS, the expected maximum concentration, if 100% lidocaine eluted, would be 6160μg/ml in the GL group and 8672μg/ml in the GB group. These calculations have been made using the formula shown in Fig. 3.
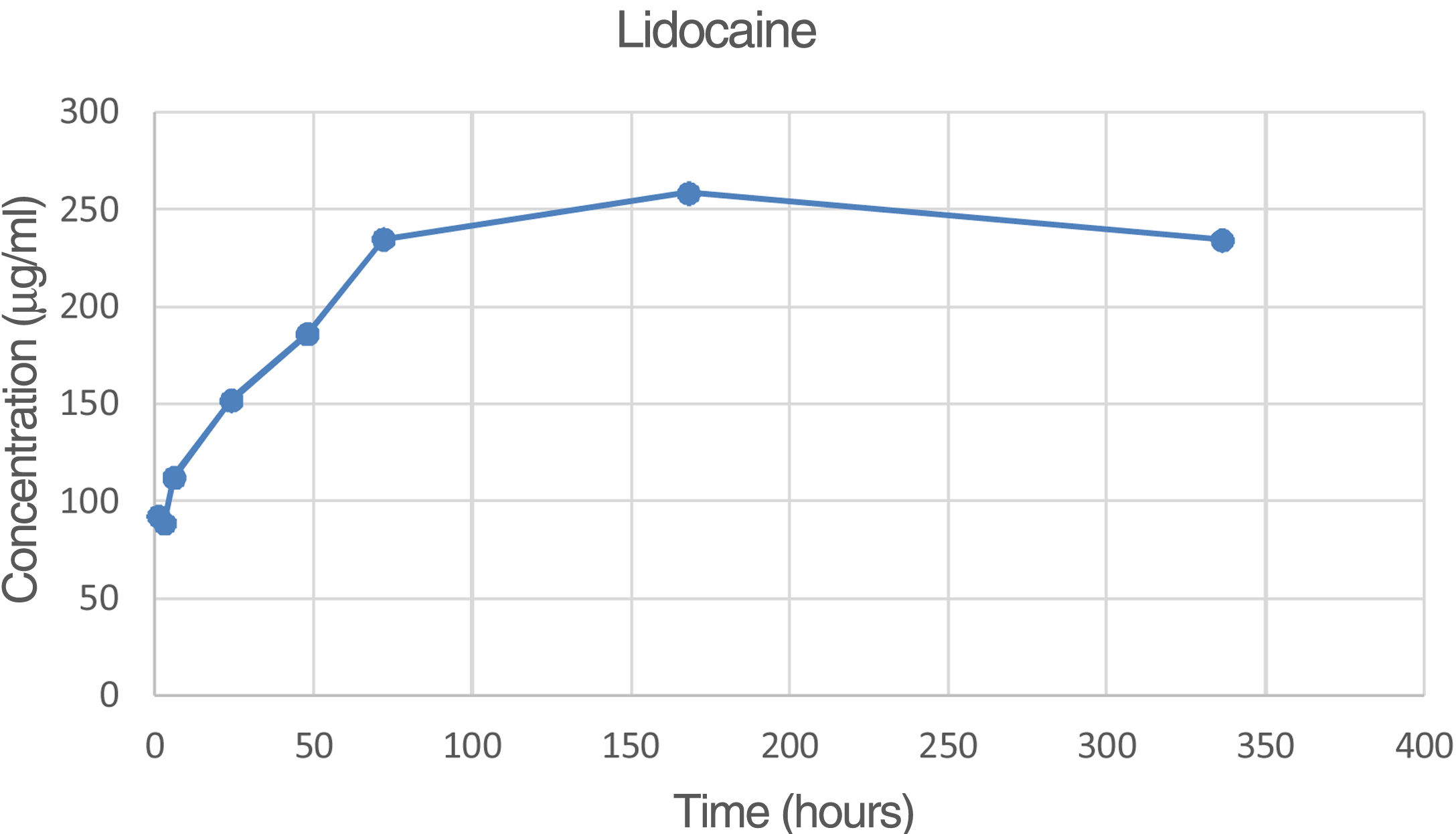
The percentage of lidocaine eluded from the PMMA bone cement in this study was 9.74% of the total amount of lidocaine per specimen at 72h and 18.73% at 336h (14 days).
Fig. 4 is the graph representing the average concentration of lidocaine eluded at the various time points.
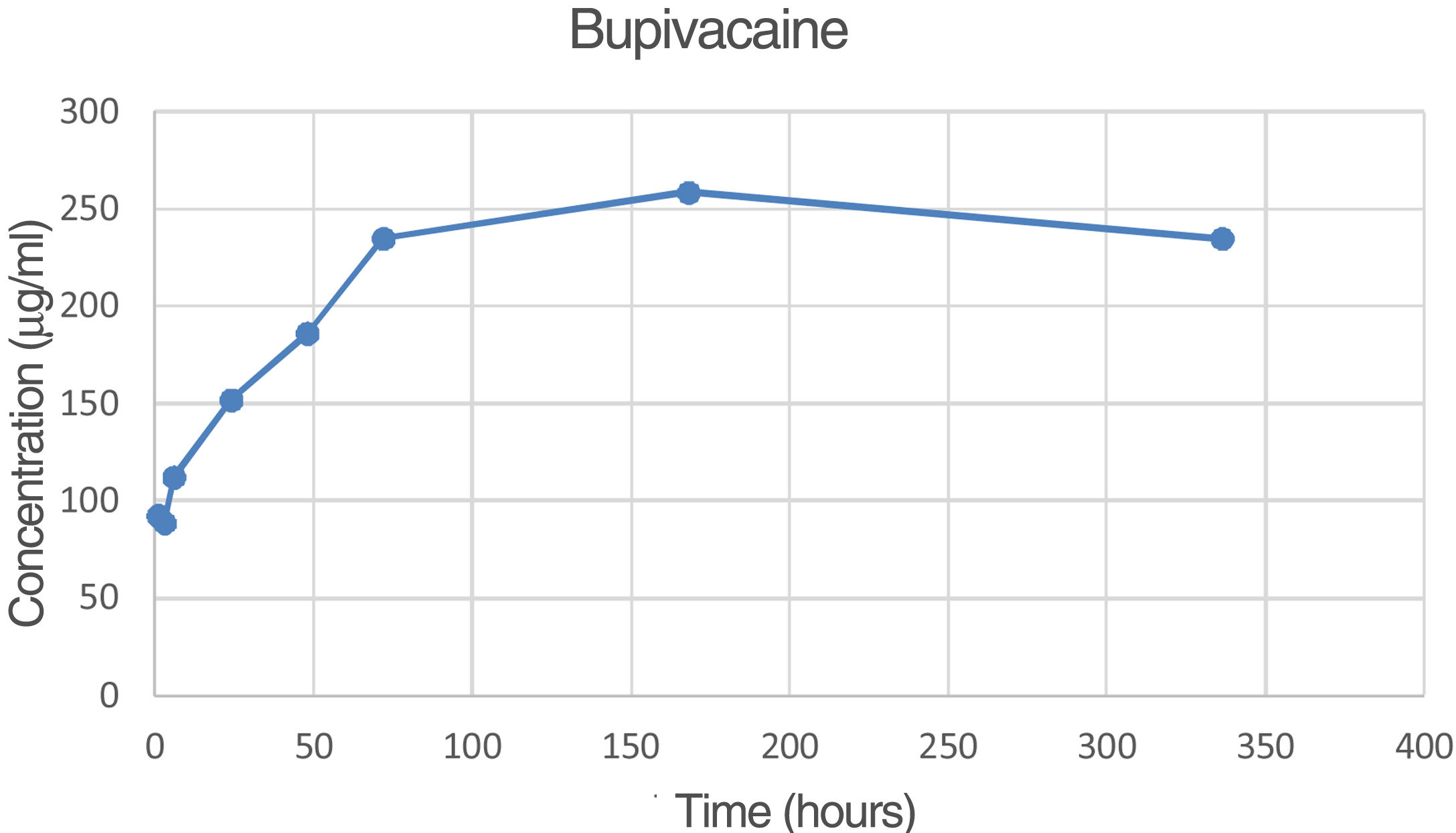
The percentage of bupivacaine eluded from the PMMA bone cement as 2.71% of the total amount of bupivacaine per specimen at 72h and 2.70% at 336h (14 days). Fig. 5 is the graphic representations of the average concentration of bupivacaine eluded at the various time points.
DiscussionTo carry out this study, we have chosen Palacos® R+G bone cement, a high viscosity cement containing gentamicin sulphate, similar to the cements routinely used in our setting. Palacos® (Heraeus) is one of the most widely used brands on the market for fixation during arthroplasty.17
We have selected two LAs to evaluate their elution in bone cement with antibiotics. On the one hand, lidocaine, as an anaesthetic having a short half-life, and on the other hand, bupivacaine, as an anaesthetic that has a longer half-life. Both have good thermal resistance, an essential condition, considering that in vitro PMMA can reach temperature peaks during curing of up to 80–90°C.11,18
Two earlier studies have been performed analysing the elution of different LAs added to PMMA bone cements. In the study by Bond et al.,8 they added lidocaine, prilocaine, bupivacaine, and tetracaine to five PMMA bone cements without antibiotic. A percentage of elution of between 0.05 and 1.10% at 72h was reported for the different LAs. Meanwhile, in the study by Balaguer et al.,14 lidocaine and bupivacaine were added to a medium viscosity PMMA bone cement containing gentamicin. They reported a percentage of lidocaine elution of 25.49% at 72h and 38.48% at 14 days. Simultaneously, they reported a bupivacaine elution rate of 3.18% at 72h and 4.53% at 14 days.
We have selected two LAs to assess their elution in bone cement with antibiotics. On the one hand, lidocaine, as an anaesthetic with a short half-life, and on the other hand, bupivacaine, as an anaesthetic with a longer half-life. Both have good thermal resistance, an essential condition, inasmuch as in vitro PMMA can reach temperature peaks during curing of up to 80–90°C.11,18
Two other studies were previously carried out and probed the elution of different LAs added to PMMA bone cements. In the study conducted by Bond et al.,8 the authors added lidocaine, prilocaine, bupivacaine, and tetracaine to five PMMA bone cements without any antibiotic. They reported elution percentages of the different LAs ranging from 0.05 to 1.10% at 72h. In the study undertaken by Balaguer et al.,14 the investigators added lidocaine and bupivacaine to a medium viscosity PMMA bone cement containing gentamicin. They documented a lidocaine elution rate of 25.49% at 72h and 38.48% at 14 days. Meanwhile, they also reported a bupivacaine elution rate of 3.18% at 72h and 4.53% at 14 days.
Our study yielded lidocaine elution percentages of 9.74% at 72h and 18.73% at14 days (336h). In the case of bupivacaine, the elution percentages were 2.71% at 72h and 2.70% at 14 days (336h).
In both study groups (LG and BG) and at both time points, the rate of elution was slower than those reported by Balaguer et al. These differences in results might be accounted for by the fact that the same bone cement was not used as in our study and by the use of a different dose of LA. Despite having used higher doses of LA in our study, the elution percentage was smaller. This finding might be attributed to the fact that the elution of the drugs from the bone cement takes place from the surface10 and may be limited regardless of the higher drug dose.
Our results and those of Balaguer et al. reveal higher elution rates compared to those described by Bond et al. In the Bond study, bone cement without antibiotic, physiological saline instead of PBS, and different geometrical characteristics of the samples were used.
As for the interpretation of our results and from a theoretical point of view, we must bear in mind two main factors: the maximum total amount of drug eluted and the dose of drug needed to block the A and C fibres that are responsible for pain conduction.
If we were to use all the cement (40.8g), the maximum eluted dose of lidocaine would be reached at 14 days (336h) with an elution percentage of 17.66%, corresponding to 0.438g of LA. For bupivacaine, the maximum dose would be reached at 7 days (168h) with an elution percentage of 2.98%, corresponding to 0.106g bupivacaine.
In the case of lidocaine, the maximum dose would exceed the toxic dose of 3.5mg/kg in a 70kg adult. In contrast, bupivacaine would not exceed the toxic dose of 2mg/kg in a 70kg adult.19 In this regard, we must bear in mind that this interpretation disregards the phenomena of absorption, metabolism, and elimination of LAs that would be present in in vivo studies.
If we interpret the elution results obtained at 72h, we observed that the amount of lidocaine eluted corresponds to 0.22g and that of bupivacaine to 0.09g. These figures are close to the doses typically used in anaesthetic blocks of 0.20g of lidocaine and 0.14g of bupivacaine.
Concerning the dose of LA required to achieve a therapeutic effect, we can refer to classic electrophysiological studies20–24 that determine the amount of LA in vitro that blocks nerve conduction in the A and C fibres responsible for the transmission of painful stimuli. These in vitro studies have determined doses of 0.084–0.8mM25,26 in the case of lidocaine and 0.048–0.200mM24,27 in the case of bupivacaine.
The 72-h elution results obtained in our study in both the LG and BG study groups exceeded the minimum doses established for the two drugs.
LAs can be administered in the context of knee arthroplasty by means of various techniques such as anaesthetic block, LIA (local infiltration anaesthetic), intra-articular puncture, or, experimentally, using bone cement as a drug delivery system. This last-mentioned technique would avoid the adverse effects associated with the techniques listed above and could allow drug elution to be maintained over a longer period of time.
Subsequent to the elution analysis of these drugs, we should analyze how adding them to the bone cement acts on workability and mechanical properties.
Previous studies have reported that the addition of 2g of antibiotic powder by hand to the powdered component of bone cement reduces bending strength by 20% and impact strength by 23%.10 The regulations of the ISO 5833:2022 international standards for Implants for surgery-Acrylic resin cements and the Standard Specification for Acrylic Bone Cement ASTM F451-16 set minimum values for bending strength of 50MPa, compressive strength of 70MPa, and Youngs modulus of 1800MPa.
With respect to the LAs, Giordano et al.28 reported that the addition of Las to bone cement enhances ifs resistance to impact. Lotfi et al.29 informed that bone cements that set in a liquid setting that contains ropivacaine exhibit less compressive strength compared to those that cure in the air.
The number one limitation of this study is that the results cannot be generalized to in vivo conditions, given that it is an in vitro study, in addition to the fact that the sample size is small.
Our future research lines contemplate carrying out workability and mechanical tests to confirm that the addition of these drugs does not alter these properties of PMMA bone cement. At the same time, we are considering the possibility of using other anaesthetics such as levobupivacaine or ropivacaine, with a medium half-life and a very good safety profile.
Level of evidenceLevel of evidence v.
FundingThis study was funded in part by the Spanish Society of Orthopaedic Surgery and Traumatology (SECOT, acronym for its name in Spanish) with the “Ayuda a Proyectos de Investigación en COT” [Grant for Research Projects in Orthopaedic Surgery and Traumatology] of the SECOT Foundation and by the Josep Trueta Grant of the Catalan Society of Orthopaedic Surgery and Traumatology (SCCOT, acronym for its name in Catalan).
Conflict of interestsNone.
The authors have no conflict of interests to declare with regard to the obtention of the Palacos® R+G bone cements used or of the Fagron® brand of local anaesthetics (lidocaine hydrochloride and bupivacaine hydrochloride).