Decision-making in patients with vertebral metastases is highly complex. Different factors of the patient, their cancer disease and treatment options are involved in it. Treatment schemes and strategies have been modified with the evolution of knowledge and treatment of disseminated oncological disease. This paper analyzes the bibliography that has been used for decision-making in the last three decades, as well as the evolution to the schemes that we could consider contemporary.
La toma de decisiones en pacientes con metástasis vertebral es de gran complejidad. En ella intervienen distintos factores del paciente, de su enfermedad oncológica y de las opciones de tratamiento. Los esquemas y las estrategias de tratamiento se han ido modificando con la propia evolución del conocimiento y tratamiento de la enfermedad oncológica diseminada. En este trabajo se analiza la bibliografía que se ha empleado para la toma de decisiones en las 3 últimas décadas, así como la evolución a los esquemas que podríamos considerar contemporáneos.
The clinical contexts in which the specialist in Orthopaedic Surgery and Traumatology (OST) has to make decisions in patients with vertebral metastases (VM) vary, but they can essentially be grouped into two.
The first is spinal cord injury with acute neurological deficit due to VM. In this context there is an urgent need to make a decision and sometimes not all the relevant clinical information is available, such as a complete extension study, and the primary tumour might not be known. In this context, surgery or urgent conventional radiotherapy treatment must be considered. It is not the purpose of this article to go into the details of the decisions to be taken in this context.
The second context, which is broader and more common, includes all other situations in which the decision can be made with more time to gather all the necessary clinical information. It includes, among others, patients with spinal cord compression (SCC) situations without neurological deficit, and patients with pathological vertebral fractures. For some of these patients, VM may be the first manifestation of their oncological disease. The aim of this article is to review the relevant literature on decision-making in this second context over the last 30 years, and the progression to contemporary regimens.
For years, treatment decisions made with the most widely used algorithms were limited to whether or not surgical treatment was required, and whether more or less aggressive surgery was indicated.1,2 These regimens applied a principle of proportionality established by estimating the theoretical survival of the patient with VM. The advances in knowledge and treatment options for disseminated cancer disease have changed the paradigm and management regimens for patients with VM.
Decision-making has been divided into three blocks that follow a chronological sequence and show progression.
Decision-making based on estimation of survivalOne of the most widely used and written about scoring systems for survival, the Tokuhashi score,3 was published in the early 1990s. This scoring system analysed six relevant aspects of the patient with VM: the patient's general condition, the number of bone metastases, the number of VM, metastases to the major internal organs, neurological status, and the primary site of the cancer. Each of these six aspects was scored from 0 to 2, and a treatment strategy was proposed based on the total score obtained (from 0 to 12 points), which was expected to relate to the patient's potential survival. Only patients with higher scores (9–12 points) were candidates for excisional surgery (en bloc resection). Patients with a very poor prognosis (0–5 points) were not candidates for surgery and would be treated with palliative care. Patients with intermediate scores (5–9 points) would be referred for less aggressive surgery.
A few years after its publication, other authors highlighted a clinical context that was not correctly covered by the scoring system.4 Patients starting with VM without a known primary tumour had a worse prognosis than as categorised by the original Tokuhashi scale, which gave this context an intermediate score for the primary tumour site category (1 point, along with kidney, liver, and uterine cancer).
This issue was revised by Tokuhashi's group in the second version of the scoring system, published in 2005.1 The primary tumour category was then expanded from 3 options (0–2 points) to 5 options depending on prognosis (0–5 points), and scoring the context of unknown primary tumour among the worst (1 point) (Table 1). The authors evaluated this revised version themselves retrospectively in 246 patients, partly included in the series of the original version of the scoring system, and prospectively in 118 patients. The scoring system gave a score (0–15 points) to estimate the theoretical survival of the patient, which would help in making the treatment decision (Fig. 1). According to this work, patients with scores of 0–8 points had a prognosis of less than 6 months of life, patients with high scores of 12–15 points had the highest prognosis of more than one year. The group with intermediate scores of 9–11 had a prognosis above 6 months. Work on the revised scoring system reports a predictive success of 85% in the worst prognosis group and a success of 95.5% in the best prognosis group. This survival estimation efficacy of the revised Tokuhashi scoring system was revalidated by the same authors in 2009.5
Revised Tokuhashi scale.
| General condition (Karnofsky score) | |
|---|---|
| Poor (10%–40%) | 0 |
| Moderate (50%–70%) | 1 |
| Good (80%–100%) | 2 |
| No. of non-spinal bone metastases | |
|---|---|
| ≥3 | 0 |
| 1–2 | 1 |
| 0 | 2 |
| No. of vertebral metastases | |
|---|---|
| ≥3 | 0 |
| 1–2 | 1 |
| 0 | 2 |
| Visceral metastases | |
|---|---|
| Unresectable | 0 |
| Resectable | 1 |
| No visceral metastases | 2 |
| Primary tumour | |
|---|---|
| Lung, osteosarcoma, stomach, bladder, oesophagus, pancreas | 0 |
| Liver, gallbladder, unidentified | 1 |
| Other | 2 |
| Kidney, uterus | 3 |
| Rectum | 4 |
| Thyroid, breast, prostate, carcinoid tumour | 5 |
| Neurological lesion | |
|---|---|
| Complete | 0 |
| Incomplete | 1 |
| No deficit | 2 |
Each of the six aspects assessed receives a score, which, when added together, allows the aggressiveness of the treatment to be weighted according to the estimated survival.
Source: Tokuhashi et al.1
Treatment proposal from Tokuhashi's work for the patient with VM.1
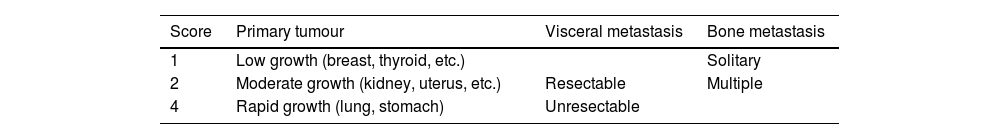
In parallel, a VM management strategy proposed by Tomita et al.2 was published in 2001. The design of this instrument was based on analysis of a retrospective cohort of 67 patients treated by their group between 1987 and 1991 in which they calculated the relative risk (RR) related to the prognosis of some aspects they considered relevant, and then attributed weighted scores to the RR. The second phase of the design was the evaluation in a prospective cohort of 61 patients treated between 1993 and 1996. The Tomita strategy only takes into consideration three aspects (Table 2): the type of primary tumour, the presence of visceral metastases, and the presence of bone metastases. The treatment proposal, proportional to theoretical survival, includes a spectrum of options of greater or lesser aggressiveness and surgical complexity (Table 3).
Tomita's scoring system.
| Score | Primary tumour | Visceral metastasis | Bone metastasis |
|---|---|---|---|
| 1 | Low growth (breast, thyroid, etc.) | Solitary | |
| 2 | Moderate growth (kidney, uterus, etc.) | Resectable | Multiple |
| 4 | Rapid growth (lung, stomach) | Unresectable |
Three aspects are assessed, which receive a score, so that a lower total score implies a better prognosis.
Source: Tomita et al.2
Treatment proposal from Tomita's scoring system.
| Score | Survival | Goal of treatment | Surgical strategy |
|---|---|---|---|
| 2 | 50 months | Long term | Wide marginal resection surgery |
| 3 | Local control | ||
| 4 | 25 months | Medium term | Marginal or intralesional resection surgery |
| 5 | Local control | ||
| 6 | 15 months | Short term | Palliative surgery |
| 7 | Palliative | ||
| 8 | <6 months | Terminal | Medical and analgesic support |
| 9 | Palliative | Palliative care | |
| 10 |
Potential scores, survivals achieved, and treatment proposal are shown.
Source: Tomita et al.2
In 2005, in the same issue of Spine in which the revised Tokuhashi scoring system1 was published, Boriani's group commented on the use of these survival-based scores.6 These systems did not consider two fundamental issues of the VM patient, i.e., the presence of comorbidities that may determine outcome and responsiveness to other non-surgical treatments, referring to rapidly evolving oncological treatments.
Over the years, several studies have been published comparing the usefulness or efficacy of these prognostic scores, comparing versions, different scores, and even comparing seven different scoring systems.7 The German group led by Benjamin Ulmar was one of the first to positively assess the efficacy of the Tokuhashi and Tomita scales, applying them retrospectively to a series of patients treated at their centre between 1984 and 2005.8–10 The Danish paper published by Wang et al. also positively assessed the usefulness of the revised Tokuhashi scoring system, applied to 448 surgical patients between 1992 and 2009.11 However, this study, which assessed the scoring system, reported an overall efficacy of 64.7%, significantly lower than that reported by Tokuhashi, and less than 60% in the group with the worst prognosis.1,5
Pointillart et al. from the Bordeaux group also found in their population of 142 surgical patients that the revised Tokuhashi score was not as effective in estimating survival as reported by its authors.12 This group explained in their article that their decision-making on VM involves different aspects, such as the patient's symptomatology, the mechanical instability of the VM, and the presence or absence of SCC, relegating the prognostic score to a less relevant role.
We also evaluated the predictive efficacy of the revised Tokuhashi scoring system in our setting, obtaining data of lower prognostic capacity than that originally reported by the Tokuhashi group and with worse outcomes in the intermediate and worse prognosis categories.13
A review of the literature on the prognostic efficacy of the revised Tokuhashi scoring system was published in 2016.14 This work grouped and analysed the main series published on this issue, finally including 10 papers of which 5 have been previously cited.5,9,11–13 The predictive data from the original paper by Tokuhashi5 are the most effective (88% overall), followed by the paper by Ulmar et al.9 (71%), and the remaining papers have a lower prognostic ability. Statistical analysis of the 10 series, grouping 1686 patients, shows overall predictive success of 63%, with higher predictive ability of the best prognosis group (77.21%) and lower predictive ability of the intermediate (55.32%) and worst prognosis groups (64.10%). In addition, they highlight the heterogeneity of the series, with a predominance of fully surgical series, which may imply a selection bias in patient characteristics. They conclude a clear loss of prognostic accuracy of this instrument.
The most important reason for this loss of efficacy of prognostic scoring systems is the advance in the treatment of disseminated cancer disease, which has improved survival for some tumours with VM. Lung cancer has the worst possible score on the revised Tokuhashi scoring system (a score of 0 on an item scored from 0 to 5). Christian Hessler's German group found that patients with VM secondary to lung cancer who were following current oncological treatment options had better survival than expected by the revised Tokuhashi score.15 These authors warned of the risk of underestimating survival and making therapeutic decisions based on this.
This concern about underestimating survival was considered by other groups, who questioned the validity of prognostic scoring systems designed with series of patients treated before 2005, who had not had the opportunity to be treated with current options such as immunotherapy.16
Furthermore, greater knowledge of the behaviour and variants of the different primary tumours has helped us understand that prognosis is variable within each category, depending on their molecular biology and markers, as in the case of breast cancer.17
Despite the controversy regarding the variability of predictive efficacy, there are authors who continue to defend the use of these prognostic tools as part of decision-making.18
Decision-making based on relevant features of vertebral metastases and treatment alternativesIn addition to survival, two other aspects have been gaining in importance, both cited by Pointillart et al., such as the presence of mechanical instability and the degree of SCC.12 Instability is defined as the loss of the ability to bear physiological loads without deformation and may be related to pain experienced by the patient with VM or neurological compression. It can also be the cause of a pathological fracture, with minimal loads or movements. The Spinal Instability Neoplastic Score (SINS) is the most commonly used instrument to assess instability. This score has proven very useful and assesses six important aspects of VM, some of which are evaluated in radiological tests such as computed tomography (Table 4).19,20 A score of less than 6 establishes that the VM is stable, 13 or more establishes severe instability, and 7–12 establishes potential instability.
SINS of mechanical instability in VM.
| Location of the lesion in the spine | |
|---|---|
| Junctional (occipital–C2, C7–T2, T11–L1, L5–S1) | 3 |
| Mobile spine (C3–C6, L2–L4) | 2 |
| Semi-rigid spine (T3–10) | 1 |
| Rigid spine (S2–S5) | 0 |
| Pain with movement/loading of the spine | |
|---|---|
| Yes | 3 |
| No (occasional not mechanical) | 1 |
| No pain | 0 |
| Type of lesion | |
|---|---|
| Lytic | 2 |
| Mixed (lytic/blastic) | 1 |
| Blastic | 0 |
| Radiographic spinal alignment | |
|---|---|
| Subluxation/translation present | 4 |
| De novo deformity (kyphosis/scoliosis) | 2 |
| Normal alignment | 0 |
| Vertebral body collapse | |
|---|---|
| >50% collapse | 3 |
| <50% collapse | 2 |
| No collapse with >50% body involved | 1 |
| None of the above | 0 |
| Posterolateral involvement of the spinal elements (facet, pedicle, or costovertebral joint fracture) | |
|---|---|
| Bilateral | 3 |
| Unilateral | 1 |
| No involvement | 0 |
A score 0–5 is deemed stable, 13 or more unstable, and 6.12 intermediate.
Source: Fisher et al.19
The degree of SCC is another relevant aspect in decision-making on the management of VM.12 The Bilsky scale describes the degree of ESCC and has proven very useful (Fig. 2).21,22
Bilsky's epidural spinal cord compression scale.21 Neurological compression can be described as mild (grade 0 and 1) or severe (grade 2 and 3). Grade 0 is an intraosseous VM, contained within the vertebral body. In grade 1 there is no deformation of the spinal cord, further divided into three categories: in grade 1a there is contact of the VM with the dura mater, in grade 1b there is contact associated with deformation of the contour of the dura mater and in grade 1c there is deformation and collapse of the subarachnoid space, with no cerebrospinal fluid (CSF) visible at the site of compression. In grade 2 there is compression and deformation of the spinal cord, but CSF is still visible in some non-compressed areas. In grade 3 the cord is compressed, displaced, and CSF is no longer visible in the subarachnoid space.
The advent of new treatment alternatives has broadened the range of options for managing VM. Conventional radiotherapy (RT) was conditioned or limited by the radiation administered to structures adjacent to the vertebra, such as the spinal cord, and by the existence of some tumours resistant to the effect of radiation (classically renal cancer, thyroid cancer, hepatocellular carcinoma, colon cancer, non-small cell lung cancer, sarcomas, and melanomas). However, the advent of extracranial stereotactic body radiation therapy (SBRT) has been a paradigm shift.23 A potential advantage of SBRT is that it allows irradiation with higher doses than RT, up to ablative doses and with sub-millimetre precision, thus minimising irradiation of tissues surrounding the VM. It allows better local control than RT, and is more effective in tumours that are classically resistant to RT. The epidural space can be a cause of local recurrence if it is invaded by tumours, and therefore if there is epidural invasion (such as Bilsky 2–3 type ESCC), SBRT must be preceded by a surgical procedure aimed at cleaning the epidural space sufficiently. This surgery is known as separation surgery,24 which consists of creating a ventral cavity, not compromising the thecal sac, with sufficient decompression between the VM and the thecal sac, with a circumferential free space of about 2–3mm. It is accompanied by stabilisation, sometimes percutaneous. Separation surgery combined with new radiotherapy techniques has achieved good local control data and low recurrence rates, with improvement in patients’ quality of life and without having to subject them to overly aggressive surgery.
These advances in knowledge and the greater availability of therapeutic options, not surgical management alone, have opened up decision-making to variables other than just theoretical life expectancy after diagnosis of VM. Various multidisciplinary algorithms have been published for decision-making in VM.25,26 One of the most widely used currently is the NOMS (Neurologic, Oncologic, Mechanical, and Systemic) framework (Fig. 3). The first aspect assessed in this framework is neurological (N), and a distinction is made between low-grade (Bilsky 0–1c) and high-grade (Bilsky 2–3) SCC. The next step is to assess the oncological aspect (O), in which the radiosensitivity of the lesion to conventional radiotherapy is evaluated. VM of classically radiosensitive tumours will benefit from initiation of treatment with conventional RT. VMs of radioresistant tumours with compression (Bilsky 0–1c) will benefit from SBRT. VMs of radioresistant tumours with significant compression (Bilsky 2–3) will benefit from separation surgery along with SBRT following surgery. The third section or step, mechanics (M), assesses the presence of instability (SINS>12), which is an indication for surgical treatment, whether or not neurological compression is present. Intermediate contexts of instability (SINS 7–12) must be taken into consideration individually, depending on the rest of the algorithm. In the last section, systemic (S), the patient's general condition and ability to overcome the previously mentioned treatments are weighted, with the help of a non-surgical specialist (medical oncology or internal medicine).
NOMS algorithm for decision-making in the patient with VM.27
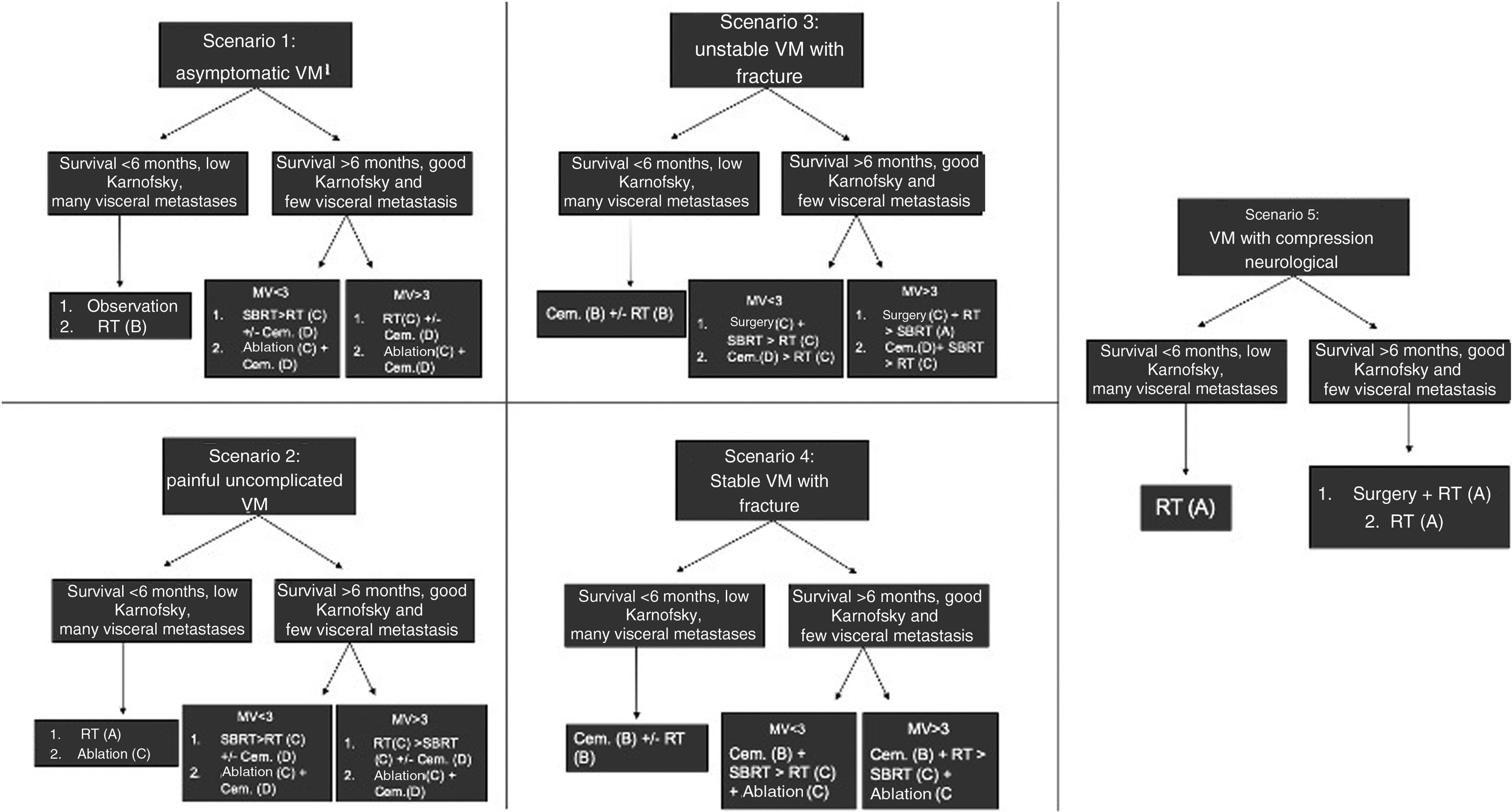
Other algorithms have included more therapeutic options than those presented in NOMS, such as percutaneous ablation and cementation, and have considered other clinical scenarios. The decision-making strategy described by The Metastatic Spine Disease Multidisciplinary Working Group considers five common clinical scenarios of VM, depending on the occurrence or not of pathological fractures, and offers different current management options and the grades of recommendation of the scientific evidence (Fig. 4).28
Clinical scenarios and treatment options proposed by the international Metastatic Spine Disease Multidisciplinary Working Group, with the grade of recommendation of the scientific evidence in brackets.8
For years, tumour committees have been commonplace in hospitals. These committees were created to provide a global vision and multidisciplinary perspective of a complex pathology, and to offer patients collegiate responses, agreed by consensus between different specialties.
The participation of the different specialties allows the treatment of patients with VM to be individualised and optimised.29–31 Despite the existence of new protocols or decision-making algorithms, decision-making tends to take place in committee and with a clearly multidisciplinary perspective.
This practice is highly dependent on the characteristics of the centre, its resources, and the culture of joint decision-making. Its main drawback is the difficulty of using the committee in emergency contexts, but it can be very useful in other clinical contexts. The experience in centres in our setting is encouraging and shows us that a multidisciplinary approach, in teams and jointly, is probably the most enriching and appropriate for a highly complex pathology, in which decisions must often be tailored to each individual patient.31
DiscussionDecision-making resources in VM have changed, as has the approach to cancer treatment, towards individualisation and personalisation.
The first instruments were based exclusively on the patient's potential life expectancy, without considering aspects that we now consider important.1,2,7 Analysis of those original studies shows heterogeneous, retrospective series and treatments that today we might consider obsolete, their context was different to the current one. There is no doubt that the concept of proportionality is fundamental to optimise the management of the patient with VM, but this proportionality must be constructed with more aspects than survival. It should not be forgotten that VM is a disseminated oncological disease, and it is the disease control options that will fundamentally determine patient survival, usually through medical treatments with chemotherapy and/or immunotherapy.
Furthermore, the literature shows that prognostic efficacy has been declining with variable and even poor outcomes in some series.12–14 There are many reasons for these disparate outcomes, the most likely being the better treatment of patients in the later series and the heterogeneity between surgical and conservative management series. In light of the outcomes of these series, and as Boriani noted, the major drawback is the lack of consideration of alternative treatment options.5 In recent years, new alternatives have appeared, enriching the arsenal but making it difficult to provide a single prescription for VM. Separation surgery combined with SBRT has become a powerful tool, which allows good local control but also, from a purely surgical perspective, has the advantage of being reproducible surgery, which can be performed in a multitude of hospitals.24 Not all surgeons and hospitals can perform vertebrectomies or large reconstructions, but performing separation surgery does appear to be a more universally achievable objective.
The advent of minimally invasive procedures such as ablation is of particular interest in patients with oligometastatic disease and can be combined with percutaneous cementation, procedures that can be performed on an outpatient basis.8 It is worth reflecting on how many patients in the original series of the scoring systems might have benefited from these options today, instead of relatively aggressive surgery, with its connotations of associated morbidity. There is no doubt that these options should be included in our decision tree.
The NOMS protocols and the protocol described by The Metastatic Spine Disease Multidisciplinary Working Group enrich decision-making with aspects that have become increasingly relevant.27,28 The passage of time and their evaluation will tell us how effective they are in improving local control and quality of life in patients with VM. Including these protocols in expert committees to assist in consensual and collegial decision-making seems to the natural way forward. However, it takes time to consider all these aspects, to collect the information and to process it in committee. They are therefore mainly geared towards the non-urgent patient.
Quite different is the situation of the urgent patient with a VM and a spinal cord injury with acute neurological deficit. In this context the variable “time” is important, almost as important as the variable “available resources”, of which several can be listed, such as the availability of urgent diagnostic tests such as MRI, availability of a competent surgical team, the possibility of transferring to another centre if needed, or specialists to consult. This context is handled differently depending on the centres in our setting, the health network to which they belong, or the existence of on-call spine surgery teams. It is also important to remember that no protocol includes the different scenarios that may arise in relation to resources, which is why this review of the literature has focused on the most homogeneous context, which may be the patient with non-urgent VM.
In this non-urgent setting, where there is a certain amount of time to gather all the necessary clinical information, decision-making cannot be based solely on classical survival scores, as they are no longer relevant for the management of VM in today's context of medical and radiation oncology.
Level of evidenceLevel of evidence IV.
Conflict of interestsThe authors have no conflict of interests to declare.