Several epidemiological studies have suggested that the prevalence of the onset of primary Sjögren's Syndrome in the elderly (EOpSS) is significantly higher (between five to eight times) than in other age groups. However, when a literature review was performed, the number of patients with EOpSS was much lower than epidemiologically expected. An evaluation was performed on Sjögren (sicca) syndrome, including immunological markers, labial salivary glands biopsy, and some extra-glandular manifestations. These could be confounding factors in the elderly patients, much more so than in other age groups, and lead to a misdiagnosis of EOpSS. This article presents a review of the most common difficulties that may be present in the recognition of EOpSS, and highlights the lack of elderly patient-centred studies as the most important unmet need.
Varios estudios epidemiológicos han sugerido que la prevalencia del síndrome de Sjögren primario (SSp) en la población de edad avanzada (Elderly-Onset primary Sjogren's Syndrome [EOpSS], según la clasificación inglés) es considerablemente mayor (entre 5 y 8 veces) que en grupos de edad diferente. Sin embargo, una revisión sistemática de la literatura mostró que el número de pacientes con EOpSS era mucho menor de lo que se esperaba epidemiológicamente. La evaluación del síndrome de sicca, los marcadores inmunológicos, la biopsia de las glándulas salivales labiales y algunas manifestaciones extraglandulares podrían convertirse en factores de confusión en pacientes de edad avanzada mucho más frecuentemente que en personas de otros grupos de edad, lo que favorecería un diagnóstico erróneo del EOpSS. En este artículo se revisan las principales dificultades que pueden afectar al reconocimiento del EOpSS, destacando la falta de estudios centrados en el paciente anciano como la necesidad insatisfecha más importante.
Elderly-onset primary Sjögren's syndrome (EOpSS) has an estimated overall prevalence of approximately 3%, but epidemiological data are very heterogeneous and prevalence depends on variables such as geographic areas, simple sizes, and diagnostic criteria.1,2 The age used to define the population is itself a variable: more than 60 years, more than 65, more than 70, according to different researchers. First data were presented by Drosos et al. in 19883: in 62 healthy volunteers with a mean age of 81 years (range: 67–95 years), EOpSS was confirmed in 4.83% of the study group. In another study conducted in 2008, Haugen et al. showed that in an elderly group (aged 71–74 years) EOpSS was confirmed in 3.39% according to the 1993 European Community Study Group (ECGS) classification criteria, and in 1.40% according to the revised 1996 European classification criteria. In this study, the prevalence of pSS in the younger group (aged 40–44 years) was lower, totaling 0.44% and 0.22%, respectively.4 In general, epidemiological studies highlighted that the prevalence of pSS in the elderly population is higher (between five to eight times) than in other age groups.5–7
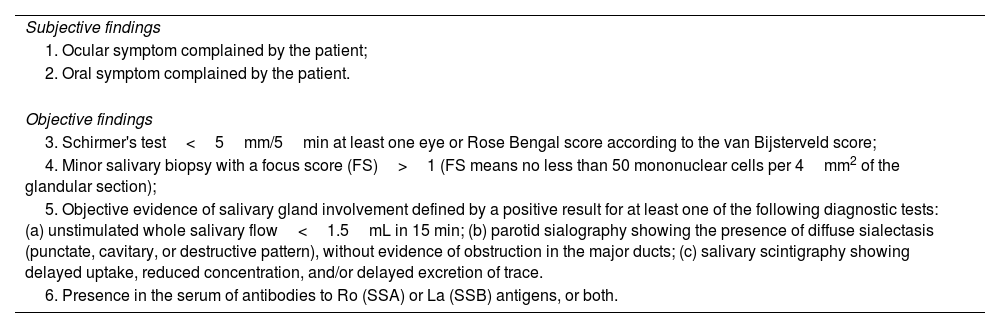
Classification criteriaAll principal cohorts in literature are based on the classification criteria proposed by ECGS in 1993 and by American European Consensus Group (AECG) in 2002. As known, these criteria were designed for entry into clinical trials. The target population consisted of persons with signs and symptoms suggestive of SS. In particular, the AECG criteria considered six items, two of which were subjective (ocular and oral symptom complaints by the patients) and four based on objective findings (Table 1).
AECG criteria.
| Subjective findings |
| 1. Ocular symptom complained by the patient; |
| 2. Oral symptom complained by the patient. |
| Objective findings |
| 3. Schirmer's test<5mm/5min at least one eye or Rose Bengal score according to the van Bijsterveld score; |
| 4. Minor salivary biopsy with a focus score (FS)>1 (FS means no less than 50 mononuclear cells per 4mm2 of the glandular section); |
| 5. Objective evidence of salivary gland involvement defined by a positive result for at least one of the following diagnostic tests: (a) unstimulated whole salivary flow<1.5mL in 15 min; (b) parotid sialography showing the presence of diffuse sialectasis (punctate, cavitary, or destructive pattern), without evidence of obstruction in the major ducts; (c) salivary scintigraphy showing delayed uptake, reduced concentration, and/or delayed excretion of trace. |
| 6. Presence in the serum of antibodies to Ro (SSA) or La (SSB) antigens, or both. |
AECG: American European Consensus Group; FS: Focus Score.
In patients without any potentially associated disease, pSS may be defined as the presence of four of the aforementioned six items (histopathology and autoantibodies are mandatory) or presence of three of the four objective criteria.8
At the end of 2016, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) presented jointly established pSS diagnostic criteria. Unlike the AECG criteria, these criteria were based on objective tests, taking into account symptoms as inclusion criteria. Of all the laboratory tests, diagnostic importance was attributed only to SSA/Ro autoantibodies (3 points). Positive serology for anti-SSB/La without positive serology for anti-SSA/Ro, and positivity for antinuclear antibodies (ANA) and rheumatoid factor were no longer considered. Histopathological examination of labial salivary gland (LSG) biopsies with the assessment of FS remained an important element (FS>1 gives 3 points).9 The diagnosis is made if a patient presents with a sum of ≥4 points, but a cut-off of 5 points instead of 4 raises the specificity of the criteria from 89% to 98%.10
Both in AECG criteria and in ACR/EULAR criteria, established exclusion criteria must be evaluated: head and neck radiation treatment, active hepatitis C virus infection (confirmed using PCR), acquired immune deficiency syndrome, sarcoidosis, amyloidosis, graft versus host disease, and immunoglobulin G4-related disease. Additionally, in the evaluation of dry eye symptoms, patients using eye drops for glaucoma daily and those who have had corneal surgery or cosmetic eyelid surgery in the last 5 years are scored 0 points.8,9
What happens when these criteria are applied to the elderly population? In the real world, the application of these proposed criteria to the elderly comes up against a whole series of criticalities.
- 1.
Sicca syndrome: Both in the criteria proposed by the AECG and those by the ACR/EULAR collaborative group, reported symptoms of ocular and/or buccal dryness are the first step. These symptoms are common among the elderly, and their prevalence may reach up to 30% in persons over the age of 65 years.11 However, when these symptoms are evaluated through objective tests, a weak confirmation is found.12 Diagnostic tests have several confusing elements in the elderly. For example, older age is associated with a reduction of tear and/or saliva production,13,14 and this reduction may affect the results of Schirmer test or the assessment of unstimulated whole saliva flow rate. In two population-based survey of health elderly people, the prevalence of an abnormal Schirmer test ranged from 12 to 58%.13,15 So, Schirmer's test may be less useful than ocular staining score as a confirmation test for keratoconjunctivitis sicca in EOpSS patients.16
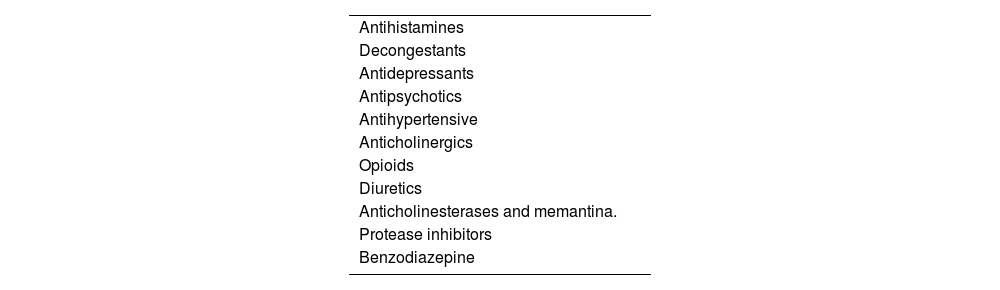
Furthermore, multimorbidity and polypharmacy (including over-the-count drugs) are very common in elderly patients.17–20 Some drugs reduce the secretion of tears or saliva.21,22 These drugs include anticholinergics, antidepressants (tricyclic or selective serotonin reuptake inhibitors), antihypertensives (terazosin, prazosin, clonidine, and atenolol), antihistamines, antireflux drugs, diuretics, benzodiazepines (Table 2). Among these, anticholinergic drugs are the most important confounding factors, so that patients who are normally taking these drugs should be evaluated for objective signs of salivary hypofunction and eye dryness after a sufficient time of their withdrawal.
- 2.
LSG biopsy: As well-known, a focal lymphocytic sialadenitis (FLS) with >50 mononuclear cells in a peri-ductal or peri-vascular localization is considered the most specific histopathological finding. The term FLS refers to the histopathological pattern of the presence of one or more foci in the biopsies, while the tissue surrounding the foci is composed mainly of unaffected parenchyma. Given the heterogeneous distribution of the inflammatory infiltrate and glandular damage, analysis of four to seven glands from each patient is suggested in order to obtain a reasonably sized sample, and analysis of a glandular surface area of at least 8mm2 is recommended.23,24
In all ages of life, the evaluation of LSG biopsies is far from easy and straightforward. Vivino et al. reported that a second expert evaluation of 58 LSG re-analysed by a single centre led to revision of the initial diagnosis in 53% of the patients.25 More recently, Costa et al. reported a multicenter cohort study in which minor salivary gland biopsies were analyzed with a standard blinded assessment by two different pathologists at 2-month interval. The analysis included the measurement of FLS and detection of germinative centres (GC)-like structures. The inter-observer variability comparison revealed poor agreement for the detection and calculation of focus score and detection of FLS, lack of concordance for the presence of duct dilation and (less for) fibrosis. In more than 12% of the cases, the second evaluation by trained pathologists led to a diagnosis change.26 Moreover, some life habits may be confusing factors. For example, cigarette smoking is negatively associated with FS>1 in lower lip biopsy in patients with pSS, and may also negatively influence the presence of anti-SSA/Ro antibodies in circulating blood.27 A protocol published in 201128 by the Sjögren's International Clinical Collaborative Alliance (SICCA) highlighted that these foci must occur adjacent to normal appearing acini.
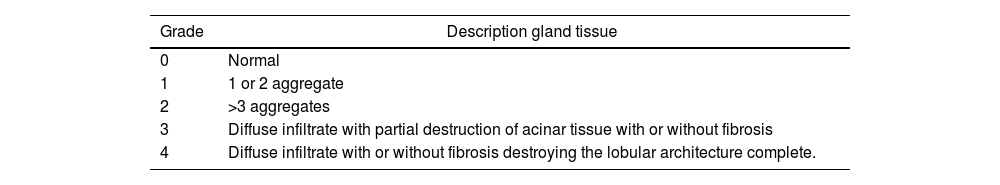
According to literature review, LSG biopsy is performed less frequently in EOpSS patients than in adults, and in some studies it has been not reported.6,29 In older persons, the presence of age-related biopsy findings may realize confounding features. For example, some investigators found that acinar atrophy and fibrosis is possible in healthy individuals aged over 65 years, FS was higher in older age groups, and the increased area of fat tissue in the LSG biopsies is not specific associated with pSS but is a selective feature of ageing.14,30–33 On the other hand, Kihuchi et al. highlighted that, while there were significant differences in frequency of acinar atrophy between the sublingual and submandibular salivary glands of subjects younger and older than 75, the labial glands showed no such variation.34 The use of a grading score taking the destruction of acinar tissue and fibrosis into account (such as the one proposed by Tarpley et al. and listed in Table 3) may be of greater usefulness in the elderly patient when pSS is suspected.
Tarpley's grading system for LSG biopsies.35
| Grade | Description gland tissue |
|---|---|
| 0 | Normal |
| 1 | 1 or 2 aggregate |
| 2 | >3 aggregates |
| 3 | Diffuse infiltrate with partial destruction of acinar tissue with or without fibrosis |
| 4 | Diffuse infiltrate with or without fibrosis destroying the lobular architecture complete. |
LSG: Labial Salivary Gland.
Compared to healthy controls, the LSG biopsies of pSS patients show more acinar atrophy and fibrotic changes.36,37 More recently, the Sjögren's histopathology workshop performed by the EULAR Sjogren's Syndrome Experimental and Translational Investigative Alliance (ESSENTIAL) study group provided a consensus guidance for the use of LSG histopathology in clinical trials. The diagnostic importance of foci that are adjacent to normal parenchyma was emphasized and several recommendations were proposed. However, the level of these recommendations is low.38
As these data highlight, there is a strong need to achieve a consensus among experts on how to differentiate pSS lesions from the age-related degenerative and atrophic lesions of salivary glands. Other histopathological markers besides FS have been proposed, but whether and how these markers may contribute to classification and diagnosis of EOpSS remains unclear. In the same way, salivary gland ultrasonography to the diagnosis of pSS showed very promising results, but data regarding EOpss are little more than anecdotal.39,40
- 3.
Immunological markers:
The antibodies most frequently present in pSS are antibodies against the small ribonucleoproteins SSA/Ro and SSB/La. In 1999, some invetigators highlighted that patients diagnosed before 45 years of age have higher anti-SSA and SSB autoantibody concentrations (62.5%) than patients with EOpSS (20.8%).41 More recently, a lower frequency of anti-SSA and anti-SSB was confirmed in patients with an elderly diagnosis (>70 years) from the Big Data International Sjögren Cohort.42,43
The absence of the auto-antibodies included in classification criteria in biopsy-proven patients characterizes the seronegative subset of pSS by definition. Seronegative pSS is not uncommon. According to data from Sjögren Big Data Project, approximately 20% of pSS patients are seronegative.44 In comparison with seropositive pSS, seronegative patients have an older age at diagnosis, a higher frequency of fatigue and pain, a lower frequency of systemic manifestations, a lower risk of lymphoma. No significant differences in results of signs and symptoms of glandular involvement are present.44–47 In particular, the possibility that one-third of patients with chronic fatigue syndrome having sicca symptoms fulfil criteria for SS,45 and that the frequency of fatigue and chronic pain could favour the diagnosis of so-called “functional somatic syndromes”43 in older patients with seronegative pSS, deserve to be highlighted for the implications they may have in clinical practice.
- 4.
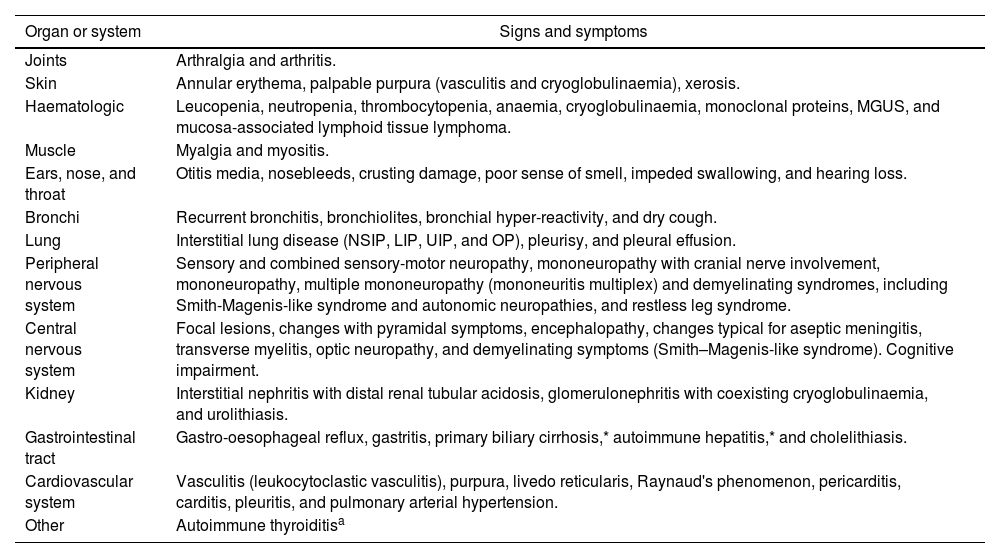
Extraglandular manifestations: Beyond sicca syndrome, pSS is a complex systemic disease. The most common extraglandular manifestations are listed in Table 4.
Table 4.The most common extraglandular manifestations of pSS.
Organ or system Signs and symptoms Joints Arthralgia and arthritis. Skin Annular erythema, palpable purpura (vasculitis and cryoglobulinaemia), xerosis. Haematologic Leucopenia, neutropenia, thrombocytopenia, anaemia, cryoglobulinaemia, monoclonal proteins, MGUS, and mucosa-associated lymphoid tissue lymphoma. Muscle Myalgia and myositis. Ears, nose, and throat Otitis media, nosebleeds, crusting damage, poor sense of smell, impeded swallowing, and hearing loss. Bronchi Recurrent bronchitis, bronchiolites, bronchial hyper-reactivity, and dry cough. Lung Interstitial lung disease (NSIP, LIP, UIP, and OP), pleurisy, and pleural effusion. Peripheral nervous system Sensory and combined sensory-motor neuropathy, mononeuropathy with cranial nerve involvement, mononeuropathy, multiple mononeuropathy (mononeuritis multiplex) and demyelinating syndromes, including Smith-Magenis-like syndrome and autonomic neuropathies, and restless leg syndrome. Central nervous system Focal lesions, changes with pyramidal symptoms, encephalopathy, changes typical for aseptic meningitis, transverse myelitis, optic neuropathy, and demyelinating symptoms (Smith–Magenis-like syndrome). Cognitive impairment. Kidney Interstitial nephritis with distal renal tubular acidosis, glomerulonephritis with coexisting cryoglobulinaemia, and urolithiasis. Gastrointestinal tract Gastro-oesophageal reflux, gastritis, primary biliary cirrhosis,* autoimmune hepatitis,* and cholelithiasis. Cardiovascular system Vasculitis (leukocytoclastic vasculitis), purpura, livedo reticularis, Raynaud's phenomenon, pericarditis, carditis, pleuritis, and pulmonary arterial hypertension. Other Autoimmune thyroiditisa MGUS: monoclonal gammopathy of undetermined significance; NSIP: nonspecific interstitial pneumonia; LIP: lymphocytic interstitial pneumonia; UIP: usual interstitial pneumonia; .OP: organizing pneumonia.
As known, both AECG and ACR/EULAR criteria emphasized the importance of the impairment of the salivary and lachrymal functions, and did not adequately considered the extraglandular manifestations of pSS. In the clinical practice, some patients may have systemic manifestations unrelated to sicca syndrome.48 This seems particularly frequent in patients with EOpSS. In the largest reported cohort, these patients showed a higher risk for presenting activity at articular, pulmonary, muscular and peripheral nervous system domains.43 When LSG biopsy is performed, an association between severe systemic manifestations and high FS is usually found, while association between systemic manifestations and immunological markers is more weak.49–52
Literature reviewA comprehensive literature search was conducted in Medline and PubMed bibliographic databases in order to identify all the studies on cohorts of patients with EopSS. This systematic review was made on 31 October 2019 according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The following main search terms were used: primary Sjogren syndrome AND/OR elderly onset Sjogren syndrome. References from all of the selected studies were also examined, while reviews, abstracts, and studies on secondary Sjogren's syndrome were excluded.
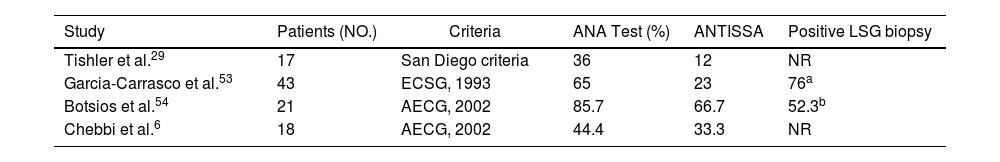
Four studies were identified, reporting on cohorts of patients with EopSS, totaling 99 patients.6,29,53,54 In these studies, disease onset was determined based on the occurrence of symptoms strongly suggestive of pSS; in three of the studies, elderly onset was set at age 65 years, but in the García-Carrasco et al. study, it was set at 70 years. Each study used the diagnostic criteria commonly being used at the time of publication. The most significant data of these four studies are listed in Table 5.
Data in the four literature studies on EOpSS patients.
| Study | Patients (NO.) | Criteria | ANA Test (%) | ANTISSA | Positive LSG biopsy |
|---|---|---|---|---|---|
| Tishler et al.29 | 17 | San Diego criteria | 36 | 12 | NR |
| Garcia-Carrasco et al.53 | 43 | ECSG, 1993 | 65 | 23 | 76a |
| Botsios et al.54 | 21 | AECG, 2002 | 85.7 | 66.7 | 52.3b |
| Chebbi et al.6 | 18 | AECG, 2002 | 44.4 | 33.3 | NR |
| Xerostomia (%) | Xeropthalmia (%) | Articular (%) | Neurological (%) | Pulmonary (%) | Haematological (%) |
|---|---|---|---|---|---|
| 100 | 94 | 94 | 16 | NR | NR |
| 98 | 91 | 23 | 12 | 16 | NR |
| 71.4 | 76.1 | 66.7 | 4.7 | 4.7 | 14.2 |
| 100 | 100 | 88.8 | 44.4 | 11.1 | 5.5 |
NR: non reported; ECSG: European Community Study Group.
A high percentage of patients with ANA positivity presented in the Botsios et al. cohort; this was only partially confirmed in the other three studies. With the exception of Chebbi et al. data, the presence of anti-SSA and anti-SSB was similar in all the studies. Differences between studies were found in clinical characteristics of patients, with neurological and pulmonary involvement more often observed in the Chebbi et al. cohort and Raynaud's phenomenon more frequently observed in the Botsios et al. LSG biopsies were performed in two cohorts. Lastly, when the authors compared older-group data with younger-group data, differences were only highlighted in the study by Chebbi et al. In this study, pulmonary involvement was more frequent in the older group (although not statistically significant), whereas the difference in levels of ANA, anti-SSA, and anti-SSB was statistically higher in the younger group. In the other three studies, clinical and laboratory results of EOpSS patients were quite similar to those in younger patients. Demographic factors and differences in genetic predisposition have a potential role in explaining these differences.
In the last five years, the Sjögren Big Data Project created a multicentre registry that today includes nearly 12,000 patients from the 5 continents.55 Until today, according to our best knowledge, this consortium has not yet published final figures regarding EOpSS patients.42,43
Discussion and conclusionsAs this review article highlighted, diagnosis of EOpSS has several unmet needs.
Firstly, although some epidemiological studies have suggested that the prevalence of pSS in the elderly population is significantly higher (between five to eight times) than in other age groups, it is possible that these suggestions are misleading. Indeed, cardinal sicca symptoms, which are the hallmark of the disease, may be attributed to ageing and/or medications.56 On the other hand, it is well-known that some age-related immunological, test and histopathological findings may represent confounding factors in the clinical practice. For example, seronegative pSS seems more frequent in elderly patients than in other age groups. In these patients, the lack of LSG biopsy can favour completely different diagnoses, functional somatic syndromes among these. On the other hand, the same LSG biopsy can give false-positive results in healthy persons or not always easily decipherable findings in elderly patients with EOpSS.
Secondly, specificity and sensitivity of AECG and ACR/EULAR classification criteria should be validated also in patients with EOpSS, even taking into account that some extra-glandular manifestations when associated with specific serological or histopathological findings may be expression of EOpSS, although sicca syndrome is absent.
Finally, our review of the literature highlighted that studies focused on patients with EOpSS are scarce. In an ageing world, these studies should be much more numerous than those available. In the absence of more extensive case studies, many of the doubts and criticalities regarding the correct diagnostic process in patients with suspicion of EOpSS remain entirely entrusted to the experience of the clinician.
Founding sourceNone.
Conflict of interestThe author declares no conflict of interest.