new oral anticoagulants (apixaban, dabigatran and rivaroxaban) are the newest advance for stroke's risk reduction in atrial fibrillation. These are as effective as warfarin in preventing stroke/systemic embolism, but exists heterogenic outcomes as gastrointestinal hemorrhage, mortality reduction, minor and major haemorrhage (adverse events). Despite of this, there is a lack of cost-effectiveness models focused on adverse events.
Methodsa cost-effectiveness analysis with a third payer perspective, interventions included were apixaban, dabigatran, warfarin and rivaroxaban. Discount rate of 3%, and 10 years of temporal horizon. The Markov model is an international, validated, and modified to assess better adverse events. Major assumptions, patients with mild and moderate stroke returns to oral anticoagulation, patients with moderate and severe hemorrhage do not returns to oral anticoagulation. Probabilities and QALYs, taken from a cost-effectiveness analysis published. Costs, information from a cohort of stroke patients. Software, TreeAge pro™ and Excel™.
Resultsoverall results, 1.48 QALYs, $17 916 USD for apixaban, 1.49 QALYs, $18 122 USD for dabigatran, 1.32 QALYs, $21 966 USD for warfarin and 1.24 QALYs, $24 547 USD for rivaroxaban. The ICER for apixaban compared to dabigatran was $12 988 USD. Negative ICER for warfarin and rivaroxaban, shows that are dominated alternatives (less benefits and more costs). Apixaban is cost-effective at 70% and dabigatran at 30% of iterations in the probabilistic sensitivity analysis.
Conclusionsapixaban and dabigatran are cost-effective alternatives, apixaban is the most cost-effective alternative from adverse events perspective. Warfarin shows better results than rivaroxaban to prevent stroke in atrial fibrillation from adverse events perspective.
los nuevos anticoagulantes orales (apixabán, dabigatrán y rivaroxabán) son el avance más reciente para la reducción del riesgo de accidente cerebrovascular en la fibrilación auricular. Estos son tan efectivos como la warfarina en la prevención del accidente cerebrovascular/embolia sistémica, pero existen resultados heterogéneos como hemorragia gastrointestinal, reducción de la mortalidad y hemorragia menor y mayor (eventos adversos). Pese a ello, se carece de modelos de costo-efectividad enfocados en eventos adversos.
Materiales y métodosse hizo un análisis de costo-efectividad con una perspectiva de tercer pagador, en el que se incluyeron intervenciones como apixabán, dabigatrán, warfarina y rivaroxabán. La tasa de descuento fue del 3% y 10 años de horizonte temporal. El modelo de Markov es internacional, validado y modificado para evaluar mejor eventos adversos. Las principales suposiciones, los pacientes con accidente cerebrovascular leve y moderado vuelven a la anticoagulación oral, los pacientes con hemorragia moderada y grave no regresan a la anticoagulación oral. Probabilidades y AVAC, tomados de un análisis de costo-efectividad publicado. Los costos, información de una cohorte de pacientes con accidente cerebrovascular. Software, TreeAge pro y Excel.
Resultadosresultados generales, 1.48 QALYs, $ 17 916 USD para apixabán, 1.49 QALYs, $ 18 122USD para dabigatrán, 1.32 QALYs, $ 21 966 USD para warfarina y 1.24 QALYs, $ 24 547 USD para rivaroxabán. El ICER para apixabán en comparación con dabigatrán fue de $ 12 988 USD. El ICER negativo para warfarina y rivaroxabán muestra que son alternativas dominadas (menos beneficios y más costos). Apixabán es rentable en 70% y dabigatrán en 30% de las iteraciones en el análisis de sensibilidad probabilístico.
Conclusiónapixabán y dabigatrán son costo-efectivos; apixabán es la alternativa más costo-efectiva desde la perspectiva de los eventos adversos. Warfarina mostró mejores resultados que rivaroxabán para prevenir accidentes cerebrovasculares en fibrilación auricular desde la perspectiva de los eventos adversos.
New oral anticoagulants (apixaban, dabigatran and rivaroxaban) are the latest advance in atrial fibrillation (AF). Some of them are effective as warfarin, reducing stroke/systemic embolism (RR 0.78 95% CI 0.67-0.92) and intracranial hemorrhage (ICH) (RR 0.49 95% CI 0.36-0.66)1. But exists heterogeneous outcomes as in gastrointestinal hemorrhage (GIH)2, mortality reduction, minor and major hemorrhage3–5. Moreover, exist differences in stroke and ICH rates between them and increase of GIH compared with warfarin. These heterogeneous outcomes (especially in adverse events), higher drug cost yield us to propose —an improvement on calculation of adverse events in economic evaluations of new oral anticoagulants.
Economic evaluations as cost-effectiveness analysis (CEA) compares the relative cost and outcomes of two or more courses of action, in health care is widely used for evaluation of efficiency in drugs and others health care technologies to offer the maximum benefit of available funds6; a study by Harvard university found that a double life years could be saving if resources were allocated by cost-effective interventions7. Several CEA for new oral anticoagulants have been published, a systematic review of the literature reported incremental cost-effective ratios (ICER) of 20 426 USD±19 653 USD for dabigatran 150, $13 834 USD±3 885 USD for apixaban and $20 884 USD±13 959 USD for rivaroxaban8. Two CEA in oral anticoagulation in Colombia showed ICER's of $ 7 846 USD (COP/USD=2 954, 24 august 2018) for dabigatran 1509, and $44 524 USD for apixaban, $28 796 USD for dabigatran and $26 340 USD for rivaroxaban10.
Most of published CEA differs on the Markov model's structure, but are similar in lacking of more specific adverse events (AE) calculations; a systematic review found that the current modelling assumptions may restrict the understanding of the true impact of AE in CEA of antineoplastic drugs11, however, for oral anticoagulation this impact is unknown, despite the hemorrhage events are almost two times more than ischemic stroke in pivotal clinical trials3–5. The assessment of hemorrhage localization are important due to cost of treatment, for example, the treatment cost of mild upper GIH, severe lower GIH; mild intraparenchymal ICH and severe subdural ICH differs. We propose a CEA in oral anticoagulation in atrial fibrillation in Colombia with the improve on hemorrhage events assessment by localization of intracranial hemorrhage and gastrointestinal hemorrhage, using an international validated Markov model and costs data from a cardiovascular hospital in Colombia, with the aim of identify the most efficient alternative in oral anticoagulation.
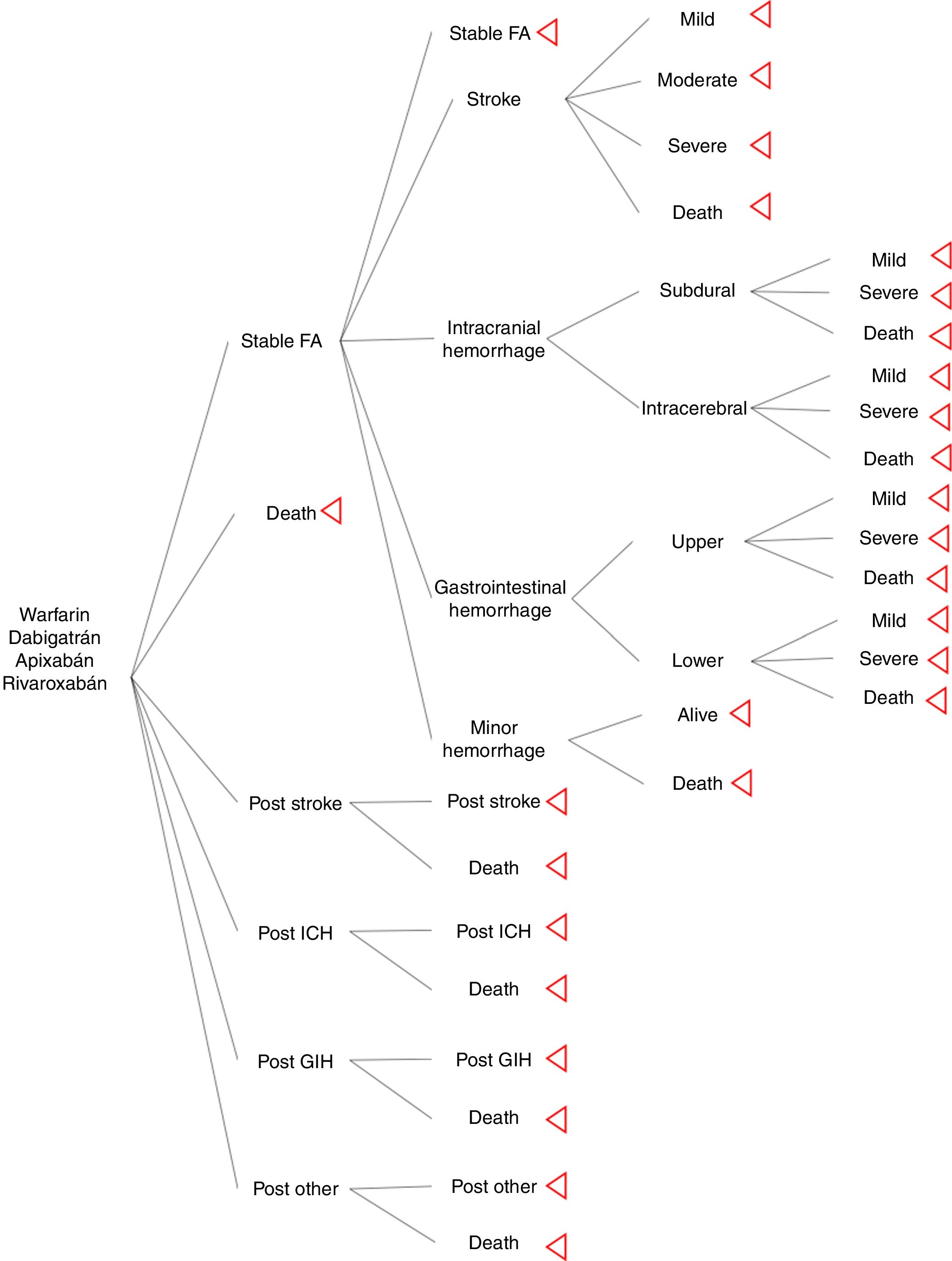
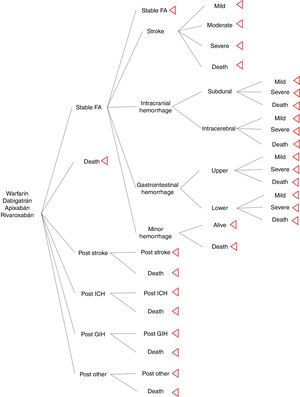
MethodsSeveral Markov models in anticoagulation are described in the literature, for the study we used the Harrington's model as a base from which we modified ICH and GIH to classify events by localization. Four treatment strategies and their associated outcomes were assessed: warfarin (INR 2-3), dabigatran (150mg twice daily), apixaban (5mg twice daily), and rivaroxaban (20mg once daily). The hypothetical cohort of patients with AF, and same characteristics as included in pivotal clinical trials. For this model rate of adherence and drug compliance were assumed the same across of the treatments. The Markov model was designed to move between health states of 1 month cycles for 10 years or until death. Cost-effective therapies were selected using a willingness-to-pay (WTP) threshold of $9 000 USD per quality-adjusted life-year (QALY) gained (approximately 3 times per capita gross domestic product) and 3% discount rate. Model's calculations and sensitivity analysis were made using TreeAge pro®.
Model structureThe model inputs of events were taken from literature. The aim was to simulate a cohort of patients with AF that has the similar adherence rate between drugs. It was used major probabilities as risk of stroke, ICH, GIH and other hemorrhage; other bleeding included retroperitoneal hemorrhage, epistaxis, and urinary tract hemorrhage12. Events risks calculations of new oral anticoagulants (NOAC) were based on information of pivotal clinical trials (ARISTOTLE, RE-LY, and ROCKET-AF); warfarin probabilities were calculated from pooled clinical trials results. Distribution of stroke rate severity was the same for NOAC and warfarin; it was extracted from Leigh's et al study13. Distribution of intracerebral, subdural (intracranial), upper, and lower (gastrointestinal) hemorrhage was taken from economic model of Leigh et al.
For the assessment of a more realistic model in oral anticoagulation, it was used information from a cohort of stroke in a cardiovascular hospital, which was useful to know the physician's believe of hemorrhage and its attitude to restart oral anticoagulation after a major hemorrhage; from this, our model excluded patients with moderate or severe GIH, all the ICH, and moderate stroke and severe stroke, this last one is not consider an adverse event for oral anticoagulation but there are a risk of hemorrhagic transformation of stroke.
UtilitiesThere is a lack of calculation for QALY's utilities in Colombia. Exists literature descriptions of the baseline utility of a patient with AF in oral anticoagulation treatment, disutilities for stroke (mild, moderate, and severe), intracranial haemorrhage (mild and severe), gastrointestinal haemorrhage (mild, moderate, and severe), other hemorrhage was used from US information.
CostsEvents costs were estimated from information of a cohort of patients with stroke in the Fundación Cardiovascular de Colombia. From resource individual information recollected from November 2015 to January 2018 of patients with stroke, we calculated event's costs considered:
- 1.
Time hospitalization (general hospitalization, intensive care unit)
- 2.
Medical supplies
- 3.
Drug costs
- 4.
Laboratory test
- 5.
Cost of medical consultation
- 6.
Cost of procedures (craniotomy, endoscopy, and colonoscopy).
- 7.
Medical fees.
Drugs’ costs were taken from the SISMED database, which is a ministry's of health database14. Drug's costs are reported in monthly Colombian pesos, for the use in the model the cost were convert in dollars using the representative rate for May of 2015. Cost of anticoagulation of warfarin and new oral anticoagulants were calculated from expert opinion of monthly controls of international normalized ratio (INR), and follow by specialist or general practitioner.
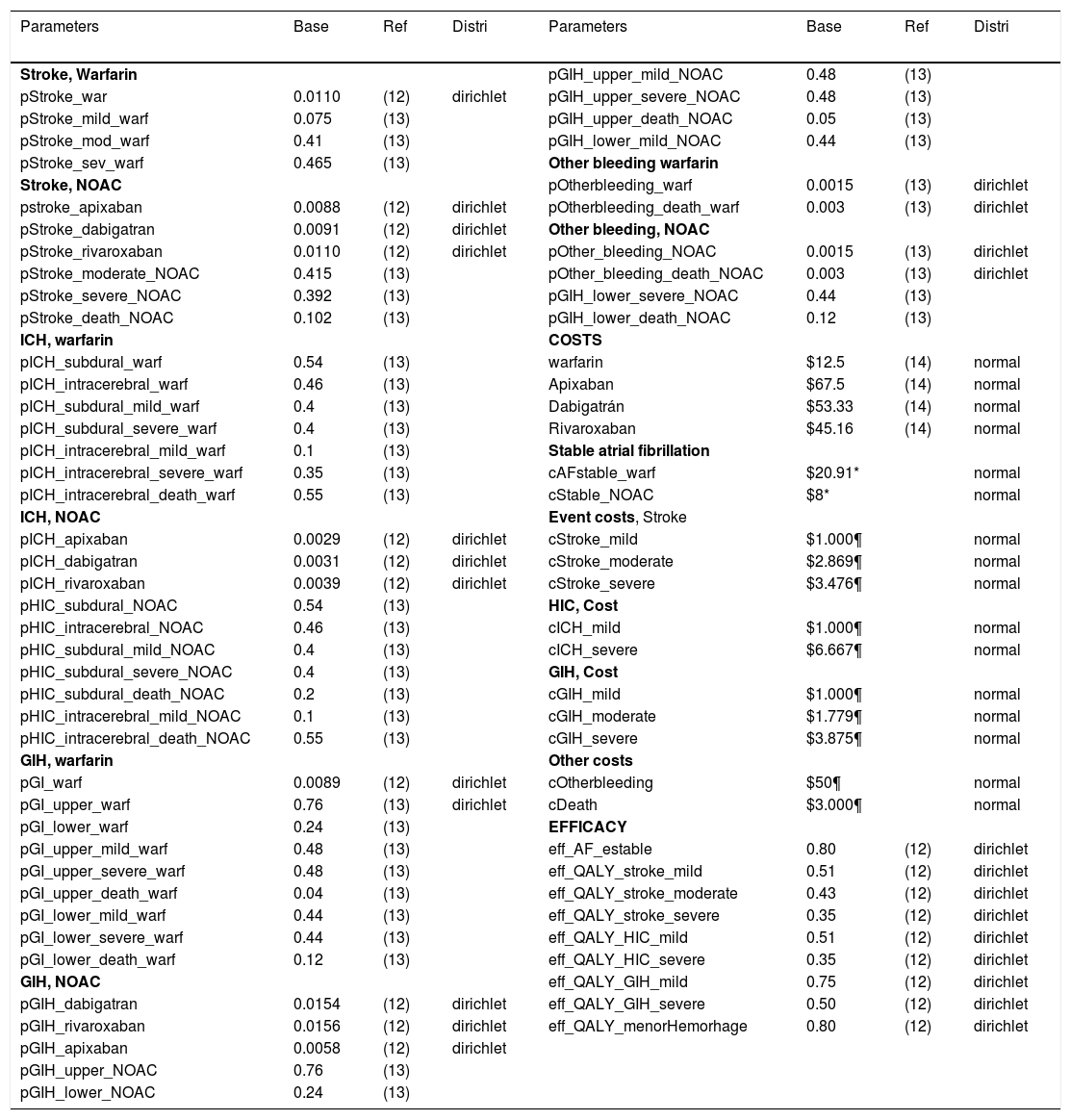
Sensitivity analysesA several sensitivity analyses were conducted. As a first step a univariate analysis was useful for identify the most influence model's parameters and their impact on the final decision. Finally, a probabilistic sensitivity analysis was done, we used dirichlet distributions for probabilities and utilities, whereas normal distribution was used for cost (Table 1).
Model parameters.
| Parameters | Base | Ref | Distri | Parameters | Base | Ref | Distri |
|---|---|---|---|---|---|---|---|
| Stroke, Warfarin | pGIH_upper_mild_NOAC | 0.48 | (13) | ||||
| pStroke_war | 0.0110 | (12) | dirichlet | pGIH_upper_severe_NOAC | 0.48 | (13) | |
| pStroke_mild_warf | 0.075 | (13) | pGIH_upper_death_NOAC | 0.05 | (13) | ||
| pStroke_mod_warf | 0.41 | (13) | pGIH_lower_mild_NOAC | 0.44 | (13) | ||
| pStroke_sev_warf | 0.465 | (13) | Other bleeding warfarin | ||||
| Stroke, NOAC | pOtherbleeding_warf | 0.0015 | (13) | dirichlet | |||
| pstroke_apixaban | 0.0088 | (12) | dirichlet | pOtherbleeding_death_warf | 0.003 | (13) | dirichlet |
| pStroke_dabigatran | 0.0091 | (12) | dirichlet | Other bleeding, NOAC | |||
| pStroke_rivaroxaban | 0.0110 | (12) | dirichlet | pOther_bleeding_NOAC | 0.0015 | (13) | dirichlet |
| pStroke_moderate_NOAC | 0.415 | (13) | pOther_bleeding_death_NOAC | 0.003 | (13) | dirichlet | |
| pStroke_severe_NOAC | 0.392 | (13) | pGIH_lower_severe_NOAC | 0.44 | (13) | ||
| pStroke_death_NOAC | 0.102 | (13) | pGIH_lower_death_NOAC | 0.12 | (13) | ||
| ICH, warfarin | COSTS | ||||||
| pICH_subdural_warf | 0.54 | (13) | warfarin | $12.5 | (14) | normal | |
| pICH_intracerebral_warf | 0.46 | (13) | Apixaban | $67.5 | (14) | normal | |
| pICH_subdural_mild_warf | 0.4 | (13) | Dabigatrán | $53.33 | (14) | normal | |
| pICH_subdural_severe_warf | 0.4 | (13) | Rivaroxaban | $45.16 | (14) | normal | |
| pICH_intracerebral_mild_warf | 0.1 | (13) | Stable atrial fibrillation | ||||
| pICH_intracerebral_severe_warf | 0.35 | (13) | cAFstable_warf | $20.91* | normal | ||
| pICH_intracerebral_death_warf | 0.55 | (13) | cStable_NOAC | $8* | normal | ||
| ICH, NOAC | Event costs, Stroke | ||||||
| pICH_apixaban | 0.0029 | (12) | dirichlet | cStroke_mild | $1.000¶ | normal | |
| pICH_dabigatran | 0.0031 | (12) | dirichlet | cStroke_moderate | $2.869¶ | normal | |
| pICH_rivaroxaban | 0.0039 | (12) | dirichlet | cStroke_severe | $3.476¶ | normal | |
| pHIC_subdural_NOAC | 0.54 | (13) | HIC, Cost | ||||
| pHIC_intracerebral_NOAC | 0.46 | (13) | cICH_mild | $1.000¶ | normal | ||
| pHIC_subdural_mild_NOAC | 0.4 | (13) | cICH_severe | $6.667¶ | normal | ||
| pHIC_subdural_severe_NOAC | 0.4 | (13) | GIH, Cost | ||||
| pHIC_subdural_death_NOAC | 0.2 | (13) | cGIH_mild | $1.000¶ | normal | ||
| pHIC_intracerebral_mild_NOAC | 0.1 | (13) | cGIH_moderate | $1.779¶ | normal | ||
| pHIC_intracerebral_death_NOAC | 0.55 | (13) | cGIH_severe | $3.875¶ | normal | ||
| GIH, warfarin | Other costs | ||||||
| pGI_warf | 0.0089 | (12) | dirichlet | cOtherbleeding | $50¶ | normal | |
| pGI_upper_warf | 0.76 | (13) | dirichlet | cDeath | $3.000¶ | normal | |
| pGI_lower_warf | 0.24 | (13) | EFFICACY | ||||
| pGI_upper_mild_warf | 0.48 | (13) | eff_AF_estable | 0.80 | (12) | dirichlet | |
| pGI_upper_severe_warf | 0.48 | (13) | eff_QALY_stroke_mild | 0.51 | (12) | dirichlet | |
| pGI_upper_death_warf | 0.04 | (13) | eff_QALY_stroke_moderate | 0.43 | (12) | dirichlet | |
| pGI_lower_mild_warf | 0.44 | (13) | eff_QALY_stroke_severe | 0.35 | (12) | dirichlet | |
| pGI_lower_severe_warf | 0.44 | (13) | eff_QALY_HIC_mild | 0.51 | (12) | dirichlet | |
| pGI_lower_death_warf | 0.12 | (13) | eff_QALY_HIC_severe | 0.35 | (12) | dirichlet | |
| GIH, NOAC | eff_QALY_GIH_mild | 0.75 | (12) | dirichlet | |||
| pGIH_dabigatran | 0.0154 | (12) | dirichlet | eff_QALY_GIH_severe | 0.50 | (12) | dirichlet |
| pGIH_rivaroxaban | 0.0156 | (12) | dirichlet | eff_QALY_menorHemorhage | 0.80 | (12) | dirichlet |
| pGIH_apixaban | 0.0058 | (12) | dirichlet | ||||
| pGIH_upper_NOAC | 0.76 | (13) | |||||
| pGIH_lower_NOAC | 0.24 | (13) |
Distri: distribution; Ref: references; NOAC: New oral anticoagulants; *: expert opinion; ¶: costs from stroke cohort study.
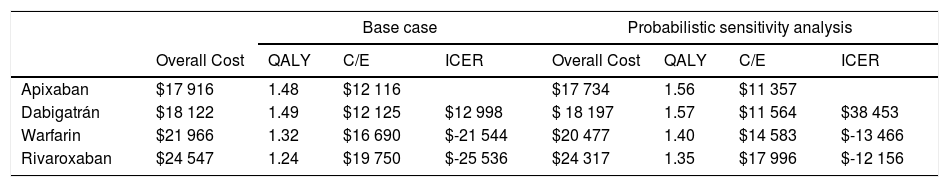
The results were: a quality adjusted life expectancy of 1.48 years and total healthcare cost of $17 916 USD for apixaban, 1.49 QALY and cost of $18 122 USD for dabigatran, 1.32 QALY and cost of $21 966 USD for warfarin, and 1.24 QALY total cost of $24 547 USD for rivaroxaban (Table 2). The incremental cost and QALY for dabigatran compared to apixaban were $206 and 0.01, the incremental cost and QALY for warfarin compared to dabigatran were $3 844 USD and -0.17, and incremental cost and QALY of rivaroxaban compared with warfarin were $2 581 USD and -0.08. In the ranking (the less costly from the most costly) warfarin and Rivaroxaban were dominated (more expensive and less QALYs). The ICER of apixaban compared to dabigatran (undominated alternatives) was $12 998 USD, and negatives ICER's for warfarin and rivaroxaban (dominated alternatives).
Study's results.
| Base case | Probabilistic sensitivity analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall Cost | QALY | C/E | ICER | Overall Cost | QALY | C/E | ICER | |
| Apixaban | $17 916 | 1.48 | $12 116 | $17 734 | 1.56 | $11 357 | ||
| Dabigatrán | $18 122 | 1.49 | $12 125 | $12 998 | $ 18 197 | 1.57 | $11 564 | $38 453 |
| Warfarin | $21 966 | 1.32 | $16 690 | $-21 544 | $20 477 | 1.40 | $14 583 | $-13 466 |
| Rivaroxaban | $24 547 | 1.24 | $19 750 | $-25 536 | $24 317 | 1.35 | $17 996 | $-12 156 |
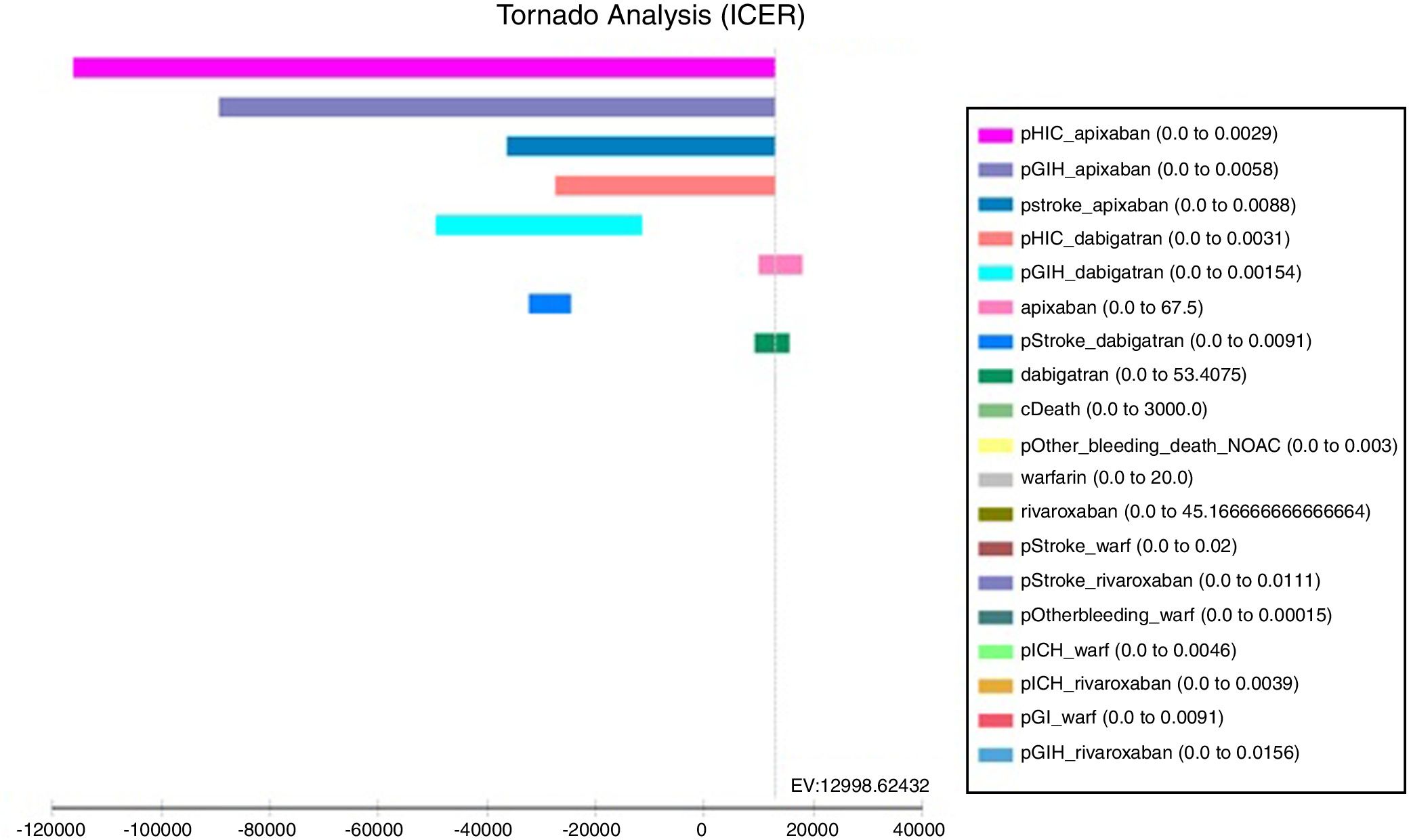
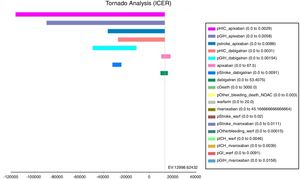
The tornado analysis (fig. 2) shows the most sensible variables of this model; probability of HIC and GIH (pHIC and pGIH) for apixaban and dabigatran were the most influential variables (fig. 1, appendix), stroke's probability for apixaban and dabigatran (pStroke) (fig. 2 appendix) also influenced in model. Apixaban and dabigatran's costs were the criteria that influenced the ICER in positive and negative way. Rest of variables were less influential.
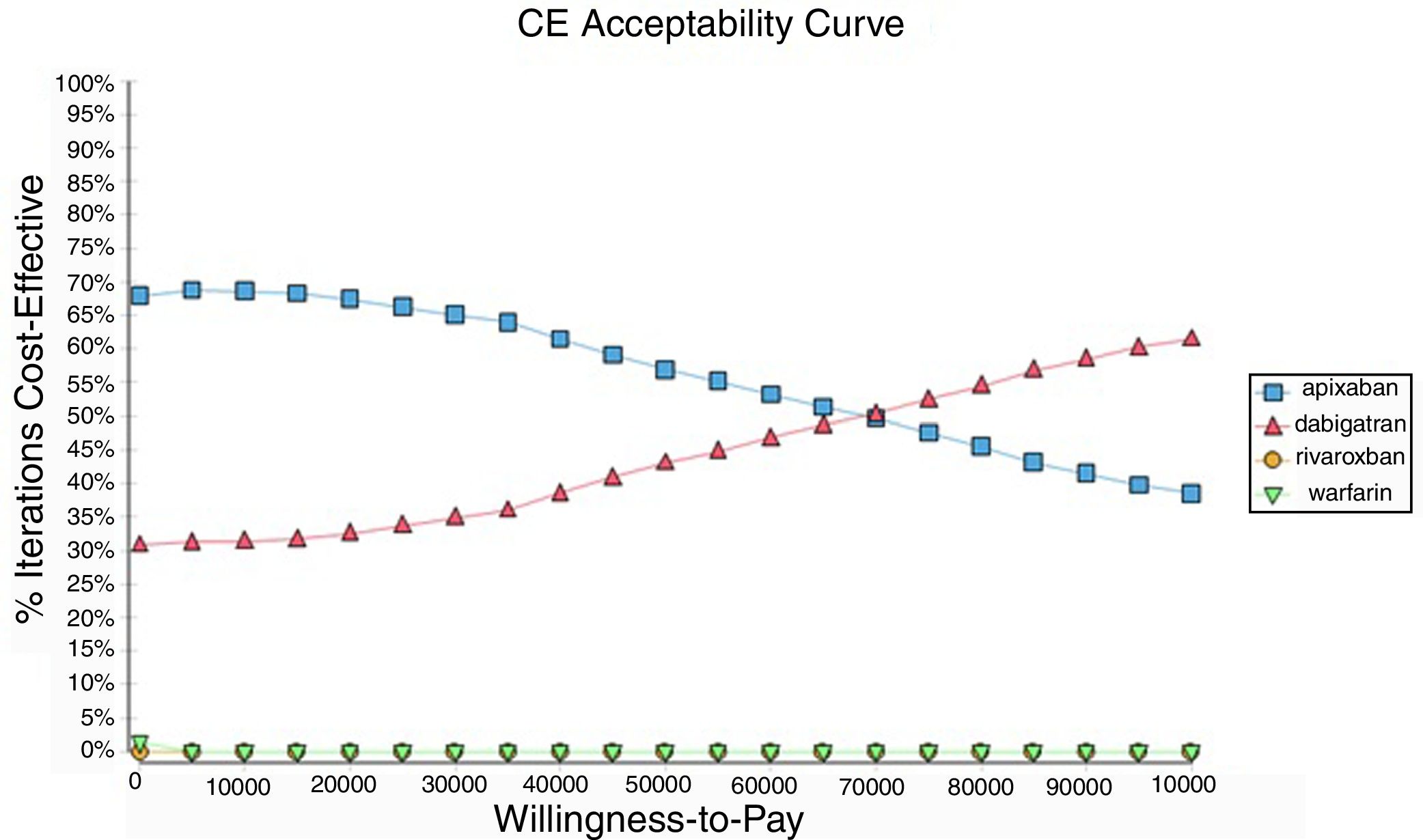
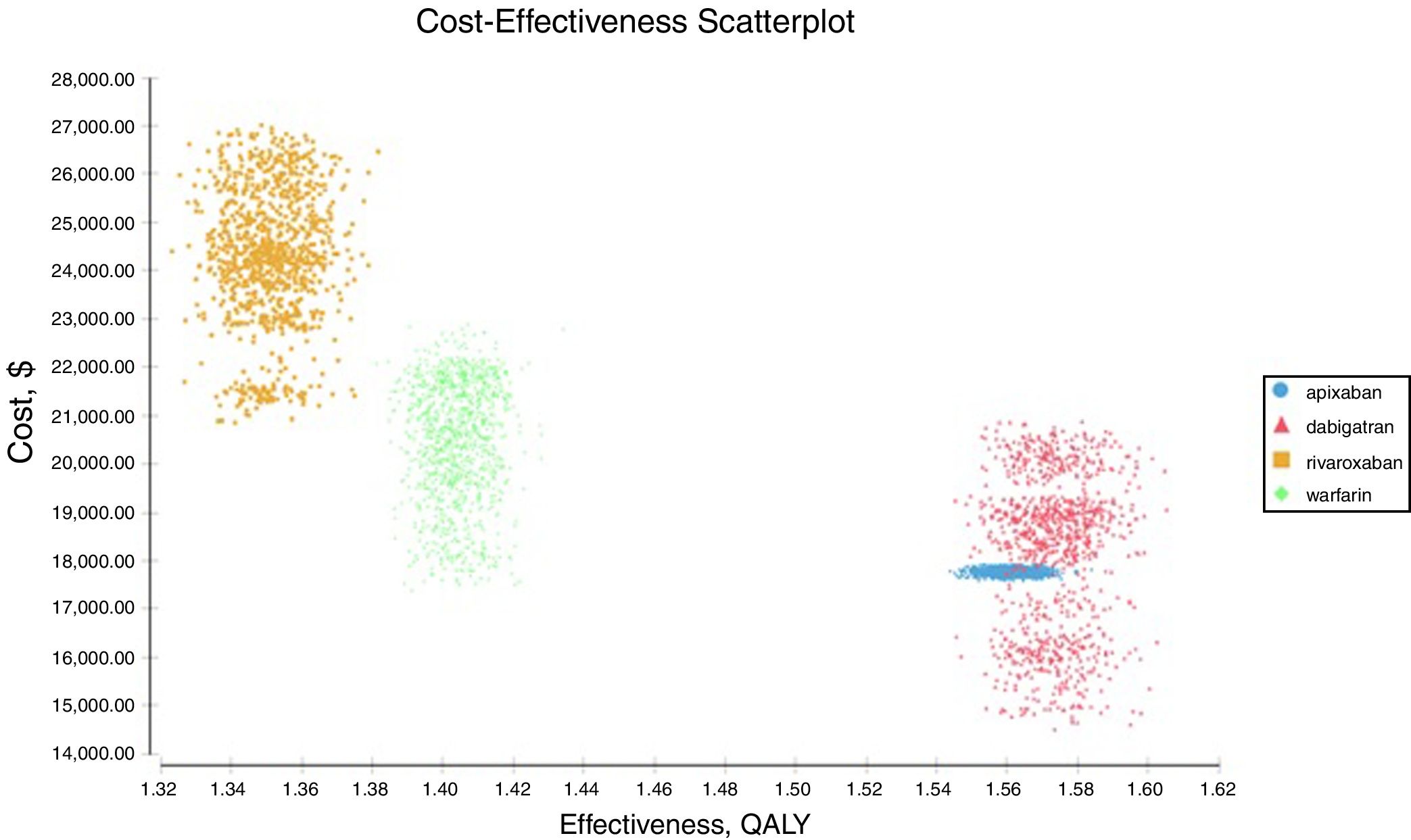
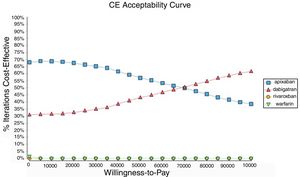
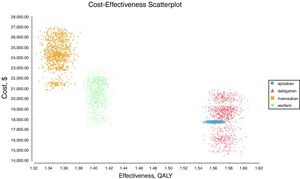
Probabilistic sensitivity analysisA series of analysis were made (Table 2), mean cost and QALYs were calculated based on probabilistic functions for the model parameters. Results are presented in Table 2, the analysis was analysed under the WTP threshold of $9 000 USD. The median cost for apixaban was $17 734 USD with gained QALY of 1.56, Dabigatran median cost was $18 197 USD with gained QALY of 1.57, warfarin median cost was $20 477 USD with gained QALY 1.40, and rivaroxaban median cost of $24 317 USD gained QALY of 1.35. The ICER's for undominated alternatives was $38.453. At willingness to pay $9 000 USD, apixaban is cost-effective at 70% of iterations, dabigatran at 30% of iterations, warfarin and rivaroxaban were not (fig. 3). The cost-effectiveness scatterplot shows the point distribution of each option, apixaban's point distribution is close between them, and it confirms the result's robustness (fig. 4).
DiscussionWe found that apixaban is the most cost-effective alternative for stroke prevention in atrial fibrillation from adverse event perspective, following by dabigatran 150, warfarin and finally rivaroxaban. This results from a less cost for apixaban and a minor difference of efficacy compared with dabigatran, and negative ICER's for warfarin and rivaroxaban indicates costlier and less efficacy alternatives. Apixaban accounted less overall cost due to smaller number of adverse events than dabigatran, warfarin and rivaroxaban, despite of greater drug's cost. The tornado type sensitivity analysis confirms that the most important model's parameters were those related to adverse events rates, this confirms the necessity of improve hemorrhage outcomes in anticoagulation models.
From this analysis warfarin was most cost-effective than rivaroxaban, driven by less overall cost (events, drug and controls costs) and best QALY's performance, the explanation is due to less gastrointestinal hemorrhage events in warfarin despite of more ICH; event rates has influence on both (cost and QALY). Clinical trials had shown an unexpected increase in gastrointestinal hemorrhage, especially among patients aged 75 years or over, a meta-analysis of 71.674 patients highlighted a 25% increase in gastrointestinal hemorrhage compared with warfarin15. This caused that warfarin had a best ICER than rivaroxaban (warfarin is dominant for rivaroxaban).
The ICERs can be used to set health priorities in three ways, (i) to minimize the expenditure to achieve a health effect target, (ii) to maximize the health benefits within budget and (iii) to consider cost-effective with an explicit threshold16. In Colombia, there is a lack of an explicit ICER to set efficiency treatments, due to the policy is focused on treatment access rather than principle of resources’ maximization, but despite of it, WHO's recommendations about thresholds (less 1 GDP is highly C-E and 1-3 GDP is C-E) for middle income countries are considered16. From a new law called “ley estatutaria de salud” that ordered to ministry of health the adoption of a technical-scientific procedure to establish which services could be no afforded, the CEA could be used as a criterion by stakeholders. Is unknown which ICER would be used in the case to choose it, considering that opportunity cost is measure better with other threshold setting techniques as those that take into account the money invested 17. Our ICER results are robust, uncertainty was measure, which allows the decision-making process —more transparent.
Study's strengths are: use of international validated anticoagulation model, the degree of complication was useful to ensure most of assumptions identified in clinical practice and hemorrhage localization, allowed the incorporation of adverse events by localization; we consider that it is a new model in oral anticoagulation, which incorporates more impact of adverse events. Model is robust, uncertainty for base case and other assumptions were measured, major changes in parameters must to be done to alter study's results. Other strength is the use of cost's information from a Cardiovascular Hospital and the bottom-up resources consumption calculation; this kind of resource utilization offer an opportunity for more detailed data collection18. Study's limitations are: use of international QALY's measurement (US), because of lack of Colombian's coefficients calculations for quantitative QALY assessment. We consider that efforts to measure coefficients in our population could be economic evaluations and patient's satisfaction studies more reliable than now, as well as national databases for costs that allow transparent decisions as those that law —indirectly claims.
Decision making process is quite complicated (by stakeholders’ perspectives and interests), but decisions on which stakeholders opinions are considered, tend to increase the acceptance 19–21. From this context, Health Technology Assessments (HTA) helps to provide input to decision making in policy and practice, its orientation is a decision making in a multidisciplinary, comprehensive nature and several perspectives; the main goal of such studies is to improve “value for money” in health care22. Multicriteria decision analysis (MCDA) is a tool to assist decision making process, which is used by national agencies to perform HTA as the Scottish Medicines Consortium (SMC). They stated criteria of clinical effectiveness and cost-effectiveness but allows other factors to play role23, ICER is important but not —the only factor. New oral anticoagulants are used in clinical practice at this moment in Colombia, this from physician's consideration, but it is no widely used due to administrative restrictions that are changing from new administrative steps. New oral anticoagulants are afforded, but restrictions to contain the expenditure could be established, in this context new oral anticoagulants could be revaluated from other criteria than economic considerations; and a roadmap with stakeholder's participation must to be used.
Roads maps establish steps and the rules to be follow, it is crucial to reach transparent decision and moreover to improve the acceptance. The inclusion of new laws in Colombia lead us to speculate what would be the model to be adopted, but we consider that CEA remain plays a role in the decision process in Colombia; nevertheless, not as has been used until now to reject a technology as the only one criterion. Model parameters from real world data from Colombian’ population have to be assess as those that allows QALY measuring and national costs. Major advances had done in Colombian’ health system to ensure drug's access for continue giving the best technology as many as possible patients, more advances are necessary to do as defining of Colombian's road maps to decision making process. In this context, exists uncertainty about the play's role of new oral anticoagulants in clinical practice in our country.
ConclusionsMost of new oral anticoagulants are cost-effective for stroke prevention in oral anticoagulation in Colombia from adverse events perspectives, apixaban is the most cost-effective and rivaroxaban the last, none one is highly cost-effective if a 9 000 USD threshold is used, but are cost-effective. However warfarin remains be the status quo by proven efficacy and the known risk's profile. From changes in Colombian's health system is unknown the play's role of new oral anticoagulants in clinical practice.
Conflict of interestNone.
Net Cardiecol, COLCIENCIAS code: 5020-53-731809
Presentation at ISPOR-CO congress 2016, Bogota, Colombia.