Since the emergence of the COVID-19 pandemic, there have been an increase in the number of reports of autoimmune diseases triggered by or related to the infection, especially in predisposed individuals, by the action of the virus itself or because of the response of the immune system.1,2
SARS-CoV-2 enters the human cells through the angiotensin-converting enzyme 2 (ACE2) and the transmembrane protease serine 2 (TMPRSS2) receptors, whose expression is high in several epithelial cells, including those of the thyroid gland, what makes it a potential target of the virus.3,4 In this way, COVID-19 can aggravate pre-existing endocrine pathology or potentiate the emergence of new abnormalities, not only during the acute phase of the infection, but also during the convalescence period.5
Although there is still little information about the impact of SARS-CoV2 on the thyroid gland,6 some cases of subacute thyroiditis related to the infection have been described. More rarely, COVID-19 appears to be associated with the emergence of new-onset or recurrent Graves’ disease (GD),3 with less than 20 cases reported until now.
GD is an autoimmune thyroid disease caused by antibodies that bind to the thyroid-stimulating hormone (TSH) receptor.5 It can result from an interplay between genetic and nongenetic factors, such as infections, psychological stress, smoking, among others.1
Hereby, we present a case of new-onset GD occurring after SARS-CoV-2 infection, in a patient without previous thyroid dysfunction, that was diagnosed and initially managed at primary care level. With this case report, we would like to provide more evidence and contribute to the establishment of a clearer relationship between SARS-CoV-2 and GD.
A 30-year-old female patient, from a nuclear type family. She has a personal history of asthma, renal lithiasis and migraine with aura. Her obstetric history includes two pregnancies, two deliveries and no miscarriage. Regarding family antecedents, she has a daughter with Celiac Disease. She denies any personal or family history of thyroid dysfunction. Her usual medication consists of a progestational oral contraceptive (Desogestrel 0.075mg) and an inhaled corticosteroid with bronchodilator (Budesonide 160μg/dose+Formoterol 4.5μg/dose).
The patient consulted Primary Health Care in November 2021 because of tachycardia, palpitations and fatigue with three months of evolution, after COVID-19 infection (August 2021). She reported progressive worsening of symptoms in the last month, identifying as exacerbating factors standing up and performing small efforts, such as talking or walking a few meters. The patient also presented dizziness, tremors, excessive sweating and intolerance to physical exercise. She denied weight loss and insomnia. This condition significantly affected her functionality and ability to work.
In this context, she had already had an ambulatory 24-h holter, echocardiogram and analytical study, with TSH, with normal results, one month ago. For the same complaints, she had already gone to the emergency department, where she had an electrocardiogram, which revealed sinus tachycardia (150 beats per minute – bpm), and a chest teleradiography, dosage of d-dimers and troponin that were normal.
Reviewing the previous history of COVID-19 infection, the patient had worsening symptoms that motivated thoracic angio-tomography, with a diagnosis of interstitial pneumonia, but without hospitalization criteria. At the time, the patient had not yet been vaccinated against COVID-19. She received the first dose only six months after infection. It should also be noted that the vaccination was given after the onset of tachycardia and palpitations.
In our consultation, on physical examination, she appeared tired, with normal blood pressure (138/89mmHg) and without fever (36.4°C). The pulse was rhythmic with a resting heart rate ranging from 130 to 140bpm, with an increase of 40bpm just while speaking. The patient had a home written record with her own saturometer with heart rates similar to those seen at the office. She had a normal peripheral oxygen saturation (97–100%) and no signs of respiratory distress. The cardiopulmonary auscultation was normal. She had no edema of the lower limbs or signs of jugular venous turgescence.
As the tests performed were normal and the symptoms of tachycardia and palpitations started after COVID-19 infection, post-infection Postural Orthostatic Tachycardia Syndrome was assumed as the most likely diagnosis. She started Bisoprolol with dose titration up to 10mg/day.
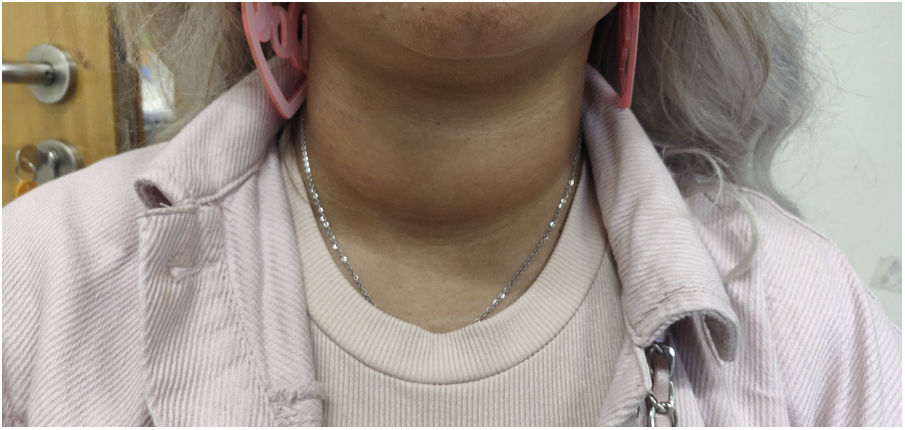
In a follow-up visit, after two weeks, the symptoms persisted, with no significant response to beta-blocker therapy. On physical examination, an increase in neck circumference was evident when compared to the previous observation (Fig. 1). On palpation, the thyroid was globose, with an elastic consistency, without thrill or nodules. There was no exophthalmia. Thus, a repeat of thyroid function tests and thyroid ultrasound study was requested.
The ultrasound showed an enlarged thyroid, homogeneous, without nodular images. There was an intense and relatively symmetric flow signal throughout the gland, suggesting goiter. Analyses showed significant hyperthyroidism, with frenation of TSH (<0.008μUI/mL) and elevation of free triiodothyronine – T3 (>20.0pg/mL) and free thyroxine – T4 (6.53ng/dL). Anti-thyroglobulin and anti-peroxidase antibodies were negative, while anti-TSH receptor antibodies were positive (57 UI/L), consistent with GD (Table 1).
Results of the analytical study evaluating thyroid function and anti-thyroid antibodies.
| Analytic parameter | Result | Reference range |
|---|---|---|
| TSH | <0.008μUI/mL | 0.550–4.780μUI/mL |
| Free T4 | 6.53ng/dL | 0.70–1.58ng/dL |
| Free T3 | >20.0pg/mL | 2.3–4.2pg/mL |
| Anti-thyroglobulin antibodies | Negative | – |
| Anti-peroxidase antibodies | Negative | – |
| Anti-TSH receptor antibodies | Positive57.0U/L | <2.9U/L |
TSH: thyroid-stimulating hormone; T4: thyroxine; T3: triiodothyronine.
Treatment with methimazole was immediately started, after exclusion of possible pregnancy, with a gradual increase in dose up to 20mg/day. In addition, she was referred to an Endocrinology consultation.
After initiation of treatment, the patient has reported significant improvement of tachycardia and palpitations. Six weeks later, she presented with facial and periorbital edema, caused by iatrogenic hypothyroidism. In the Endocrinology consultation, a block and replace strategy was chosen with a reduction of methimazole to 5mg/day and concomitant initiation of levothyroxine 50μg/day. The control analyses showed a reappearance of hyperthyroidism, which motivated the suspension of levothyroxine. Subsequently, a stabilization of the thyroid function was obtained, with recovery of the patient's autonomy and functionality.
In our case report, GD occurred after SARS-CoV-2 infection, in a patient without previous thyroid dysfunction, suggesting that COVID-19 may have played a role.
Of the cases already reported, most describe reactivation of previous disease and, less often, new-onset diagnosis, as in this patient.3 Furthermore, according to our literature review, most of the new-onset GD occurred after vaccination and only a minority of the described cases occurred after infection. Between COVID-19 infection and diagnosis of thyroid pathology, a latency period was frequently described, ranging from one to two months, according to the previously published cases.3
It is important to consider that GD is the most common cause of hyperthyroidism, especially in middle-aged and female patients, so the relationship with COVID-19 infection may be causal.2 In addition, exposure to contrast to perform pulmonary angio-tomography during the acute phase of COVID-19 infection is a bias for what we should be aware of in this case.
An increasing number of publications have pointed out COVID-19 as a potential trigger of autoimmune thyroid disease. However, this association is not yet clearly understood, and further prospective studies in this area are needed. Thus, it is important to call the attention of the health professionals to this possible association and actively research thyroid dysfunction, avoiding delay in diagnosis and treatment. Additionally, this clinical case shows that there may be a mismatch between clinical and analytical detection, since at the onset of symptoms this patient had normal thyroid function. Therefore, if there is high clinical suspicion, it may be pertinent to repeat the study in a short term.
Ethical approvalWritten informed consent was obtained from the patient.
FundingNo funding sources.
Conflict of interestThe authors declare that they have no conflict of interest.