To investigate the prognosis of patients with spontaneous remission (SR) of phospholipase A2 receptor (PLA2R)-associated membranous nephropathy (MN).
Patients and methodsPatients diagnosed with MN were recruited after examining their renal biopsy in the Renal Department of China-Japan Friendship Hospital between January 2015 and September 2021. Among them, 24 patients with SR were included in this study and follow-up.
ResultsTwenty-four patients diagnosed with SR of PLA2R-associated MN were recruited; 11 were male, and 13 were female, with a mean age of 49.5±14.5 years (range, 30–77 years). The initial 24-hour urinary total protein and serum albumin levels were 0.29±0.14g/d and 37.5±4.4g/L, respectively, and the initial serum creatinine was 65.0±15.8μmol/L. During the follow-up of 33.9±19.1 months (range, 6–73 months), 22 (91.7%) patients maintained remission; however, one patient had impaired renal function due to acute coronary syndrome and coronary angiography findings, and one patient experienced a repeated relapse caused by respiratory tract infection, at 50 and 70 months. A systematic review of the relevant literature was conducted, and records of patients with SR of PLA2R-associated MN were retrieved from 16 case reports or case series with a total of 97 cases.
ConclusionsMost patients with SR of MN had a promising long-term prognosis, with only a few cases of relapse.
Investigar el pronóstico de los pacientes con remisión espontánea en la nefropatía membranosa (MN, por sus siglas en inglés) asociada al receptor fosfolipasa A2 (PLA2R).
Pacientes y métodosPacientes con MN diagnosticados por biopsia renal en el Departamento Renal del China-Japan Friendship Hospital entre enero de 2015 y septiembre de 2021. Entre ellos, 24 pacientes con remisión espontánea fueron reclutados y seguidos.
ResultadosSe reclutaron 24 pacientes con MN en remisión espontánea asociada a PLA2R; 11 varones y 13 mujeres, con una edad media de 49,5±14,5 años (rango: 30-77 años) en el momento del diagnóstico. La proteína total y la albúmina sérica en orina de 24h iniciales fueron de 0,29±0,14 y 37,5±4,4g/l, respectivamente; la creatinina sérica inicial fue de 65,0±15,8μmol/l. Durante el seguimiento de 33,9±19,1 (rango: 6-73) meses, 22 pacientes (91,7%) mantienen la remisión. Un paciente presentó insuficiencia renal por síndrome coronario agudo y angiografía coronaria. Otro paciente tuvo una recaída causada por una infección del tracto respiratorio 2 veces, a los 50 y 70 meses. Se realizó una revisión sistemática de la literatura. Los pacientes con MN asociada con PLA2R en remisión espontánea se recuperaron en 16 informes o series de casos, de 97 casos en total.
ConclusionesLa mayoría de los pacientes con MN en remisión espontánea tuvieron un pronóstico prometedor a largo plazo, mientras que solo unos pocos casos tuvieron recaída.
Primary membranous nephropathy (pMN) has become a major glomerular disease; however, approximately one-third of the affected patients achieve spontaneous remission (SR).1 The possibility of complete spontaneous remission has been reported in a few previous related case reports or case series.2–17 Among them, one large-scale case series reported 29 cases with only 9 months of follow-up after SR.9 Therefore, the prognosis of phospholipase A2 receptor (PLA2R)-associated membranous nephropathy (MN) with SR remains unclear. To the best of our knowledge, we have tried to confirm the SR of MN from the perspective of renal histopathology for the first time and analyzed the complement activation state during remission. Further, our study highly focused on the prognosis of MN with SR and included a large number of samples and a long duration of follow-up.
Membranous nephropathy is an autoimmune renal disease, and PLA2R is the major autoantigen of pMN, which can be detected in approximately 70% of patients with pMN.18,19 In this study, we detected anti-PLA2R antibodies in sera and PLA2R antigens in renal biopsy tissues of patients with SR of PLA2R-associated MN. In addition, an increasing number of studies have shown that complement activation is closely related to MN pathogenesis. In this study, we examined the immunohistochemical expression of C1q (to measure the classical pathway), mannose-binding lectin (MBL, to measure the lectin pathway), and factor B (to measure the alternative pathway) in glomerular areas, and the status of complement activation in patients with SR of MN was compared with that in nephrotic syndrome (NS).
Materials and methodsPatientsPatients diagnosed with MN after examining their renal biopsy at the Renal Department of China-Japan Friendship Hospital between January 2015 and September 2021 were recruited for this study. We defined PLA2R-associated MN with SR as patients with a positive glomerular PLA2R antigen who achieved spontaneous remission at the time of biopsy without previous treatment with glucocorticoids or immunosuppressants. The inclusion criteria were as follows: (1) patient with a minimum of 10 glomeruli; (2) patient above 30 years; (3) patient with initial 24-h urinary total protein level<0.5g/d and serum albumin level≥30g/L; (4) patient with negative serum anti-PLA2R antibody and positive glomerular PLA2R antigen; and (5) patient with no history of treatment with glucocorticoids, immunosuppressants, rituximab, and other biological agents. The exclusion criteria include (1) patients with comorbidity of severe systemic diseases or life-threatening diseases, (2) patients with incomplete clinical or pathological data, and (3) patients with comorbid renal diseases. All patients were followed for>6 months. Follow-up started at the time of renal biopsy and ended at either one of the following (whichever arrived first): (1) March 2022 or (2) the date of relapse events. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of the China-Japan Friendship Hospital (2021-113-K71). All patients provided written informed consent at the time of biopsy, including consent to publish and report their data.
MethodsClinical data collectionPatients’ baseline characteristics were collected at the time of renal biopsy, including age, sex, albumin, proteinuria, urinary albumin-to-creatinine ratio (ACR), complement (C3, C4), serum creatinine, and estimated glomerular filtration rate (eGFR). All clinical and laboratory data were collected from the electronic medical records of our hospital.
Detection of serum anti-PLA2R antibody and glomerular PLA2R antigen(a) Serum anti-PLA2R antibodies were detected using antigen-specific enzyme-linked immunosorbent assay (ELISA) in all patients at the time of diagnosis. A standard ELISA was performed according to the manufacturer's instructions (Euroimmun), and the cutoff value was set at 20RU/ml. Patients’ sera at presentation were stored at −20°C until use. (b) Glomerular PLA2R antigens were detected by immunofluorescence using a rabbit polyclonal PLA2R antibody (Abcam, UK) in renal tissue, according to standard protocols. The specimens were embedded in paraffin, sectioned at 3μm, dewaxed, hydrated, antigen retrieved, and incubated overnight at 4°C with rabbit polyclonal antibodies (diluted 1:500) against human PLA2R, followed by FITC-conjugated antibodies (diluted 1:100). The sections were observed using immunofluorescence microscopy.
Renal histologyThe biopsy specimens were divided into three portions, processed, and evaluated according to a previously standardized protocol. Briefly, one portion was fixed in buffered formalin, processed into paraffin blocks, and stained routinely for light microscopy. The second portion was frozen for direct immunofluorescence studies to detect IgG, IgA, IgM, C3, C4, C1q, and fibrinogen levels. The third portion was processed into resin blocks, and its ultrathin slices were used for transmission electron microscopy.
Immunohistochemistry of complement componentThe complement component in renal tissue was detected by immunohistochemistry according to a previously described protocol.20 The following primary antibodies were incubated overnight at 4°C, including anti-C1q (ab268120) diluted 1:400, anti-mannose binding lectin (MBL) (ab23457) diluted 1:25, and anti-factor B (ab192577) diluted 1:400 separately, and were all from Abcam, Cambridge, United Kingdom. Phosphate buffer was used as a blank control instead of the primary antibody. The reactions were visualized by staining with horseradish peroxidase and diaminobenzidine (DAB).
Systematic review of literatureA literature search was performed on PubMed, Embase, Web of Science, and Cochrane Library, as well as on our center's database (from the establishment of the database to March 2022). The keywords used include “Glomerulonephritis, Membranous” [Mesh], “Receptors, Phospholipase A2” [Mesh] and “remission” [Mesh]. The inclusion criteria were: (1) prospective or retrospective cohort study, and (2) studies analyzing patients with proven MN based on biopsy reports with positive serum anti-PLA2R antibody or glomerular PLA2R antigen and who achieved spontaneous remission without immunosuppressive therapy during follow-ups.
Statistical analysisData are presented as median and interquartile range (IQR) for data that were not normally distributed or mean±standard deviation (SD) for normally distributed data for continuous variables. Statistical analyses were performed using SPSS 18.0.
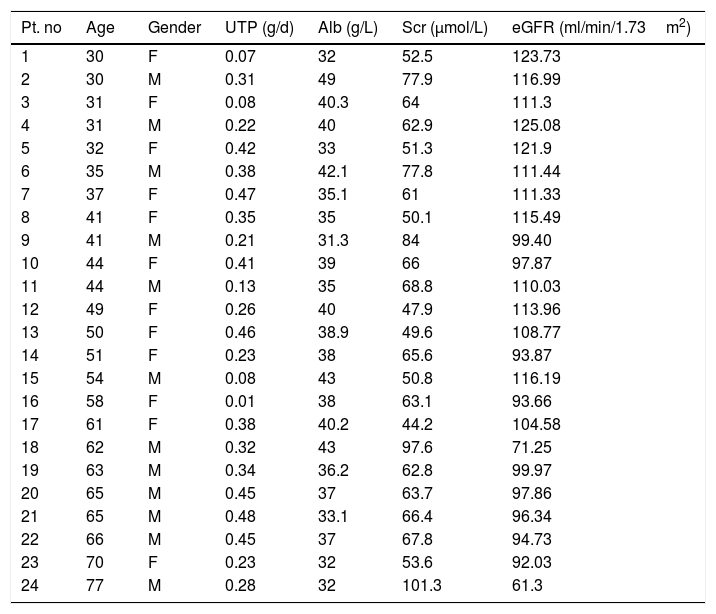
ResultsGeneral data of the patientsA total of 899 patients diagnosed with MN in the Renal Department of China-Japan Friendship Hospital between June 2015 and September 2021 were analyzed retrospectively. Among them, 24 patients with SR of PLA2R-associated MN were recruited for this study; 11 were male, and 13 were female, with an average age of 49.5±14.5 years (range, 30–77 years) at diagnosis. The initial 24-hour urinary total protein and serum albumin levels were 0.29±0.14g/d and 37.5±4.4g/L, respectively. The initial serum creatinine (Normal value: 35–106μmol/L) and eGFR values were 65.0±15.8μmol/L and 103.4±16.0ml/min/1.73m2, respectively. Normal renal function, defined as an eGFR>90ml/min/1.73m2, occurred in 22 cases (91.7%), and renal tubular injury was rare, as only one patient had elevated urinary N-acetyl-beta-glucosaminidase (NAG) levels. Anti-nuclear antibody (ANA) was negative in all patients, and the baseline mean serum Complement C3 (normal value: 70–128mg/dl) and Complement C4 (normal value: 16–47mg/dl) were 96.4±23.4mg/dl and 20.5±5.0mg/dl, respectively. For anti-PLA2R antibody, all patients were negative to ELISA, with a cutoff value of <20RU/ml. The general patient data are shown in Table 1.
General data of the patients with spontaneous remission in PLA2R associated membranous nephropathy (sort by age).
| Pt. no | Age | Gender | UTP (g/d) | Alb (g/L) | Scr (μmol/L) | eGFR (ml/min/1.73m2) |
|---|---|---|---|---|---|---|
| 1 | 30 | F | 0.07 | 32 | 52.5 | 123.73 |
| 2 | 30 | M | 0.31 | 49 | 77.9 | 116.99 |
| 3 | 31 | F | 0.08 | 40.3 | 64 | 111.3 |
| 4 | 31 | M | 0.22 | 40 | 62.9 | 125.08 |
| 5 | 32 | F | 0.42 | 33 | 51.3 | 121.9 |
| 6 | 35 | M | 0.38 | 42.1 | 77.8 | 111.44 |
| 7 | 37 | F | 0.47 | 35.1 | 61 | 111.33 |
| 8 | 41 | F | 0.35 | 35 | 50.1 | 115.49 |
| 9 | 41 | M | 0.21 | 31.3 | 84 | 99.40 |
| 10 | 44 | F | 0.41 | 39 | 66 | 97.87 |
| 11 | 44 | M | 0.13 | 35 | 68.8 | 110.03 |
| 12 | 49 | F | 0.26 | 40 | 47.9 | 113.96 |
| 13 | 50 | F | 0.46 | 38.9 | 49.6 | 108.77 |
| 14 | 51 | F | 0.23 | 38 | 65.6 | 93.87 |
| 15 | 54 | M | 0.08 | 43 | 50.8 | 116.19 |
| 16 | 58 | F | 0.01 | 38 | 63.1 | 93.66 |
| 17 | 61 | F | 0.38 | 40.2 | 44.2 | 104.58 |
| 18 | 62 | M | 0.32 | 43 | 97.6 | 71.25 |
| 19 | 63 | M | 0.34 | 36.2 | 62.8 | 99.97 |
| 20 | 65 | M | 0.45 | 37 | 63.7 | 97.86 |
| 21 | 65 | M | 0.48 | 33.1 | 66.4 | 96.34 |
| 22 | 66 | M | 0.45 | 37 | 67.8 | 94.73 |
| 23 | 70 | F | 0.23 | 32 | 53.6 | 92.03 |
| 24 | 77 | M | 0.28 | 32 | 101.3 | 61.3 |
Abbreviations: UTP, 24-h urinary total protein; Alb, albumin; Scr, serum creatinine; eGFR, estimated glomerular filtration rate.
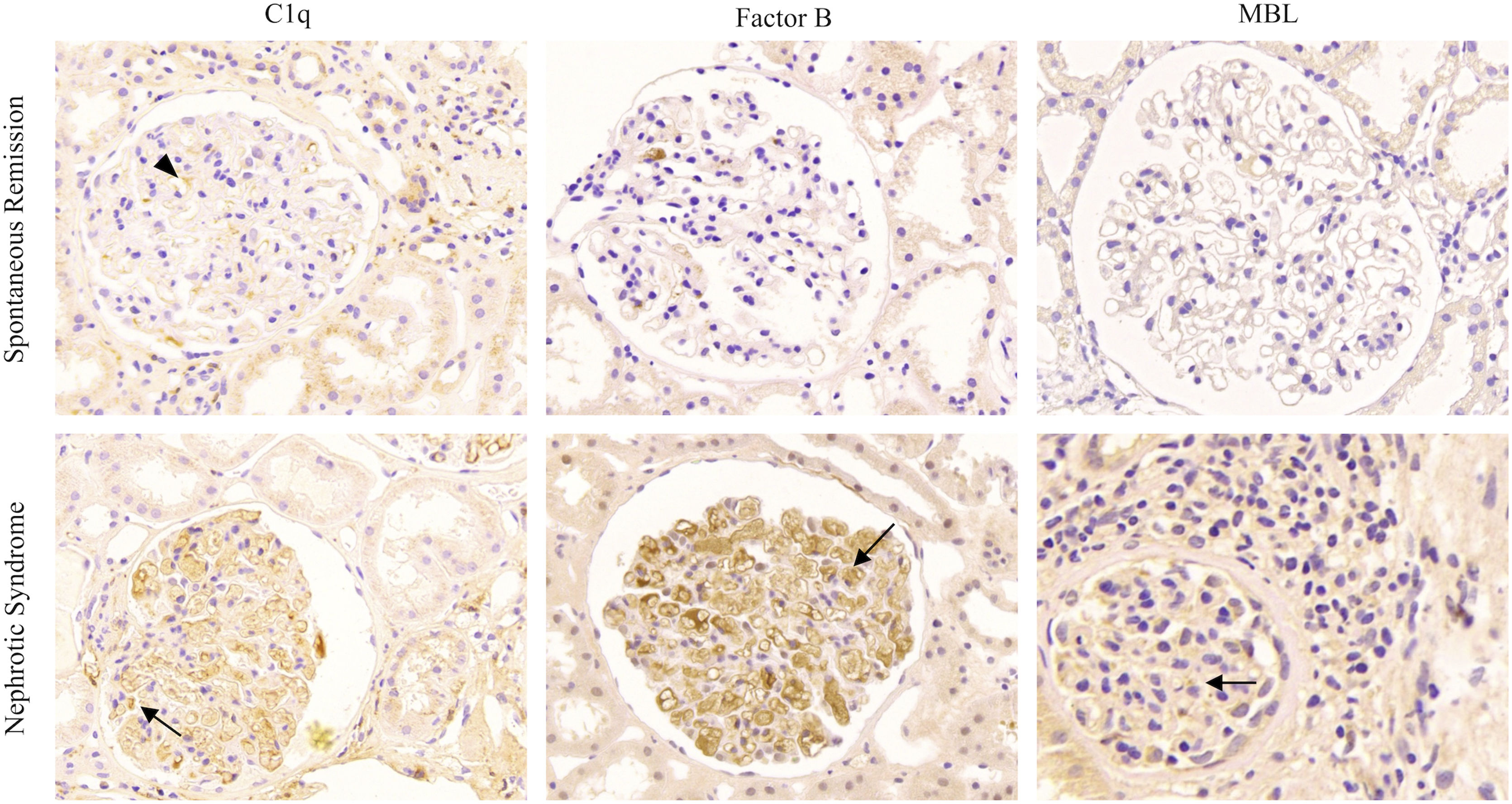
Kidney biopsies were performed in all patients, and MN was diagnosed in all specimens collected. An average number of 29.3±14.2 (range: 11–84) glomeruli were sampled for light microscopy per renal biopsy specimen. Immunofluorescence staining of the renal histology revealed PLA2R-associated MN. Light microscopy showed diffuse thickening of the basement membrane and subepithelial deposition of fuchsinophilic proteins in most specimens, together with focal segmental spike formation. IgG subclasses were identified, and the positivity rates of IgG1 and IgG4 in immunofluorescence staining were 100.0% and 87.5%, respectively. As mentioned above, immunohistochemical staining for C1q, MBL, and factor B in the glomeruli was performed to detect the complement pathway in patients with PLA2R-associated MN with SR (shown in Fig. 1). Among these patients, MBL and factor B expressions were negative in 23 patients, while C1q expression was weakly positive in one patient.
Typical renal histopathological of complement pathway activation in patients with PLA2R-associated MN. Immunohistochemical staining for C1q, factor B, and mannose-binding lectin (MBL) in the glomeruli to detect the complement pathway in patients with PLA2R-associated MN. (a) Immunohistochemical staining for C1q, factor B, and MBL in patients with spontaneous remission. The expression of C1q was slightly positive in only one patient (C1q, arrowheads). (b) Immunohistochemical staining for C1q, factor B, and MBL in patients with nephrotic syndrome (arrow).
Likewise, we recruited 24 age-, sex-, and Scr-matched patients with MN who presented with nephrotic syndrome at the initial onset as the control group. Glomerular C1q, MBL, and factor B expressions were significantly positive in most of these patients, thus suggesting that more than one complement pathway is activated during the progression of nephrotic syndrome in patients with MN, whereas it is rarely activated in patients with SR. The classical complement pathway may also play a role in the early or remission state of MN; however, more patients need to be further studied.
Treatment and outcomesAll patients with SR of pMN received optimal supportive care. Antihypertensive drugs belonging to the renin–angiotensin–aldosterone system inhibitor (RASi) group were administered to reduce proteinuria and blood pressure (BP). The RASi includes angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), and the targeted BP range is 110–130/60–80mmHg. Among the patients, four were treated with ACEIs and 15 with ARBs, a combination of ACEI and ARB was administered to two patients, and the rest were treated without RASi due to their low basal blood pressure and low urinary protein (UTP<0.3g/d).
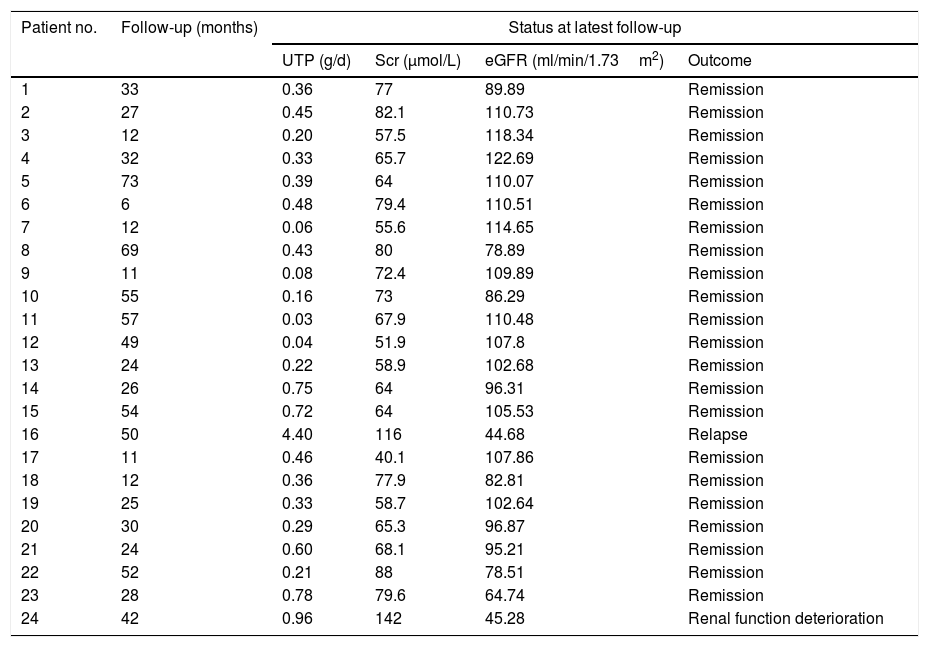
At the end of the follow-up period of 33.9±19.1 months (range: 6–73 months), 22/24 (91.7%) patients maintained remission, while relapse occurred in one case. Patient No. 16 experienced a repeated relapse caused by respiratory tract infection at 50 and 70 months, and glucocorticoids and cyclosporine were successively used for the immunosuppressive therapy; however, this patient maintained nephrotic proteinuria and deteriorated renal function. The remaining patient had renal function impairment without increased urinary protein levels at 42 months of follow-up due to acute coronary syndrome and coronary angiography. Notably, none of the patients underwent hemodialysis or died. Details of the outcomes are shown in Table 2.
The outcomes of patients with spontaneous remission in PLA2R associated membranous nephropathy.
| Patient no. | Follow-up (months) | Status at latest follow-up | |||
|---|---|---|---|---|---|
| UTP (g/d) | Scr (μmol/L) | eGFR (ml/min/1.73m2) | Outcome | ||
| 1 | 33 | 0.36 | 77 | 89.89 | Remission |
| 2 | 27 | 0.45 | 82.1 | 110.73 | Remission |
| 3 | 12 | 0.20 | 57.5 | 118.34 | Remission |
| 4 | 32 | 0.33 | 65.7 | 122.69 | Remission |
| 5 | 73 | 0.39 | 64 | 110.07 | Remission |
| 6 | 6 | 0.48 | 79.4 | 110.51 | Remission |
| 7 | 12 | 0.06 | 55.6 | 114.65 | Remission |
| 8 | 69 | 0.43 | 80 | 78.89 | Remission |
| 9 | 11 | 0.08 | 72.4 | 109.89 | Remission |
| 10 | 55 | 0.16 | 73 | 86.29 | Remission |
| 11 | 57 | 0.03 | 67.9 | 110.48 | Remission |
| 12 | 49 | 0.04 | 51.9 | 107.8 | Remission |
| 13 | 24 | 0.22 | 58.9 | 102.68 | Remission |
| 14 | 26 | 0.75 | 64 | 96.31 | Remission |
| 15 | 54 | 0.72 | 64 | 105.53 | Remission |
| 16 | 50 | 4.40 | 116 | 44.68 | Relapse |
| 17 | 11 | 0.46 | 40.1 | 107.86 | Remission |
| 18 | 12 | 0.36 | 77.9 | 82.81 | Remission |
| 19 | 25 | 0.33 | 58.7 | 102.64 | Remission |
| 20 | 30 | 0.29 | 65.3 | 96.87 | Remission |
| 21 | 24 | 0.60 | 68.1 | 95.21 | Remission |
| 22 | 52 | 0.21 | 88 | 78.51 | Remission |
| 23 | 28 | 0.78 | 79.6 | 64.74 | Remission |
| 24 | 42 | 0.96 | 142 | 45.28 | Renal function deterioration |
Abbreviations: UTP, 24-h urinary total protein; Alb, albumin; Scr, serum creatinine; eGFR, estimated glomerular filtration rate;
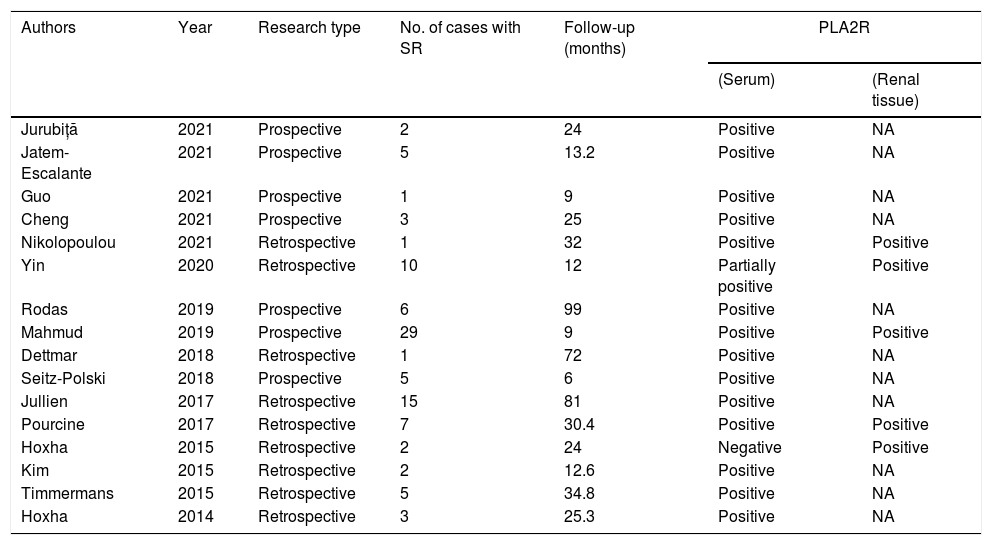
Membranous nephropathy is an autoimmune renal disease and a common cause of primary nephrotic syndrome in adults.21 Patients with MN tend to get spontaneous remission. Although the possibility of SR has been reported in previous studies on patients with PLA2R-associated MN,2–17 only a few completed SR cases have been reported, including 16 case reports or case series with a total of 97 cases (Table 3). Mahmud et al.9 performed a multicenter survey and reported 29 patients with SR of PLA2R-associated MN, with only 9 months of follow-up after remission. Similarly, Rodas et al.8 conducted a prospective observational study on patients with PLA2R-associated MN for 99 months and reported six patients with SR. Since the prognosis of PLA2R-associated MN with SR remains unclear, we decided to carry out this study at our hospital. To the best of our knowledge, this study had a large sample size and a long follow-up period for PLA2R-associated MN with SR.
Reports in literatures of patients with spontaneous remission of PLA2R associated membranous nephropathy.
| Authors | Year | Research type | No. of cases with SR | Follow-up (months) | PLA2R | |
|---|---|---|---|---|---|---|
| (Serum) | (Renal tissue) | |||||
| Jurubiță | 2021 | Prospective | 2 | 24 | Positive | NA |
| Jatem-Escalante | 2021 | Prospective | 5 | 13.2 | Positive | NA |
| Guo | 2021 | Prospective | 1 | 9 | Positive | NA |
| Cheng | 2021 | Prospective | 3 | 25 | Positive | NA |
| Nikolopoulou | 2021 | Retrospective | 1 | 32 | Positive | Positive |
| Yin | 2020 | Retrospective | 10 | 12 | Partially positive | Positive |
| Rodas | 2019 | Prospective | 6 | 99 | Positive | NA |
| Mahmud | 2019 | Prospective | 29 | 9 | Positive | Positive |
| Dettmar | 2018 | Retrospective | 1 | 72 | Positive | NA |
| Seitz-Polski | 2018 | Prospective | 5 | 6 | Positive | NA |
| Jullien | 2017 | Retrospective | 15 | 81 | Positive | NA |
| Pourcine | 2017 | Retrospective | 7 | 30.4 | Positive | Positive |
| Hoxha | 2015 | Retrospective | 2 | 24 | Negative | Positive |
| Kim | 2015 | Retrospective | 2 | 12.6 | Positive | NA |
| Timmermans | 2015 | Retrospective | 5 | 34.8 | Positive | NA |
| Hoxha | 2014 | Retrospective | 3 | 25.3 | Positive | NA |
Abbreviations: SR, spontaneous remission; follow-up, the follow up time after remission.
PLA2R is the major autoantigen of pMN, highly expressed in glomerular podocytes, and its autoantibodies are mainly IgG4 subtypes.22,23 Serum anti-PLA2R antibodies play an important role in the clinical prediction of pMN at diagnosis and have been correlated with the disease activity. Previous studies have shown that the PLA2R IgG4 subclass could improve the sensitivity and specificity of MN diagnosis.24 Serum PLA2Rab levels at baseline may also be used independently to predict the timing of clinical remission.25 The risk factors influencing the long-term prognosis of PLA2R-associated MN include male sex, increased proteinuria during the course of the disease, decreased eGFR at onset, high serum PLA2Rab levels, and C3 positive deposition in renal biopsy samples.26 In patients with pMN, IgG deposition in renal tissue may be accompanied by C1q deposition, where IgG1 is mainly in the early stage, IgG4 is mainly in the late stage, and C1q is positively correlated with IgG1.27 Thus, activation of the classical complement pathway plays a role in the early stage of the disease but is probably not a major player. Other studies have also suggested that the MBL pathway of complement activation commonly affects the prognosis of pMN, as patients with MBL deposition reach incomplete remission faster.28 Therefore, we examined the immunohistochemical expression of C1q (to measure the classical pathway), MBL (to measure the lectin pathway), and factor B (to measure the alternative pathway) in glomeruli in our study. The expression of C1q was slightly positive in only one patient, while the expression of MBL and factor B was significantly weaker in patients with nephrotic syndrome. Previous studies have shown that affinity-purified IgG4 anti-PLA2R antibodies can bind to MBL and promote C4 deposition,29 which we also confirmed in patients with nephrotic syndrome.
Numerous studies have shown the correlation between proteinuria and renal prognosis, and proteinuria might be the strongest predictor of outcome in patients with MN.30 Moreover, it has also been reported that nearly 80% of patients with subnephrotic proteinuria could survive this renal condition for more than 10 years.31 A literature survey showed that after 6 months of conservative treatment, the proportion of SR (including complete remission and partial remission) in patients with proteinuria>4g/d and patients with proteinuria>8g/d was 45% and 34%, respectively.11,32 Patients with pMN in a persistent remission state usually have promising long-term prognosis.33
All patients included in this study were assessed by clinical and laboratory criteria, which conformed to a low risk of progressive loss of kidney function, and received adequate supportive care to reduce proteinuria by RASi.34,35 Most patients remain in remission for a long period and do not receive treatment with immunosuppressive agents, including glucocorticoids. The identification of patients with expected SR is significant for those with a low risk of progressive loss of kidney function, and the recurrence of MN should not be underestimated. A retrospective cohort study of 395 patients with MN showed that 42% of patients with subnephrotic proteinuria developed nephrotic syndrome within 1 year of follow-up, and 61% of patients developed nephrotic syndrome at 4 years of follow-up.31 The patients with SR of MN in our study with low initial proteinuria (<0.5g/d) had a low recurrence rate and slow progression of renal function loss. During follow-up, only one patient experienced renal function loss due to contrast medium, and one patient experienced relapse due to respiratory tract infection. Comprehensive prevention of infection may benefit patients with MN in remission. We speculate that the initial urinary protein level of <0.5g/d in patients with SR of MN is of great significance for lower recurrence rates and long-term renal prognosis. This study had several limitations, which included its relatively small sample size, single-center design, and limited follow-up time. Therefore, a multicenter study with larger sample size and longer follow-up period should be carried out in the future.
ConclusionIn conclusion, our study investigated the prognosis of patients with SR of PLA2R-associated MN. We found that most patients with baseline urinary protein levels of <0.5g/d had a good prognosis and lower recurrence rate. Also, we found that respiratory tract infection was one of the causes of MN recurrence in patients with remission.
Take-home-message of complementComplement activation is involved in the development of MN. In particular, activation of the classical complement pathway in glomeruli might accelerate the disease progression; however, it is not significant in patients with SR. Therefore, proteinuria and the titers of PLA2R, together with complement, should be used to predict SR in patients with MN.
Authors’ contributionsX.W. and J.Z. contributed to the research idea, literature search, study design, statistical analysis and article draft. G.M.Z. and S.M.J. were responsible for revising the article. X.M.Z., J.Y.L. and H.M.G. contributed to the data collection and interpretation of the results. W.G.L. contributed important intellectual content during manuscript drafting or revision. All authors helped revising the paper and read and approved the final version of the manuscript.
Consent for publicationAll patients provided written informed consent including consent to publish and report individual patient data.
Data availability statementThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and informed consentStudy approval statement: The research was in compliance with the Declaration of Helsinki. The study has been approved by the clinical research ethics committee of the China-Japan Friendship Hospital. However, since it was a retrospective study and patient's identity was anonymous, the study had been granted an exemption from requiring ethics approval document and inform consent to participate statement, which got a record number as 2021-113-K71.
Funding sourcesNot applicable.
Conflict of interestNone.
Not applicable.