To evaluate the effect on creatinine clearance (CG-CrCl, Cockcroft–Gault equation) of switching to boosted protease inhibitor (PI) monotherapy in patients receiving a triple drug antiretroviral regimen containing TDF.
MethodsAll patients who had received a TDF-containing regimen for at least one year and had been switched to PI monotherapy were included. A rapid decrease in CG-CrCl during exposure to TDF was defined as a decrease in CG-CrCl at least five times higher than the expected due to age (0.4ml/min/year by the years of exposure to TDF). In this subgroup of patients, we considered improvement if the last value of CG-CrCl on PI monotherapy was 10% higher than the last value of CG-CrCl before switching to PI monotherapy. A multivariate logistic regression was constructed to identify factors associated to renal improvement after switching to bPI monotherapy.
Results64 patients included. The median (IQR) annual change in CG-CrCl during PI monotherapy was significantly lower than the median (IQR) annual change while exposed to TDF [−0.9 (−4.7 to +2.8) ml/min vs. −4 (−8 to −1) ml/min, p=0.001]. 44 patients experienced a rapid decline during TDF exposition. After switch to PI monotherapy, 15/44 (34%, 95% CI: 21–50%) had an improved CG-CrCl and 16/44 (36%, CI 23–52%) experienced a further decline in CG-CrCl. The only variable associated to CG-CrCl improvement was a more rapid CG-CrCl decline in the last year of exposure to TDF.
ConclusionSwitching to PI monotherapy partially reversed CG-CrCl decrease associated to TDF use, especially in patients with a more rapid decline while receiving TDF.
Evaluar el efecto de la retirada de TDF en el aclaramiento de creatinina medido mediante la fórmula de Cockcroft-Gault (CG-ClCr) en pacientes que simplifican a monoterapia con un inhibidor de la proteasa (IP) potenciado.
MétodosSe incluyeron todos los pacientes que habían recibido un regimen con TDF durante al menos un año y que posteriormente habían sido simplificados a monoterapia. Se definió como rápida disminución del CG-CrCl durante la exposición a TDF a una disminución del CG-CrCl de al menos 5 veces mayor de lo esperado para la edad (0.4ml/min/año por los años de exposición al TDF). En este subgrupo de pacientes, se consideró mejoría si el último valor del CG-CrCl durante la exposición a monoterapia era un 10% más alto que el último valor de CG-CrCl antes de la simplificación. Se construyó una regresión logística multivariante para identificar los factores asociados a mejoría del CG-ClCr.
ResultadosSe incluyeron 64 pacientes. La mediana del cambio anual en el CG-CrCl durante la exposición a monoterapia fue significativamente inferior a la mediana del cambio anual durante la exposición a TDF (p=0.001). 44 pacientes presentaron una rápida disminución del CG-CrCl durante la exposición a TDF. Después de la simplificación, 15/44 (34%, IC 95%: 21–50%) presentaron una mejoría del CG-CrCl y 16/44 (36%, IC 23–52%) continuaron con un empeoramiento en el CG-CrCl. La única variable asociada con mejoría fue haber presentado una disminución más rápida del CG-CrCl en el último año de exposición a TDF.
ConclusiónLa simplificación a monoterapia revierte parcialmente la disminución del CG-CrCl asociada al TDF, especialmente en los pacientes que presentan una disminución más rápida durante la exposición a TDF.
Tenofovir (TDF) is a nucleotide reverse transcriptase inhibitor, excreted through glomerular filtration and active tubular secretion.1 In a recent meta-analysis, TDF-containing regimens were associated with a statistically significant loss of renal function of modest magnitude.2
The European AIDS Clinical Society Guidelines3 recommend stopping TDF if there is a progressive decline in creatinine clearance (CG-CrCl) not explained by other causes. However it is not clear if CG-CrCl recovers completely after stopping TDF. The reversibility of TDF-related renal impairment has been evaluated in three retrospective studies.4–6 In these studies, renal function improved after TDF discontinuation but renal impairment was not fully reversible.
Boosted protease inhibitor (PI) monotherapy has demonstrated to be effective in maintaining long-term viral suppression in the majority of patients. PI monotherapy avoids the long-term toxicity associated with nucleoside/nucleotide analogs.7 Therefore, switching to PI monotherapy might be an option in patients with a progressive TDF-associated renal decline. None of the published studies has systematically evaluated the reversibility of TDF-associated renal impairment in patients switching away from a TDF-containing regimen to PI monotherapy. The aim of our study was to evaluate the effect on CG-CrCl of switching to PI monotherapy in patients receiving a TDF-containing regimen.
MethodsWe performed a retrospective cohort study of all patients attending our HIV Unit who had received a TDF-containing regimen for at least one year and had been switched to PI monotherapy. During this period all creatinine determinations were done with a modified Jaffe method. We excluded patients with less than two annual determinations of creatinine during TDF therapy or during PI monotherapy, patients without a serum creatinine measurement within 3 months prior to starting TDF and/or those whose follow-up after the switch to PI monotherapy was less than 5 months. This limit was chosen because five months was the median time to maximum improvement in renal function after TDF cessation in Wever's study.5
Our renal function measurement was the estimated creatinine clearance calculated by the Cockcroft–Gault equation (CG-CrCl). CG-CrCl was recorded at 6 months intervals from the last value before starting TDF to the last available value while the patient was still receiving PI monotherapy.
A rapid decrease in CG-CrCl during exposure to TDF was defined arbitrarily as a decrease in CG-CrCl at least five times higher than the one expected due to age. We calculated the expected CG-CrCl decline multiplying 0.4ml/min/year by the years of exposure to TDF. Estimating CG-CrCl loss related to age was based on the results of an observational study in healthy Caucasian volunteers.8 Renal function outcomes after switching to PI monotherapy in patients with a rapid decrease in the CG-CrCl during exposure to TDF was analyzed separately. We defined the categorical variable improvement of CG-CrCl in this subgroup of patients if the last value of CG-CrCl during exposure to PI monotherapy was 10% higher than the last value of CG-CrCl before switching to PI monotherapy. The study was approved by the Ethics Committee for Clinical Research of La Paz Hospital.
Statistical methodsPatient characteristics were described using median (IQR) for continuous variables and frequency (%) for categorical variables. A logistic regression model with a predictive approach was constructed to identify factors associated to renal improvement (with dichotomous outcome) after switching to PI monotherapy in the group of patients with a significant decrease in the CG-CrCl during treatment with TDF. We analyzed the following variables: age, sex, hypertension, diabetes, hepatitis C, months on TDF, months on PI in triple therapy, use of didanosine, dose of ritonavir (100 or 200mg.) on PI monotherapy, CD4 count, CG-CrCl at starting TDF, CG-CrCl at switch to PI monotherapy and change of CG-CrCl in the last year of TDF. Variables with a p value of <0.1 in the univariate analysis were retained in the multivariate analysis. Data were analyzed using SPSS version 18.0, p-values <0.05 were considered significant.
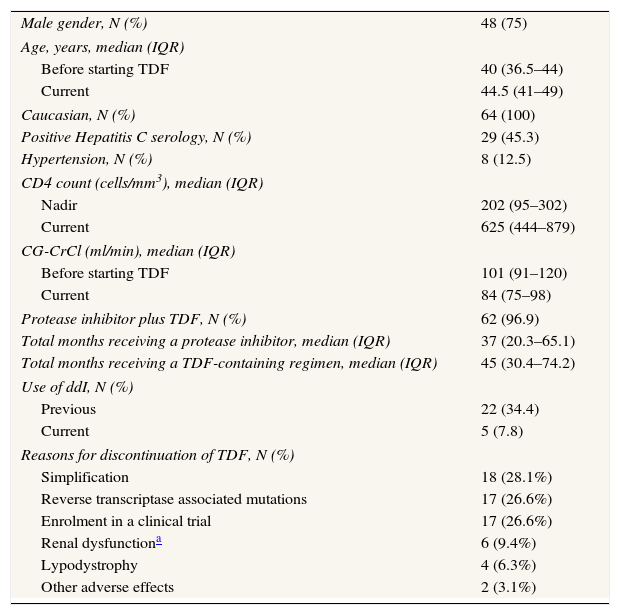
ResultsWe included 64 patients (Table 1). Forty-six patients switched to lopinavir/ritonavir monotherapy, 17 to darunavir/ritonavir monotherapy and one to atazanavir/ritonavir monotherapy. Most patients continued on the same PI after the switch to PI monotherapy. Four patients on lopinavir/ritonavir in triple therapy switched to darunavir/ritonavir monotherapy. The median time on PI monotherapy was 30 (19.9–38.6) months.
Patients characteristics at the time of switch to boosted protease inhibitor monotherapy (N=64).
| Male gender, N (%) | 48 (75) |
| Age, years, median (IQR) | |
| Before starting TDF | 40 (36.5–44) |
| Current | 44.5 (41–49) |
| Caucasian, N (%) | 64 (100) |
| Positive Hepatitis C serology, N (%) | 29 (45.3) |
| Hypertension, N (%) | 8 (12.5) |
| CD4 count (cells/mm3), median (IQR) | |
| Nadir | 202 (95–302) |
| Current | 625 (444–879) |
| CG-CrCl (ml/min), median (IQR) | |
| Before starting TDF | 101 (91–120) |
| Current | 84 (75–98) |
| Protease inhibitor plus TDF, N (%) | 62 (96.9) |
| Total months receiving a protease inhibitor, median (IQR) | 37 (20.3–65.1) |
| Total months receiving a TDF-containing regimen, median (IQR) | 45 (30.4–74.2) |
| Use of ddI, N (%) | |
| Previous | 22 (34.4) |
| Current | 5 (7.8) |
| Reasons for discontinuation of TDF, N (%) | |
| Simplification | 18 (28.1%) |
| Reverse transcriptase associated mutations | 17 (26.6%) |
| Enrolment in a clinical trial | 17 (26.6%) |
| Renal dysfunctiona | 6 (9.4%) |
| Lypodystrophy | 4 (6.3%) |
| Other adverse effects | 2 (3.1%) |
TDF, tenofovir; ddI, didanosine; CG-CrCl, creatinine clearance according to the Cockcroft–Gault equation.
During exposure to TDF the median annual CG-CrCl change was −4 (−8 to −1) ml/min and the incidence rate of a decrease of CG-CrCl of at least 25% was 11 (95% CI: 7–15) per 100 patients-years. After switch to PI monotherapy, the median annual CG-CrCl change was −0.9 (−4.7 to +2.8) ml/min. The annual change in CG-CrCl during PI monotherapy was significantly lower than the annual change while exposed to TDF (p=0.001).
Patients with rapid CG-CrCl decline during TDF exposureDuring exposure to TDF, 44 patients (68.8%) experienced a rapid decline of CG-CrCl. In this group of patients, the median CG-CrCl declined from 109 (95–121) ml/min to 81 (69–95) ml/min, for a median of exposition to TDF of 42 (31.5–72.3) months. There were no relevant differences between the characteristics before starting TDF of the subgroup of patients with rapid CG-CrCl decline and the total sample (data no shown).
In patients with a rapid CG-CrCl decline while exposed to TDF the median annual CG-CrCl change was −7 (−11 to −4) ml/min. After switch to PI monotherapy, the median annual change of CG-CrCl was significantly lower: −0.4 (−3 to +4) ml/min (p<0.001).
After switch to PI monotherapy, 15 (34%, 95% CI: 21–50) of the 44 patients with rapid CG-CrCl decline while receiving TDF had an improvement of the CG-CrCl. Time receiving PI monotherapy was not different in patients with and without CG-CrCl improvement: median exposures were 20.5(11.3–32.7) vs. 30.3(21.8–36.8) months, respectively (p=0.06). The median time to maximum improvement in CG-CrCl was 11 (6–14) months. Of the 15 patients with CG-CrCl improvement, only four reached a CG-CrCl above their pre-TDF CG-CrCl.
In the group of 29 patients without CG-CrCl improvement, the median maximum CG-CrCl value reached during PI monotherapy was 83 (80–101) ml/min, which was similar to the median CG-CrCl value at the time of switch to PI monotherapy (82ml/min, IQR 77–98ml/min). Of these 29 patients, 13 and 3 patients had a CG-CrCl decline above 10% and 25%, respectively, at the end of follow-up after switch to PI monotherapy.
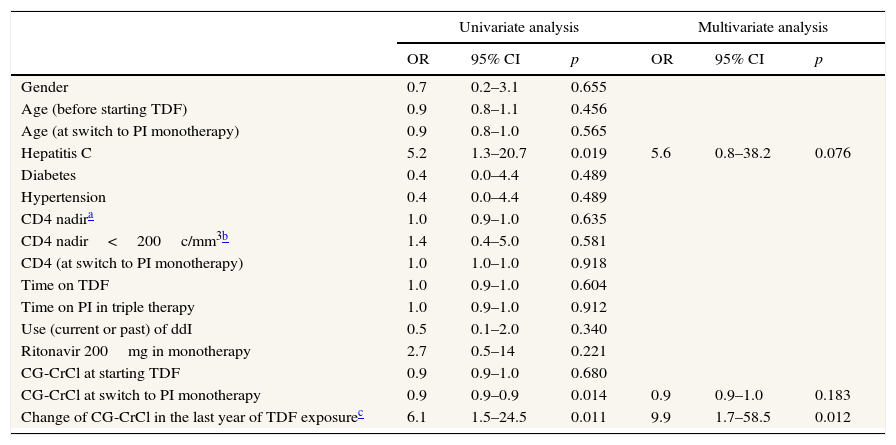
Factors associated to CG-CrCl improvement after switch to PI monotherapyIn the multivariate analysis (Table 2), the only variable associated to CG-CrCl improvement after switching to PI monotherapy was a more rapid CG-CrCl decline (greater than 10ml/min) in the last year of exposure to TDF [p=0.01; OR 9.9 (1.7–58.5)].
Factors associated with improvement in creatinine clearance (N=44).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Gender | 0.7 | 0.2–3.1 | 0.655 | |||
| Age (before starting TDF) | 0.9 | 0.8–1.1 | 0.456 | |||
| Age (at switch to PI monotherapy) | 0.9 | 0.8–1.0 | 0.565 | |||
| Hepatitis C | 5.2 | 1.3–20.7 | 0.019 | 5.6 | 0.8–38.2 | 0.076 |
| Diabetes | 0.4 | 0.0–4.4 | 0.489 | |||
| Hypertension | 0.4 | 0.0–4.4 | 0.489 | |||
| CD4 nadira | 1.0 | 0.9–1.0 | 0.635 | |||
| CD4 nadir<200c/mm3b | 1.4 | 0.4–5.0 | 0.581 | |||
| CD4 (at switch to PI monotherapy) | 1.0 | 1.0–1.0 | 0.918 | |||
| Time on TDF | 1.0 | 0.9–1.0 | 0.604 | |||
| Time on PI in triple therapy | 1.0 | 0.9–1.0 | 0.912 | |||
| Use (current or past) of ddI | 0.5 | 0.1–2.0 | 0.340 | |||
| Ritonavir 200mg in monotherapy | 2.7 | 0.5–14 | 0.221 | |||
| CG-CrCl at starting TDF | 0.9 | 0.9–1.0 | 0.680 | |||
| CG-CrCl at switch to PI monotherapy | 0.9 | 0.9–0.9 | 0.014 | 0.9 | 0.9–1.0 | 0.183 |
| Change of CG-CrCl in the last year of TDF exposurec | 6.1 | 1.5–24.5 | 0.011 | 9.9 | 1.7–58.5 | 0.012 |
PI, protease inhibitor; TDF, tenofovir; ddI, didanosine; CG-CrCl, creatinine clearance according to the Cockcroft–Gault equation.
In this retrospective cohort study, we have found that switching to PI monotherapy ameliorate the loss of CG-CrCl associated to prolonged treatment with TDF-containing regimens. This result suggests that a decline in the CG-CrCl during TDF therapy is partially reversible after switch to PI monotherapy.
The majority of our patients did not develop renal insufficiency during TDF treatment. However, the median annual decline in CG-CrCl was of −4ml/min which is almost 10 times greater than expected just by the influence of age.8 The decline of CG-CrCl during the exposition to TDF observed in our study is similar to other cohorts.9,10
In the subgroup of patients with a rapid decrease of renal function during TDF, the CG-CrCl stabilized in the majority of patients after TDF cessation. Only one third of these experienced a recovery greater than 10% of renal function, but also 36% had a creatinine clearance decline in spite of TDF withdrawal.
In comparison with our results, Wever and colleagues5 found a higher percentage of reversibility and an earlier recovery after cessation of TDF. Unlike our study, patients included in Wever's study had experienced a more rapid loss of kidney function and had ceased TDF because of a CG-CrCl<60ml/min. As it happened in our study, Wever et al. showed that a more rapid decline in CG-CrCl on TDF was independently associated to greater recovery of renal function. Therefore a prior more rapid decline in renal function might explain the greater recovery of renal function in Wever's study. In the Jose et al. paper6 similar declines in the eGFR were observed during TDF exposure [−3.1ml/min/1.73m2/year] and also 38.6% of patients with a decline in eGFR did not recover filtration rate after TDF cessation comparable to the 36% observed in our study. By the contrary in our study we were not able to identify risk factors related to incomplete recovery [higher eGFR at baseline, lower eGFR after TDF discontinuation and duration of TDF exposure]. This could be partially explained by different measurements of renal function (MDRD and CG-CrCl are not interchangeable especially with filtration rates in the normal range) and the lower sample size of our study.
In the analysis of factors associated to renal improvement, Wever et al. found that having received a PI with TDF was independently associated to a higher CG-CrCl recovery after TDF cessation. We could not determine whether treatment with a PI along with TDF is a predictive factor of renal function recovery, because 97% of our patients had received a PI with TDF. There are some data coming from cohort studies showing an association between use of PI (lopinavir and atazanavir) with renal function decline.11,12 So, our results may not to be generalized to other treatment settings.
In univariate analysis, the positive hepatitis C serology was significantly associated with CG-CrCl improvement. However, this association was lost in multivariate analysis. It is likely that the presence of cirrhosis and other comorbidities associated to HCV infection are risk factors of renal decline during TDF therapy. Therefore, the HCV/HIV coinfection may be to lead a rapid loss of CG-CrCl and to be a confounding factor. More studies are necessary to evaluate the toxicity of TDF in patients with HCV/HIV coinfection.
This report has the limitations of a retrospective non-comparative design and small sample size. It is possible that other factors associated to renal damage have not been completely recorded in the clinical reports. However, as we evaluated a paired sample of patients the effect of the comorbidities is maintained during follow-up, so their presence had minimum impact on renal function improvement after discontinuation of TDF. Creatinine clearance was measured by Cockcroft–Gault equation. We acknowledge this is not the most accurate way to estimate creatinine clearance especially in patients with normal glomerular filtration rate. Nevertheless this equation is widely used in most of pivotal trials evaluating drug effects on renal function and dose-adjustment is based on GC results in most of the Summary of Product Characteristics (SmPC). The proteinuria and fractional excretion of phosphorus were not requested systematically in the patients during follow-up, so we could not assess the impact of the withdrawal of TDF on the tubular function. In patients who had collected urine, no cases of crystalluria or interstitial nephritis associated with treatment with darunavir and atazanavir was reported. On the other hand, we considered arbitrarily a rapid decrease in CG-CrCl during exposure to TDF as a CG-CrCl loss higher than 2ml/min/year. There is no consensus on the definition of rapid loss of renal function which makes it difficult to compare our results with other published series.13 Patient's follow-up could have not be long enough to capture precisely CG-CrCl changes. To our knowledge this is the first study that has evaluated the effect of PI monotherapy in the reversibility of the decline in the CG-CrCl during TDF therapy. However, it has the disadvantage that most of the patients included were treated with a TDF plus PI, so our results may not be applicable to a wider TDF-exposed population.
In conclusion, switching to PI monotherapy partially improved the CG-CrCl decline associated to TDF-containing regimens. Improvement was greater in those patients with a more rapid CG-CrCl decline while receiving TDF.
Conflict of interestThe authors declare no conflict of interest.
Sources of supportDr. Miriam Estebanez and Dr. Francisco X Zamora are supported by a grant from the FIS Rio Hortega program financed by Fondo de Investigaciones Sanitarias, Ministerio de Ciencia e Innovación. Dr. Ignacio Pérez-Valero is an investigator of Red de Investigación en SIDA (RIS) [AIDS Research Neatwork] RD 12/0017.
IdiPAZ AIDS and infectious diseases investigator group is partially supported by “Red de Investigación en SIDA” (AIDS Research Network) (RIS)RD07/0006/2007.