The aim of this study was to assess the clinical usefulness of therapeutic drug monitoring (TDM) of voriconazole (VOR) in a university hospital.
MethodsA retrospective review was conducted on the clinical records of 52 patients treated with VOR and on whom TDM was performed. Steady-state trough plasma VOR concentration was measured at least 5 days after starting treatment. The therapeutic range of plasma VOR concentration was defined as 1–5.5μg/mL.
ResultsThe most frequent underlying conditions in the study population were lung transplant (48.1%) and hematological malignancies (26.9%). At the first TDM in each patient, VOR levels were outside the therapeutic range in 16 (30.7%) cases: <1μg/mL in 10 (19.2%) and >5.5μg/mL in 6 (11.5%). Eleven patients (21.2%) experienced severe muscle weakness and had considerable difficulty walking. All these patients were receiving concomitant treatment with corticosteroids. Age younger than 30 years (p=.005) and cystic fibrosis as the underlying disease (p=.04) were factors associated with low VOR levels. Almost all patients who had VOR concentrations >1μg/mL at the first TDM had a successful outcome (96%).
ConclusionsPlasma VOR concentrations were outside the therapeutic range at the first TDM in 30% (16/52) of patients. Age younger than 30 years and cystic fibrosis were factors associated with low VOR levels. The potential interactions between corticosteroids and VOR should be highlighted, as they could be responsible for a high rate of muscle weakness observed in our patients. Prospective trials are needed to investigate VOR TDM and corticosteroid pharmacokinetics.
Nuestro objetivo fue evaluar la utilidad clínica de la monitorización de la concentración plasmática (TMD) de voriconazol (VOR) en un hospital universitario.
MétodosRevisión retrospectiva de las historias clínicas de 52 pacientes tratados con VOR en los que se realizó TDM. El intervalo terapéutico de la concentración plasmática de VOR fue definida entre 1μg/mL y 5.5μg/mL.
ResultadosLas condiciones subyacentes más frecuentes en la población de estudio fueron trasplante de pulmón (48,1%) y neoplasias hematológicas (26,9%). En la primera determinación de TMD de VOR estaban fuera del intervalo en 16 (30,7%) casos: <1μg/mL en 10 (19,2%) y>5,5μg/mL en 6 (11,5%). Once pacientes (21,2%) experimentaron debilidad muscular, éstos pacientes recibían tratamiento concomitante con corticosteroides. Los Factores asociados con bajos niveles de VOR observados fueron la edad menor a 30 años (p= 0,005) y la fibrosis quística (p=0,04). Casi todos los pacientes que tenían concentraciones VOR>1μg/mL en la primera TDM tuvieron un resultado satisfactorio (96%).
ConclusionesEn 30% (16/52) de los pacientes, las concentraciones plasmáticas de VOR estaban fuera del intervalo terapéutico en la primera TDM. La edad menor a 30 años y la fibrosis quística fueron factores asociados con niveles bajos de VOR. Observamos una posible interacción entre corticoesteroides y voriconazol con debilidad muscular asociada en los pacientes tratados con ambos fármacos. Se necesitan estudios clínicos prospectivos en relación a las interacciones entre corticoesteroides y voriconazol.
Voriconazole (VOR) is a broad-spectrum triazole antifungal that acts against a wide range of fungal pathogens, including Aspergillus and Candida spp., resulting in a first-line indication for the treatment of invasive aspergillosis.1 VOR is mainly metabolized via CYP2C19 and secondarily, via CYP2C9 and CYP3A4.2 Large inter and intraindividual variability has been observed in VOR plasma concentrations, regardless of the route of administration.2–6 Pharmacokinetic studies in volunteers and adult patients have shown that VOR exhibits variability and a nonlinear profile. This is likely the result of saturation of hepatic metabolism of the drug3,7 and other factors, such age, concomitant liver disease, interacting medications, and the ability to metabolize VOR via the CYP2C19 P450 enzyme because of polymorphisms in the gene encoding this enzyme.8,9
Various studies have suggested a relationship between VOR exposure and treatment efficacy and toxicity.10–14 Therapeutic drug monitoring (TDM) of VOR may lead to improved patient outcomes.2,11,15
In 2008, we implemented routine TDM of VOR as a complementary tool in the management of immunocompromised patients. The objective of this study was to determine the clinical usefulness of VOR TDM in daily clinical practice in a third-level hospital.
Materials and methodsA retrospective study was performed in all patients who underwent determination of VOR plasma levels during a prophylaxis regimen16,17 or treatment with this antifungal drug as part of their standard clinical care. At present, prophylaxis against invasive aspergillosis is not routinely recommended in all solid organ transplant recipients.17
In our cohort we have selected patients who received VOR as prophylaxis, because this patients are colonized by Aspergillus spp. and were at risk of invasive aspergillosis.
The study was performed in the Hospital Universitari Vall d’Hebron (Barcelona, Spain) from January 2008 to December 2010, is a 1100-bed teaching hospital, with more than 58,000 admissions/year and approximately 50 lung transplants are performed each year. The laboratory and pharmacy records were reviewed to identify patients for inclusion. In all patients, VOR was administered as a loading dose of 6mg/kg/twice a day (2 doses) by intravenous (i.v.) route, followed by 4mg/kg/twice daily (i.v.) thereafter, or as oral formulation (v.o.) 400mg twice daily (2 doses) followed by 200mg twice daily (v.o.), thereafter. Steady-state trough plasma levels of VOR were measured at least 5 days after the start of treatment, at 30min before the next dose. Blood samples drawn before achieving steady-state were excluded.
For the purposes of the study the therapeutic range of plasma VOR concentration was set at 1–5.5μg/mL in patients receiving antifungal treatment10,15 and 0.5–5.5μg/mL in patients receiving prophylaxis.18 The following data were extracted from the medical records: demographic information, underlying disease, VOR indication, posology, potentially interacting medications, VOR plasma concentrations, timing of dose relative to TDM, dose modifications performed, adverse events, and outcomes. Patients with cystic fibrosis as a cause for lung transplantation were classified as cystic fibrosis in the section on underlying disease (Table 1). In patients treated with tacrolimus, VOR dose was reduced by one-third the original dose before the drug was started, in accordance with the recommendations of the package insert. VOR TDM was performed to adjust doses according to published guidelines and the literature1,10,11 and at the discretion of the treating physician. Successive TDM was carried out after modifying dose, switching to oral route, when the infectious process showed a poor response, or drug toxicity was suspected. Invasive fungal infection was defined based on the criteria of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG)19 and the international society for lung and heart transplantation (ISHLT) consensus criteria for the lung transplant population. Colonization was defined as recovery of fungi in sputum or bronchoalveolar lavage or bronchoalveolar aspirated fluid without evidence of invasive fungal infection. Lack of response to VOR therapy was established by persistent fungal infection after 14 days of treatment or by progression of infection (based on clinical and radiological evidence or death due to fungal infection). Complete response was defined by resolution of all clinical signs and symptoms attributable to fungal infection, complete or very nearly complete (>50%) radiographic resolution, and negative culture results.
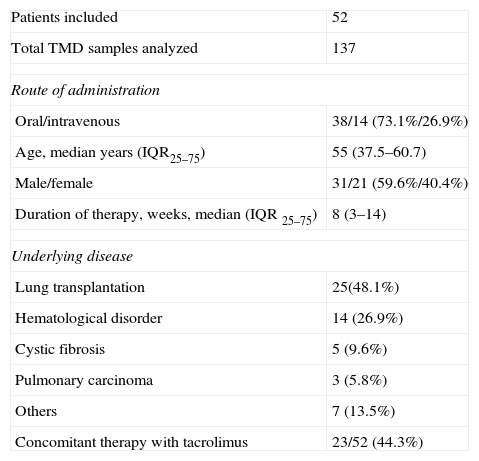
Demographic and clinical characteristics of 52 patients with voriconazole therapeutic drug monitoring.
| Patients included | 52 |
| Total TMD samples analyzed | 137 |
| Route of administration | |
| Oral/intravenous | 38/14 (73.1%/26.9%) |
| Age, median years (IQR25–75) | 55 (37.5–60.7) |
| Male/female | 31/21 (59.6%/40.4%) |
| Duration of therapy, weeks, median (IQR 25–75) | 8 (3–14) |
| Underlying disease | |
| Lung transplantation | 25(48.1%) |
| Hematological disorder | 14 (26.9%) |
| Cystic fibrosis | 5 (9.6%) |
| Pulmonary carcinoma | 3 (5.8%) |
| Others | 7 (13.5%) |
| Concomitant therapy with tacrolimus | 23/52 (44.3%) |
Abbreviations: TMD, therapeutic drug monitoring; IQR25–75, interquartile range.
Voriconazole assay: VOR concentrations were measured using a validated high-pressure liquid chromatography (HPLC) method, modified according to previously published data.6 Chromatography results were analyzed using Empower chromatography software (Waters, Milford, MA, USA). The limit of quantification, defined as the lowest amount of drug that could be quantified in a plasma sample with ±20% accuracy and precision, was 0.25μg/mL. The linear range was 0.25–20μg/mL. Data analysis: A descriptive analysis was performed. Dichotomous variables were compared using the chi-square or Fisher exact test. Continuous variables were expressed as the median and interquartile range (IQR) (25th–75th percentile). All percentages were calculated as the number over the number patients with available data. Univariate analysis was applied to identify factors leading to low VOR plasma concentrations. Data analysis was performed with SPSS Statistics 16 (IBM SPSS, Chicago, IL).
ResultsFifty-two patients were included (21 women and 31 men) with a median age of 55 years (IQR 35.7–60.7). The most frequent underlying condition was lung transplantation. Demographic data and underlying diseases are shown in Table 1. Among 52 patients who underwent VOR TDM, 47 (90.4%) received VOR as treatment for the following conditions: 40 (85.1%) for aspergillosis infection (tracheobronchitis 24, pulmonary aspergillosis 10, aspergilloma 2, disseminated aspergillosis 1, cutaneous aspergillosis 1, and 2 galactomannan-positive patients with hematological disease), 2 (4.3%) for fluconazole-resistant candidiasis, 1 (2.1%) for cryptococcal meningitis infection, and 4 (8.5%) for other fungal infections (cutaneous infection by Nattrassia mangiferae, cutaneous infection by Scedosporium spp., tracheobronchitis by Scopulariopsis spp.). The 5 (9.6%) remaining patients, who received VOR as prophylaxis, were colonized by Aspergillus spp. and were at risk of invasive aspergillosis. They included 1 patient with a hematological disorder, 2 with lung carcinoma undergoing chemotherapy, and 2 with pulmonary disease.
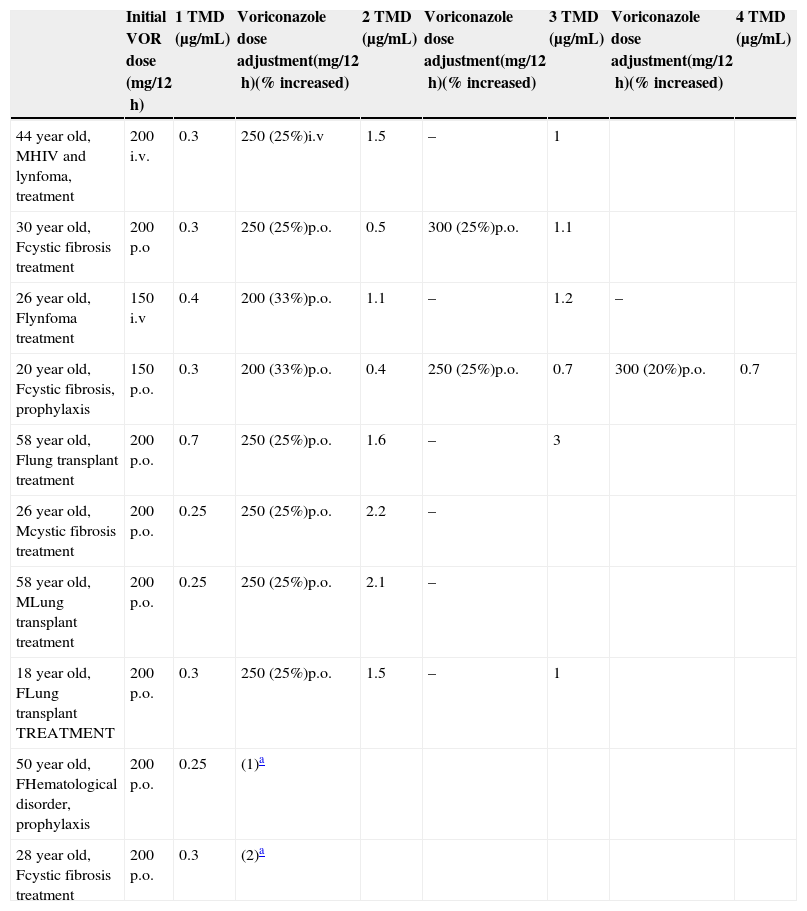
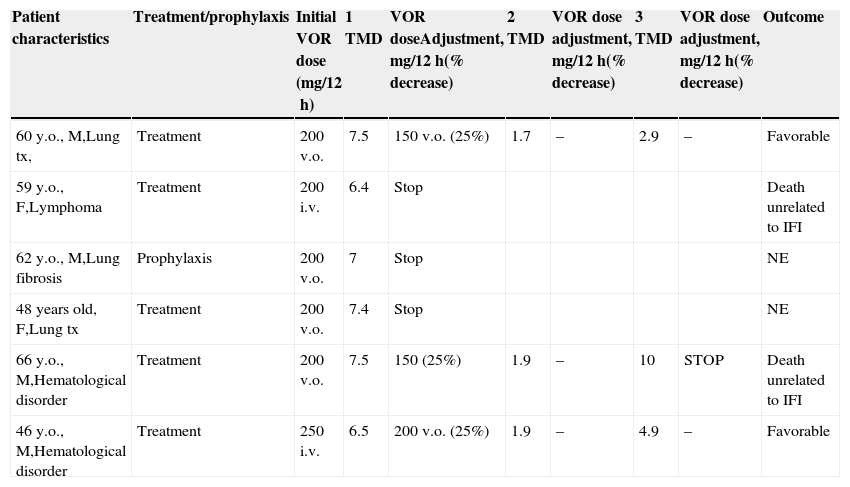
VOR was administered intravenously in 14 (26.9%) cases and orally in 38 (73.1%). Four patients received 2 antifungal drugs as initial therapy. The median duration of therapy was 8 weeks (IQR 3–14). The first trough levels were measured at a median of 6 days (IQR 5–15) after starting therapy. One hundred forty samples from the 52 patients, 2.7 samples per patient (IQR 2–3.75), were used for TDM. In the first TDM determination, 16 (30.7%) of the 52 patients presented VOR levels outside the desired range. In the 47 patients receiving VOR for treatment, TDM levels were <1μg/mL in 8 (17%) cases and >5.5μg/mL in 5 (10.6%) patients. In the 5 patients receiving VOR prophylaxis, VOR trough level was <0.5μg/mL in 2 cases and >5.5μg/mL in 1 patient. In 8 of the 10 patients with low levels, VOR dose was increased by 25% to 50% of the initial dose, achieving desired concentrations in all but one (Table 2). The median time to achieve desired plasma levels in these patients was 16 days (IQR 14–19). In the other 2 patients, VOR was discontinued; in one of them, because of progression of his hematological disease and in the other, VOR was changed to itraconazole. In the 6 patients with TMD VOR levels above the established range, the drug was temporarily suspended in 3 clinically asymptomatic patients and was later reintroduced with a 25% dose reduction, whereas in the 3 other patients, who had neurological symptoms suggesting toxicity, antifungal treatment was changed (Table 3).
Patients with voriconazole blood levels <1μg/mL in the steady-state.
| Initial VOR dose (mg/12h) | 1 TMD (μg/mL) | Voriconazole dose adjustment(mg/12h)(% increased) | 2 TMD (μg/mL) | Voriconazole dose adjustment(mg/12h)(% increased) | 3 TMD (μg/mL) | Voriconazole dose adjustment(mg/12h)(% increased) | 4 TMD (μg/mL) | |
|---|---|---|---|---|---|---|---|---|
| 44 year old, MHIV and lynfoma, treatment | 200 i.v. | 0.3 | 250 (25%)i.v | 1.5 | – | 1 | ||
| 30 year old, Fcystic fibrosis treatment | 200 p.o | 0.3 | 250 (25%)p.o. | 0.5 | 300 (25%)p.o. | 1.1 | ||
| 26 year old, Flynfoma treatment | 150 i.v | 0.4 | 200 (33%)p.o. | 1.1 | – | 1.2 | – | |
| 20 year old, Fcystic fibrosis, prophylaxis | 150 p.o. | 0.3 | 200 (33%)p.o. | 0.4 | 250 (25%)p.o. | 0.7 | 300 (20%)p.o. | 0.7 |
| 58 year old, Flung transplant treatment | 200 p.o. | 0.7 | 250 (25%)p.o. | 1.6 | – | 3 | ||
| 26 year old, Mcystic fibrosis treatment | 200 p.o. | 0.25 | 250 (25%)p.o. | 2.2 | – | |||
| 58 year old, MLung transplant treatment | 200 p.o. | 0.25 | 250 (25%)p.o. | 2.1 | – | |||
| 18 year old, FLung transplant TREATMENT | 200 p.o. | 0.3 | 250 (25%)p.o. | 1.5 | – | 1 | ||
| 50 year old, FHematological disorder, prophylaxis | 200 p.o. | 0.25 | (1)a | |||||
| 28 year old, Fcystic fibrosis treatment | 200 p.o. | 0.3 | (2)a |
Abbreviations: TMD, therapeutic drug monitoring; VOR, voriconazole; F, female; M, male; i.v. intravenous; p.o., orally.
Patients with voriconazole blood levels >5.5μg/ml in the steady-state.
| Patient characteristics | Treatment/prophylaxis | Initial VOR dose (mg/12h) | 1 TMD | VOR doseAdjustment, mg/12h(% decrease) | 2 TMD | VOR dose adjustment, mg/12h(% decrease) | 3 TMD | VOR dose adjustment, mg/12h(% decrease) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 60 y.o., M,Lung tx, | Treatment | 200 v.o. | 7.5 | 150 v.o. (25%) | 1.7 | – | 2.9 | – | Favorable |
| 59 y.o., F,Lymphoma | Treatment | 200 i.v. | 6.4 | Stop | Death unrelated to IFI | ||||
| 62 y.o., M,Lung fibrosis | Prophylaxis | 200 v.o. | 7 | Stop | NE | ||||
| 48 years old, F,Lung tx | Treatment | 200 v.o. | 7.4 | Stop | NE | ||||
| 66 y.o., M,Hematological disorder | Treatment | 200 v.o. | 7.5 | 150 (25%) | 1.9 | – | 10 | STOP | Death unrelated to IFI |
| 46 y.o., M,Hematological disorder | Treatment | 250 i.v. | 6.5 | 200 v.o. (25%) | 1.9 | – | 4.9 | – | Favorable |
Abbreviations: TMD, therapeutic drug monitoring; VOR, voriconazole; F, female; M, male; i.v. intravenous; p.o., orally.
NE: non evaluable; tx, transplantament.
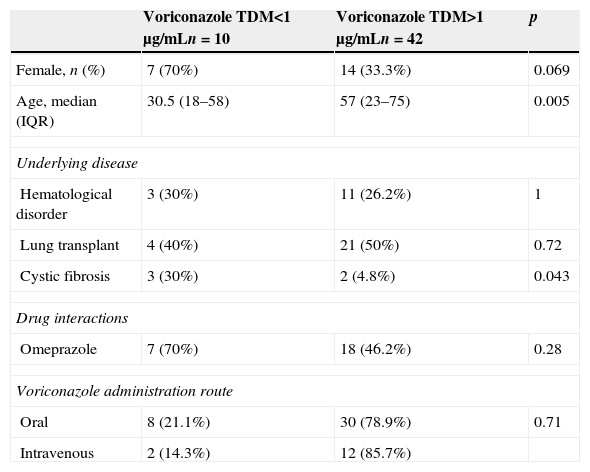
Regarding adverse events, complex visual hallucinations occurred in 4 (7.7%) patients, 3 of whom showed supratherapeutic levels. All patients completely recovered after discontinuation of VOR. Eleven patients (21.2%) experienced severe muscle weakness and had considerable difficulty walking. All these patients were lung transplant recipients and were receiving concomitant treatment with corticosteroids (median prednisone dose, 13.5mg/day; IQR 7.5–20). Seven out of 52 (13.4%) patients presented visual disturbances as photopsias, which resolved spontaneously. In 4 patients (7.7%) transaminases increased (<10 normal value); hepatic impairment normalized after drug withdrawal. Occurrence of these complications was unrelated to levels outside the therapeutic range. In 13 of 23 patients receiving tacrolimus, plasma levels of this immunosuppressant drug were higher than 15ng/mL (therapeutic range, 5–15ng/mL). Five of these patients showed creatinine increases up to 5mg/dL. On analysis of factors that could contribute to VOR concentrations falling outside the therapeutic range, we found that median age was 30.5 years in patients with low VOR levels (<1μg/mL) and 57 years in those with levels of >1μg/mL. Furthermore, 3 of 5 patients with cystic fibrosis showed subtherapeutic VOR concentrations. Univariate analysis identified age <30 years (p=0.005) and cystic fibrosis (p=0.04) as factors associated with low VOR concentrations (<1μg/mL) (Table 4). Of the 47 patients that received VOR as treatment, 35 were evaluated in relation to outcome. Six of the 8 patients in whom VOR TDM level was <1μg/mL were evaluated: in 4 (66%) cases, therapy outcome was successful, whereas the remaining 2 (33.3%) patients showed progression of fungal disease. In the 34 patients in whom VOR TDM level was 1–5.5μg/mL, 25 were evaluated: therapy was successful in 24 (96%) patients and 1 (4%) case presented disease progression. In the 5 patients with an initial TDM >5.5μg/mL, 4 were evaluated, and all had a successful outcome. In the remaining 12 patients, outcome was not evaluated because 5 presented progression of the underlying disease, the physicians decided to change the antifungal drug in 5 other cases, and antifungal treatment was stopped in 2 patients.
Univariate analysis of associated factor of voriconazole TMD <1μg/mL.
| Voriconazole TDM<1μg/mLn=10 | Voriconazole TDM>1μg/mLn=42 | p | |
|---|---|---|---|
| Female, n (%) | 7 (70%) | 14 (33.3%) | 0.069 |
| Age, median (IQR) | 30.5 (18–58) | 57 (23–75) | 0.005 |
| Underlying disease | |||
| Hematological disorder | 3 (30%) | 11 (26.2%) | 1 |
| Lung transplant | 4 (40%) | 21 (50%) | 0.72 |
| Cystic fibrosis | 3 (30%) | 2 (4.8%) | 0.043 |
| Drug interactions | |||
| Omeprazole | 7 (70%) | 18 (46.2%) | 0.28 |
| Voriconazole administration route | |||
| Oral | 8 (21.1%) | 30 (78.9%) | 0.71 |
| Intravenous | 2 (14.3%) | 12 (85.7%) | |
Abbreviations: TMD, therapeutic drug monitoring; VOR, voriconazole; IQR, interquartile range.
Thirty percent (16 of 52) of our patients presented VOR levels outside the therapeutic range, even though analyses were performed at steady state. These results, which are similar to those of previous studies20–23 confirm the high variability of VOR metabolism and the difficulty of predicting which patients will present the recommended concentrations. We found that cystic fibrosis (p=0.04) and age (p=0.005) were associated with low VOR levels (<1μg/mL). Cystic fibrosis is usually associated with considerable changes in drug pharmacokinetics. Patients generally have a larger volume of distribution for many drugs because of their lower fat stores and increased ratio of lean body mass to total body mass, as compared to those without this condition. Consequently, larger doses of antibacterial agents, for example, must be given to attain the same serum concentration as in healthy individuals.24 In a cohort of 35 cystic fibrosis lung transplant recipients, Berge et al.,22 reported the 30% of patients had low plasma VOR concentrations (<0.5mg/L). In a study published by Thomas et al.25 in 15 lung transplant recipients with cystic fibrosis, a median of 12 days was required for VOR to reach therapeutic blood levels. Mitsani et al. 21 reported that age has a considerable impact on VOR levels: older patients (≥60 years of age) were significantly more likely to have initial plasma levels >1.5μg/mL. Previous studies have found an apparent association between VOR concentration and clinical efficacy.10,11,13,15,20,22 Troke et al.26 analyzed data from nine clinical trials, focussing on the clinical efficacy of VOR and its relationship with plasma concentrations. With 825 patients included, the maximum clinical response rate (74%) was achieved at a mean plasma concentration of 3.0–4.0μg/mL. In a recent pharmacokinetic analysis of 505 plasma samples in 55 patients with invasive mycoses receiving recommended VOR doses, Pascual et al. proposed changing the initial VOR doses (300–400mg oral or 200–300mg i.v. BID) to achieve a concentration range of 1.5–4.5mg/L, which was identified as having the best efficacy-safety profile (>85% probability of response and <15% probability of neurotoxicity).25 According to latest recommendations25–27 considering 2μg/mL as the lower therapeutic limit of plasma VOR, 44.7% of our patient cohort (21 of 47, treatment group) had levels below 2μg/mL in the first TDM.
VOR treatment was successful in 32 of the 35 cases evaluated for outcome in our study, 4/6 (66%) with TDM <1μg/mL and 24/25 (96%) with TDM in the range of 1–5.5μg/mL. The sample and above the low number of evaluable patients in the TMD group <1μg/mL not allow us to draw statistically significant conclusions at this point, but the differences observed in our cohort between both groups are consistent with previously published studies, as suggested by other authors10,11,15,20,22,25–27 the patients with TDM >1μg/mL have a higher probability of response.
In our cohort, around 20% of patients receiving VOR experienced muscle weakness, a large percentage considering that there is only one reported case of this complication in the literature.28 In the affected patients, all of whom were lung transplant recipients, muscle weakness was not associated with supratherapeutic VOR levels, and all cases showed slow progressive improvement after withdrawal of VOR and decreasing corticosteroid dose. In a study performed in the healthy population, itraconazole was reported to considerably increase the plasma concentrations and effects of oral methylprednisolone.29 We believe that steroids likely play an important role in the pathogenesis of this adverse effect. Thus, it may be advisable to lower the steroid dose when VOR is used concomitantly. Thirteen of 23 (56%) patients had supratherapeutic tacrolimus levels, which led to acute renal failure in 5 cases. Therefore, close monitoring of both drugs should be performed. Although combined antifungal treatment is not routinely recommended as first-line therapy in aspergillosis,30 such treatment could be justified to provide coverage against Aspergillus infection until therapeutic drug levels of VOR are achieved. This could be particularly important in patients who present invasive fungal disease and those in whom subtherapeutic levels occur more often (e.g., cystic fibrosis). In our cohort 4 patients receiving combined therapy until therapeutic drug levels of VOR were achieved. Our study has the limitations of a retrospective design and a relatively small number of patients. Nonetheless, the information obtained in this detailed description of daily clinical practice can be of value to clinicians. In conclusion, in 30% (16 out of 52) of patients VOR concentration were outside the therapeutic range in the first TDM. Patients younger than 30 years old and cystic fibrosis was associated with low VOR levels of, therefore the VOR TDM is a useful tool for the management of these patients. Finally, we would like to underline the potential interactions between corticosteroids and VOR, which could be responsible of a high rate of muscle weakness observed in our patients. Prospective trials are needed to investigate VOR TDM and corticosteroid pharmacokinetics.
Conflict of interestThe authors declare no conflict of interest.