Burkholderia cepacia complex have emerged as significant pathogens in cystic fibrosis (CF) patients due to the risk of cepacia syndrome and the innate multi-resistance of the microorganisms to antibiotics. The aim of this study was to describe the antimicrobial susceptibility profiles, the genotypes and subtypes of BCC, and the clinical evolution of CF patients with BCC.
MethodsThe lung function and Brasfield and Shwachman score were assessed in 12 patients. BCC were identified and susceptibility was studied by MicroScan (Siemens). Species and genospecies of BCC were confirmed by molecular methods in a Reference Centre (Majadahonda).
ResultsBCC were identified in 12 of 70 patients (17.1%) over a ten year period. The mean age to colonization by BCC was 24.4 years (SD: 7.71). B. cenocepacia was isolated in 4 patients (33.3%), B. contaminans was isolated in 3 patients (25%), both B. vietnamiensis and B. stabilis were isolated in 2 patients (16.7%), and B. cepacia, B. multivorans and B. late were isolated in one patient (8.3%). Among the B. cenocepacia, subtype IIIa was identified in two strains, and subtype IIIb was identified in the other two strains. There was susceptibility to meropenem in 90% of BCC, 80% to cotrimoxazole, 60% to minocycline, 50% to ceftazidime, and 40% to levofloxacin.
ConclusionsB. cenocepacia was the most prevalent species among the BCC isolated in CF adult patients, and subtypes IIIa and IIIb were identified in the 50% of the strains. Meropenem and cotrimoxazole showed the best activity.
Burkholderia cepacia complex (BCC) es un patógeno emergente significativo en pacientes con fibrosis quística (FQ), debido al riesgo de síndrome cepacia y debido a su multirresistencia a los antimicrobianos. El objetivo de este estudio fue describir la sensibilidad antimicrobiana, los genotipos y subtipos de BCC, y estudiar la evolución clínica de los pacientes con FQ.
MétodosSe estudió la función pulmonar de 12 pacientes, y la puntuación Brasfield y Shwachman. BCC fue identificado y se estudió la sensibilidad mediante MicroScan (Siemens). Las especies y genoespecies de BCC se confirmaron mediante métodos moleculares en un centro de referencia (Majadahonda).
ResultadosBCC se identificó en 12 de 70 pacientes (17,1%) durante 10 años. La edad promedio de colonización por BCC fue de 24,4 años (DE: 7,71). B. cenocepacia se aisló en 4 pacientes (33,3%), B. contaminans se aisló en 3 pacientes (25%), B. vietnamiensis y B. stabilis se aisló cada una en 2 pacientes (16,7%), y B. cepacia, B. multivorans y B. late se aisló en un paciente (8,3%) cada una. Entre las cepas de B. cenocepacia, el subtipo IIIa se identificó en 2 cepas y el subtipo IIIb se identificó en otras 2. El 90% de las cepas de BCC fueron sensibles al meropenem, el 80% al cotrimoxazol, el 60% a la minociclina, el 50% a la ceftazidima y el 40% al levofloxacino.
ConclusionesB. cenocepacia fue la especie más prevalente entre los aislados de BCC en pacientes adultos, y los subtipos IIIa y IIIb se identificaron en el 50%. El meropenem y el cotrimoxazol fueron los antibióticos más activos.
Burkholderia cepacia complex (BCC) has emerged as significant pathogens in cystic fibrosis (CF) patients due to the risk of cepacia syndrome (a fatal necrotizing pneumonia with bacteremia), the organism's innate multi-resistance to antibiotics, and the transmissibility of bacterial strains between patients by social contact.1 Close and prolonged contact among CF patients and sharing nebulizers facilitates BCC acquisition and transmission.2,3 It is proven that BCC bacteria acquisition is associated to hospitalization and cross-infection by social contact CF.
The taxonomy of the genus Burkholderia has undergone several major revisions over the last decades. In the mid-1990s, “B. cepacia” strains were shown to belong to at least five different species, which were collectively referred to as the B. cepacia complex.4 Further taxonomic analyses revealed that even more species were present within the BCC and currently 17 B. cepacia complex species have been described4–7: B. cepacia, B. multivorans, B. cenocepacia, B. stabilis, B. vietanmiensis, B. dolosa, B. ambifaria, B. anthina, B. pyrrocinia, B. ubonensis, B. latens, B. diffusa, B. arboris, B. seminalis, B. metallica, B. lata and B. contaminans.8
Except for B. ubonensis, all of these species have been isolated from sputum of CF patients,6,7 with B. cenocepacia and B. multivorans as predominant.9
B. cenocepacia is subdivided in 4 subtypes (IIIa, IIIb, IIIc and IIId), that are encoded by four alleles of the gen recA and there are differences in the frequency and virulence of the strains. Furthermore, B. cenocepacia is considered to be one of the most serious pathogens because it is frequently associated with reduced survival and highest risk of developing fatal cepacia syndrome.10 BCC species are intrinsically resistant to many antibiotics such as aminoglycosides and polymyxin B and often require combination therapy to suppress infection in CF.11
Infections with BCC bacteria in CF patients are often correlated with increased morbidity and mortality, and the innate resistance of these organisms to a broad range of antibiotics complicates the treatment of infected patients.12,13 This resistance is caused by various mechanisms, including limited permeability, changes in lipopolysaccharide structure and the presence of several multidrug efflux pumps, inducible chromosomal beta-lactamases and altered penicillin-binding proteins. In addition, in vitro biofilm formation has been described for multiple B. cepacia complex strains and this may contribute to their ability to survive in the CF lung environment by providing additional protection against antibiotics.14–16
Treatment of BCC infected patients should be based preferably on the results of susceptibility tests and often include combination therapy with two or three antibiotics showing synergistic activity.12,17,18In vitro susceptibility studies on BCC strains show that breakpoint concentrations of ceftazidime, ciprofloxacin, meropenem, tetracyclines or high doses of tobramycin have a bacteriostatic activity against a considerable fraction of these strains.19–21 Consequently, these antibiotics are often used to treat BCC infected CF patients. In addition cotrimoxazol is still frequently used in the treatment of chronic BCC infections, although susceptibility testing of these complementary antibiotics revealed a poor activity against many BCC strains.18,22
The goal of this study was to assess the isolated and the susceptibility of BCC and to analyze the clinic repercussions.
MethodsThe sputum of patients with CF were analyzed to final BCC isolates, at the adult CF Unit in the Hospital La Princesa that has been operating since March 1997. These samples were processed in microbiology department in the standard procedure23; we used specific selective medium and quantitive streak, using the conventional procedure of serial dilution for the sample. The sputum underwent on a process of homogenization with N-acetylcystein before culturing. In our laboratory, the culture mediums used were: blood agar, bacitracin chocolate agar, manitol-salt agar, MacConkey agar, Sabouraud cloranfenicol agar and selective medium for B. cepacia called BCSA (Biomèrieux). The incubation time for plates was from 3 to 5 days at 35°C. Bacitracin chocolate agar was incubated in CO2 atmosphere.
The preliminary identification of the BCC strains was performed by MicroScan (Siemens) and Api 20 NE (Biomerieux). These procedures were performed according to the manufacturer's recommendations.24
Subsequently, the strains were remitted at Centro Nacional de Microbiología (Majadahonda, Madrid) for the confirmation and determination of the specie and genospecie. For this study, the following methods were carried out: Api 20 NE (biomerieux, Marcy l’Etoile) and GN2 Microplate (BIOLOG, Hayward, CA) and molecular methods. All procedures were performed according to the manufacturer's recommendations.
Chromosomal DNA extractionDNA extraction was performed with the commercial kit QIAamp DNA Mini Kit (QIAGEN, GmbH, Hilden, Germany) following the manufacturer's instructions.
PCR analysisAmplification of genes was performed in a final volume of 25μl, using the kit PuReTaq Ready-To-Go PCR Beads (Amersham Biosciences, Buckinghamshire, UK), and containing 5μl of extracted DNA and 10pmol of each primer: fD1 and rP225 for 16S rDNA, and BCR1 and BCR426 for recA. Thermal cycling was carried out in a TaKaRa PCR Thermal Cycler v. III mod TP600 (TAKARA BIO Inc., Otsu, Shiga) under the following conditions for 16S rDNA: 94°C for 5min for the first cycle, 35 cycles of 15s at 94°C, annealing for 15s at 55°C, and extension at 72°C for 1min and 50s. Conditions for amplification of recA were as follows: 94°C for 5min, 30 cycles of 30s at 94°C, annealing for 45s at 55°C, and extension at 72°C for 10min.
We visualized 2μl of each PCR product by agarose gel electrophoresis with agarose concentration adjusted at 1.5% and using 1× TAE buffer. Molecular size markers were included on all gels: Marker X 0.07–12.2kbp (Roche Applied Sciences, Mannheim, Germany) and GeneRuler 100bp DNA Ladder (Fermentas GmbH, St. Leon-Rot, Germany) for 16s rDNA and recA products, respectively.
Strains identified as B. cenocepacia were subjected to PCR method with specific primers for RecA-IIIA group (BCRG3A1 and BCRG3A2) and RecA-IIIB (BCRG3B1 and BCRG3B2) under conditions previously described.24
Ten μl of PCR products were visualized by agarose gel electrophoresis at the same conditions described above.
Nucleotide sequence analysis16s rDNA and recA PCR products were sequenced using fD1 and rP2 and BCR1 as primers, respectively. Sequencing reactions were prepared by means of Big Dye Terminator v 3.1 (Applied Biosystem, USA) in a final volume of 10μl in according with the manufacturer's instructions, and analyzed with ABI PRISM 3100 genetic analyzer capillary electrophoresis system (Applied Biosystem, USA). Sequences were assembled using SeqMan 3.61 software (DNA Star, Inc, Madison, WI, USA). Analysis also involved the use of Basic Local Alignment Search Tool (BLAST: www.ncbi.nlm.nih.gov) to establish the correct gene identity.
PFGE analysisStrains relationship was analyzed by pulsed-field gel electrophoresis (PFGE). Plugs preparation, lysis, cell washing and restriction digestion were performed as described previously25,26 with slight differences. The restriction enzyme XbaI (40U, Fermentas GmbH, St. Leon-Rot, Germany) was used. PFGE was performed following the protocol described for Stenotrophomonas maltophilia by Valdezate et al.,27 and using DRIII Chef System (Bio-Rad Laboratories, Hercules, USA) and lambda phage concatemers (Biolabs, New England, UK) as molecular weight marker. Images were obtained with Quantity One v. 4.6.1 software (BioRad). Images analysis were performed visually and isolates were regarded as genotypically indistinguishable if they had an identical banding pattern.
Antibiotic susceptibility was performed by microdilution with MicroScan and disk diffusion simultaneously. Both methods were considered as CLSI breakpoints.28 For ciprofloxacin and imipenem, the breakpoints of levofloxacin and meropenem were used, respectively. The following antibiotics were studied: ceftazidime, ciprofloxacin, levofloxacin, cotrimoxazol, minocycline, imipenem and meropenem.
Patients with BCC were studied with followings variables: age (at the beginning and currently), sex, weight (at the beginning and currently), gen mutation Cystic Fibrosis Transmembrana Regulator, evolution of respiratory function that was determined by the percentage according to theoretical value of the volume breathing out in the first second (FEV1) from their first isolation as well as the radiological punctuations of Brasfield and clinics of Shawchman at the beginning of the BCC isolation and current.
Co-colonization with other microorganisms was assessed.
The Brasfield score was evaluated with 0–5 (from low to high) according to radiological signs: air entrapment, linear shadows, nodular cystic lesions, segmental or lobar consolidation and the overall impression of the severity. The global punctuation obtained was subtracted to 25. The lowest value achieved corresponded to more severe radiology. The chest radiology and its Brasfield score correspondent were conducted each year.
Clinical score Shwachman evaluated 4 items with maximum punctuation of 25 each: general activity, physical examination, growth and nutrition and chest-X-ray. The ideal punctuation was 100 and the status of the patients was classified according to the punctuation: excellent (86–100 points), good (71–85 points), slight (56–70 points), moderate (40–55 points) or serious (less than or equal to 40).
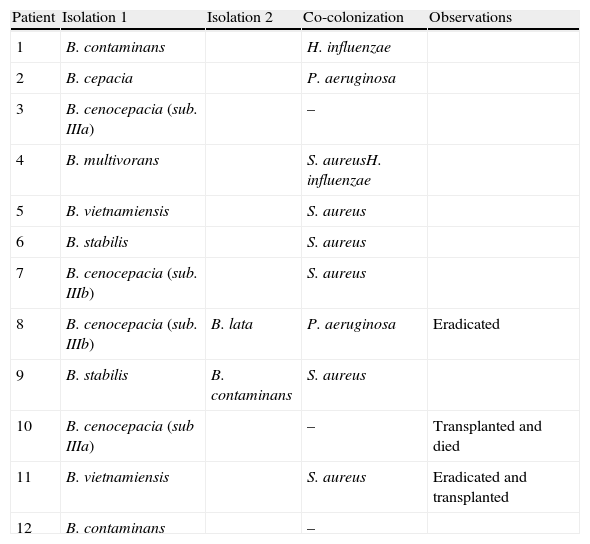
ResultsBCC was isolated in 12 of 70 adult CF patients (17.1%) during 10 years. Two of the patients had a lung transplant, one of them died after the transplant and the other, BCC was eradicated in 2005 before the transplant that was in 2011. BCC was eradicated in other patient in 2009. These patients were studied only for a few months, and so clinic evolution was not registered. B. cenocepacia was isolated in 4 patients (33.3%), B. contaminans in 3 patients (25%), B. stabilis in 2 patients (16.7%), B. vietnamiensis in 2 patients (16.7%), B. cepacia in one patient (8.3%), B. multivorans in one patient (8.3%) and B. lata in one patient (8.3%). Among B. cenocepacia, the subtype IIIa was identified in two patients out of 4 (50%), and subtype IIIb in the other two patients (50%). One patient had at first B. cenocepacia and then had a B. lata. Similarly, other patient had B. stabilis and then had B. contaminans. In our study, 50% of patients with BCC had Staphylococcus aureus strains (Table 1).
Isolations of the various Burkholderia cepacia complex species and co-colonization with other CF-pathogens in patients chronically.
| Patient | Isolation 1 | Isolation 2 | Co-colonization | Observations |
| 1 | B. contaminans | H. influenzae | ||
| 2 | B. cepacia | P. aeruginosa | ||
| 3 | B. cenocepacia (sub. IIIa) | – | ||
| 4 | B. multivorans | S. aureusH. influenzae | ||
| 5 | B. vietnamiensis | S. aureus | ||
| 6 | B. stabilis | S. aureus | ||
| 7 | B. cenocepacia (sub. IIIb) | S. aureus | ||
| 8 | B. cenocepacia (sub. IIIb) | B. lata | P. aeruginosa | Eradicated |
| 9 | B. stabilis | B. contaminans | S. aureus | |
| 10 | B. cenocepacia (sub IIIa) | – | Transplanted and died | |
| 11 | B. vietnamiensis | S. aureus | Eradicated and transplanted | |
| 12 | B. contaminans | – |
PFGE analysis shows that the same strain was isolated in each CF patient but it was different between patients, so this certifies that there was no cross transmission.
90% of BCC were sensible to meropenem, 80% to cotrimoxazol, 60% to minocycline, 50% to ceftazidime and 40% to levofloxacin, 20% to ciprofloxacin, and 10% to imipenem.
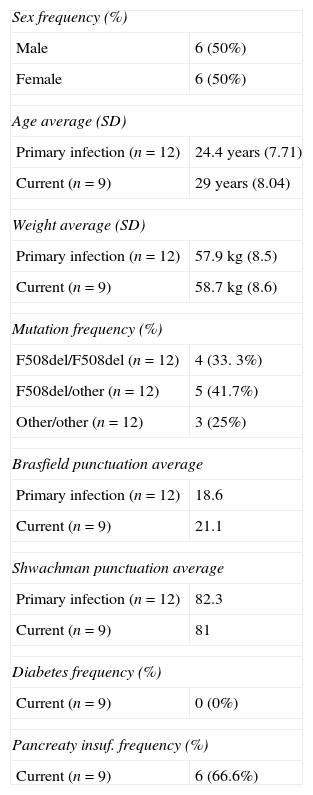
The 50% of the CF patients were male and the average age that these patients had their first isolation of BCC was 24.4 (SD: 7.71). The 41.7% of the patients had F508del/other mutation, 33.3% had F508del/F508 mutation and 25% had other/other mutation.
At the beginning Brasfield and Shwachman score was calculated for all patients included in this study, and the average punctuation was 18.6 and 82.3. However, the current Brasfield and Shwachman score is conducted with patients colonized. The average punctuation was 21.1 and 81. Only 1 patient had diabetes and 6 patients had pancreatic insufficiency. Table 2 shows the clinical characteristics of the CF patients who had BCC.
Clinical characteristics of cystic fibrosis patients.
| Sex frequency (%) | |
| Male | 6 (50%) |
| Female | 6 (50%) |
| Age average (SD) | |
| Primary infection (n=12) | 24.4 years (7.71) |
| Current (n=9) | 29 years (8.04) |
| Weight average (SD) | |
| Primary infection (n=12) | 57.9kg (8.5) |
| Current (n=9) | 58.7kg (8.6) |
| Mutation frequency (%) | |
| F508del/F508del (n=12) | 4 (33. 3%) |
| F508del/other (n=12) | 5 (41.7%) |
| Other/other (n=12) | 3 (25%) |
| Brasfield punctuation average | |
| Primary infection (n=12) | 18.6 |
| Current (n=9) | 21.1 |
| Shwachman punctuation average | |
| Primary infection (n=12) | 82.3 |
| Current (n=9) | 81 |
| Diabetes frequency (%) | |
| Current (n=9) | 0 (0%) |
| Pancreaty insuf. frequency (%) | |
| Current (n=9) | 6 (66.6%) |
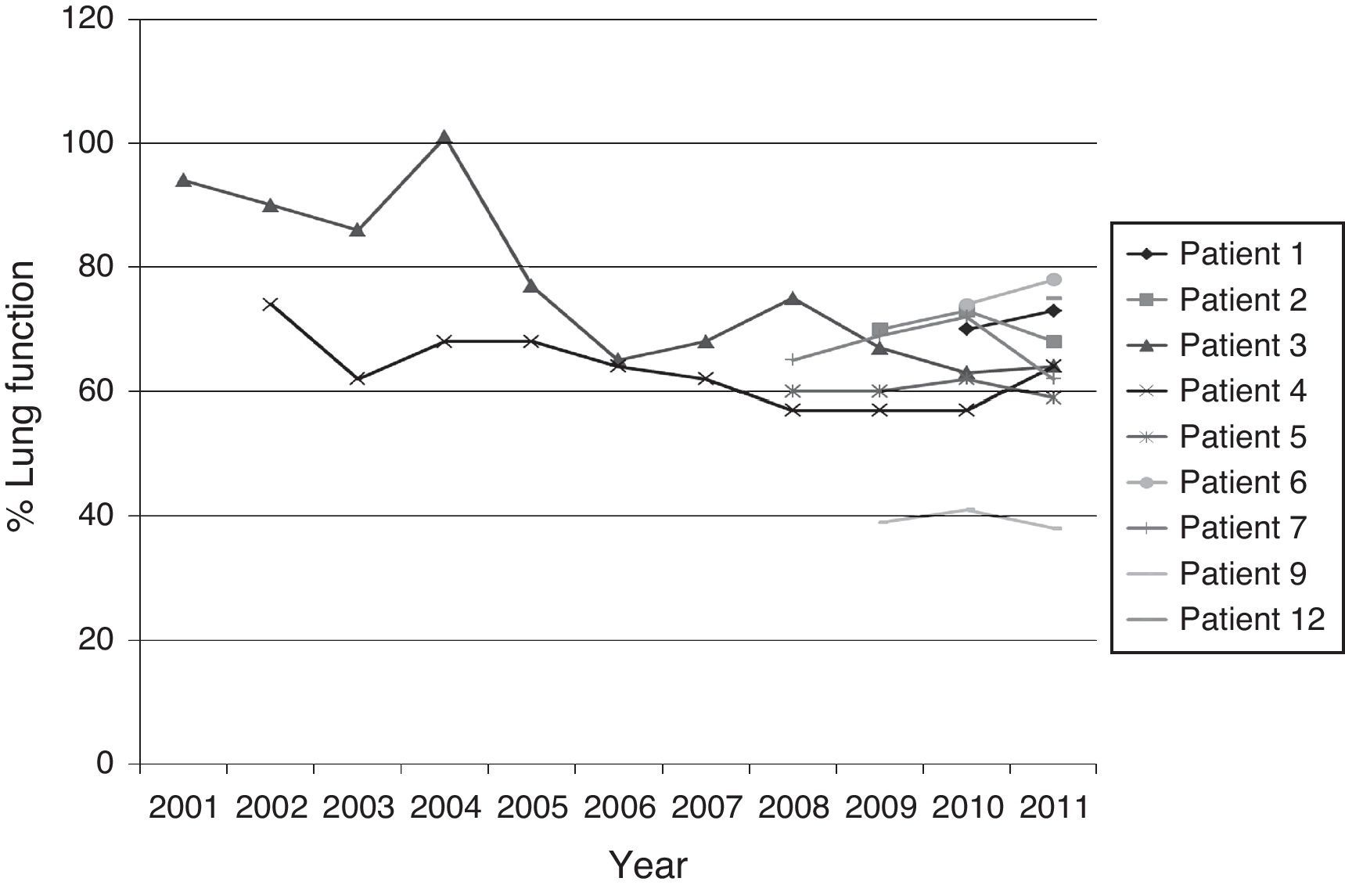
Fig. 1 shows the evolution of lung function (%FEV1) of the patients who had more than an isolation of BCC.
DiscussionThere are few studies related to clinical evolution of patients with an isolated species of BCC, and there is a general idea that the B. cenocepacia species is associated to greater morbidity and mortality in CF patients.19 Therefore, there are not many recent data about patients with BCC in Spain. A study in Hospital Universitario de Cruces shows an increase of colonization incidence with BCC.29
It is important that identification of BCC species is carried out in specialized center, because facultatives help to supervise a close monitoring of these patients who are colonized by these species, as they can evolve adversely.
A study performed by Van Pelt et al.30 shows that API 20 NE was more accurate than MicroScan. 90% of isolates were correctly identified using API 20NE versus 68% using MicroScan. They suggest the use of BCSA plates for the initial isolation of B. cepacia directly from clinical material. The sensitivity of these growth media appeared to be excellent (96%); the specificity was not 100%. It was quite striking to find that for the automated assays, as MicroScan, the accuracy was insufficient. The major outcome of the present analysis is the fact that molecular identification by PCR-RFLP analysis is superior to the biochemical and microbiological species identification procedures, although it should be emphasized that results obtained with API20 NE were satisfactory.30
In our study, the B. cenocepacia, B. contaminans, B. vietnamiensis, B. stabilis, B. multivorans, B. cepacia and B. lata were isolated from the respiratory secretions of 12 CF patients examined and B. cenocepacia was the most prevalent species like most of data described in European literature of CF patients.19 However, this discovery does not agree with a Portugal study (Susana Correia et cols), in which the most frequent species were B. cepacia 57% and B. stabilis 13%. It is maybe due to using non-sterile saline solutions intrinsically contaminated by B. cepacia.19 These contaminated solutions were detected by Infarmed during a routine microbiological inspection.19 In our study B. cepacia and B. stabilis were isolated in lower proportion like other studies in Europa and America.19
Among B. cenocepacia isolation, there was the same proportion between B. cenocepacia subtypes IIIa and IIIb.
One of the patients had B. cenocepacia as first isolation, B. lata as second isolation and then was eradicated. Another patient had B. vietnamiensis in 2004 that was eradicated, and lung transplantation was done in him in 2011, and another patient was isolated with B. cenocepacia subtype IIIa in 2004, but he died in the same year after a lung transplant. The survival results of lung transplant are worst in patients who are colonized with B. cenocepacia, so some units of transplant contraindicate the transplant in that case. It has been recognized that B. cenocepacia carries a worse prognosis compared with B. multivorans with shorter survival when matched with Pseudomonas aeruginosa controls.31 Cepacia syndrome has been reported with both of these species.26 The exact pathophysiology of this syndrome is poorly understood, and the precise mortality rate in not known, although it is thought to approach 100%.32
Our patients have low FEV1 values. A great number of patients had already deteriorated prior to BCC isolation as a result of colonization by other pathogenic agents and disease progression. In clinically stable patients no significant alterations were seen in FEV1 values and nutritional state. There is evidence that lung colonization might not be detected by standard cultures for some time (up to 2 years) after BCC acquisition.33 In addition, it is not clear if, in cases of intermittent isolation, there is a re-infection by a new strain or if there is a recrudescence of the initial strain.20 In the majority of cases in our different isolation, no replacement of the initial BCC strains was seen.
Cases were registered in which the same strain (with the same genotype), which persistently infected a patient over several years, was eradicated by antibiotic therapy or had no apparent impact on the clinical picture of other patients. It is not clear why strains of the different BCC species differ in their persistence, epidemiology and pathogen potential in CF and why the same strains can be associated to very different clinical evolutions.19 It depends on factors inherent in each individual patient, on co-colonization by other pathogens and other factors still to be identified, stressing the importance of undertaking studies of this type.19
Antibiotic resistance is considered an important virulence factor of BCC organisms.14 Although therapy is usually guided by antimicrobial susceptibility testing, eradication of BCC organisms is rarely achieved.21 Multiple hypotheses have been formulated to explain this failure, including inadequate antibiotic concentrations or inactivation of the antibiotic in sputum, impaired host defenses in CF patients, biofilm formation, “inoculum” effect and in vivo growth rate of these organisms.34
Elke et al. reported in a Belgium study that meropenem, minocycline and ceftazidime were the most active antibiotics against BCC isolation and ciprofloxazin and trimethoprim/sulfamethoxazole had the lowest activity.35 However, our study found that meropenem was the most active with 90% of susceptibility, followed by trimethoprim/sulfamethoxazole, minocycline and ceftazidime with 80%, 60% and 50%, respectively. Although BCC organisms are typically resistant against aminoglycosides, high doses of tobramycin inhibited the majority of tested strains. Nebulized tobramycin yielding high peak concentrations in sputum is increasingly used for treating CF patients.36–38
Consequently, these higher concentrations should be taken into account when evaluating the usefulness of this antibiotic.35 Several reports confirm that nebulized tobramycin shows great promise in the treatment of BCC-infected CF patients: for example, Weidmann et al.39 recently described the complete eradication of BCC organisms from the lungs of CF patients by using a combination of nebulized tobramycin and amiloride. In addition, a combination therapy with nebulized and intravenous meropenem and tobramycin also resulted in the successful treatment of a female CF patient suffering from cepacia syndrome, although the sputum samples of the latter patient remained positive for B. cenocepacia.39
BCC species are intrinsically resistant to many antibiotics such as aminoglycosides and polymyxin B and often require combination therapy to suppress infection in CF.11 The antibiotics polymyxin, gentamicin and vancomycin are used at high concentrations in B. cepacia Selective Agar, a highly effective medium for their isolation from CF sputum.11 Nzula et al.13 compared the antibiotic susceptibility of six BCC species and concluded that it was highly variable except for innate polymyxin resistance and not linked to the taxonomic status of the isolates examined. Efflux, the secretion of chromosomal beta-lactamases and the impermeability of the outer envelope of BCC bacteria have been implicated in antibiotic resistance.14
In contrast, the molecular basis for biocide resistance in BCC bacteria has been poorly studied despite these organisms being linked with many instances of contamination in disinfectants and other anti-infective solutions.40
The mission and therefore the challenge posed by the identification of BCC species for routine clinical microbiology laboratories are different. Strains isolated on selective media and tentatively identified as belonging to the BCC using commercial systems should be confirmed with the classical biochemical tests described.41
The early detection of BCC is extremely important both for the CF patient as well as for the CF community. However, a recent study5 indicated that less than half of W.S. centers surveyed employ “B. cepacia”-specific selective media or incubate cultures for extended periods, both of which improve the yield of this organism. The use of these up-to-date culture techniques is technically not demanding and should be the expected standard of care in every CF center worldwide.
In this study, we only eradicated B. cenocepacia in one patient; another patient died immediately post transplant, and neither patient had cepacia syndrome and suffered an important clinical deterioration, although the period of observation was short because they acquired BCC recently.
The improved diagnosis of infections caused by members of the BCC and other B. cepacia-like organisms will help with the interpretation of the results from clinical outcome studies, and by doing so will provide crucial information regarding the pathogenicity and/or transmissibility of specific strains involved.5
Conflict of interestNone of the authors have any conflict of interest to declare.