Thyrotoxic periodic paralysis (TPP) is a complication of hyperthyroidism mainly reported in Asian males. It is uncommon in other races, although its incidence is increasing in Western countries. We report the case of a Caucasian patient diagnosed with thyroid hyperfunction who experienced episodes of loss of strength in the lower limbs.
The patient was a 35-year-old male with an unremarkable personal history who attended the emergency room reporting a loss of strength in the proximal lower limbs, with no pain or sensory changes, mainly occurring in the evening and related to intense work activity. The episode had occurred at other times during the previous three months. Clinical history found hyperhidrosis, distal tremor, and occasional palpitations, which did not occur at the same time as muscle weakness. The main finding in neurological examination was an asymmetric decrease in lower limb strength with generalized hypoactive bone and tendon reflexes. Urgent supplemental tests requested found hypokalemia (2.7mequiv./L, NV: 3.8–5.2mequiv./L), elevated creatine kinase level (253U/L, NV: 45–135U/L), and sinus tachycardia at 110bpm. The condition improved with water and electrolyte replacement, and the patient was admitted for a complete work-up.
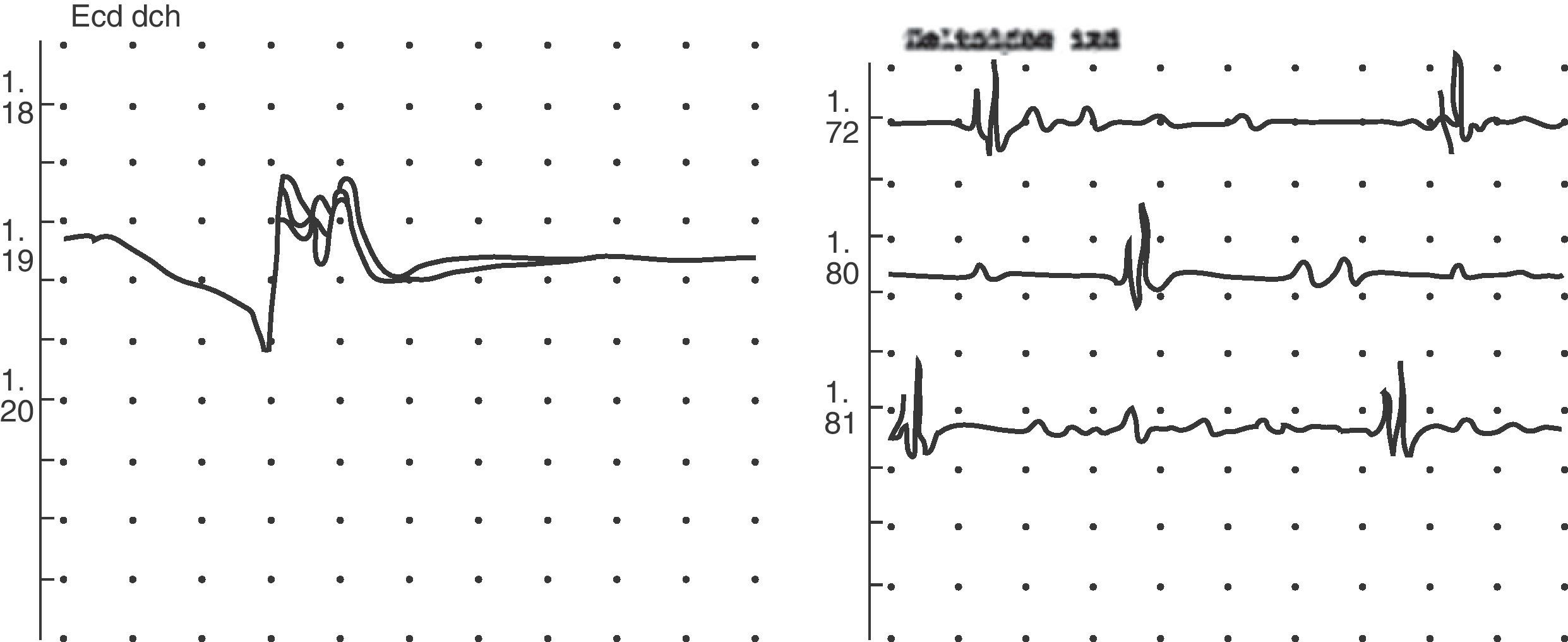
Medullary compression and infection were ruled out as the cause during his hospital stay, and electromyography consistent with non-specific myopathy of a probable metabolic origin suggested muscle disease (Fig. 1). Primary hyperthyroidism was also found. On neck examination, grade 2 diffuse goiter was palpated. Supplemental tests for antithyroid peroxidase antibodies (TPO) and anti-TSH receptor antibodies (TSI) were positive, and thyroid gammagraphy showed an increased gland size with homogeneous distribution of the radioactive agent. The patient was diagnosed with Graves disease, and treatment was started with thiamazole 30mg and propranolol 40mg daily. The patient's course was satisfactory, and he is currently symptom-free and in an euthyroid state after a therapeutic radioiodine dose (Table 1). Because of the clinical characteristics of the condition, its triggers, the finding of hypokalemia, the absence of a family history of periodic paralysis, and symptom improvement after antithyroid treatment was started, the condition was diagnosed as thyrotoxic hypokalemic periodic paralysis.
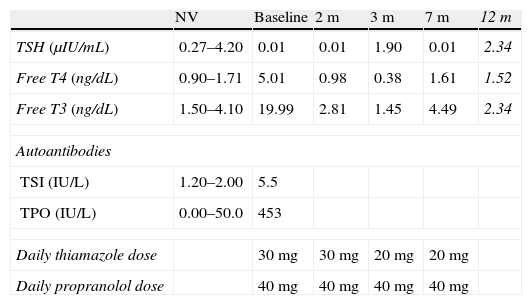
Laboratory parameters and treatment diagnosis and monitoring.
| NV | Baseline | 2m | 3m | 7m | 12m | |
| TSH (μIU/mL) | 0.27–4.20 | 0.01 | 0.01 | 1.90 | 0.01 | 2.34 |
| Free T4 (ng/dL) | 0.90–1.71 | 5.01 | 0.98 | 0.38 | 1.61 | 1.52 |
| Free T3 (ng/dL) | 1.50–4.10 | 19.99 | 2.81 | 1.45 | 4.49 | 2.34 |
| Autoantibodies | ||||||
| TSI (IU/L) | 1.20–2.00 | 5.5 | ||||
| TPO (IU/L) | 0.00–50.0 | 453 | ||||
| Daily thiamazole dose | 30mg | 30mg | 20mg | 20mg | ||
| Daily propranolol dose | 40mg | 40mg | 40mg | 40mg | ||
Values after the therapeutic dose of 15mCi of radioiodine are given in italics.
T4: thyroxine; TPO: peroxidase antibodies; TSH: thyrotropin; TSI: anti-TSH receptor antibodies; T3: triiodothyronine; NV: normal values.
TPP is a well-known complication in Asian populations, where it occurs in 1.8–1.9% of hyperthyroid patients.1 However, there has been a recent increase in the number of cases of TPP in Western countries.2 Most cases reported in Europe occur in Mediterranean areas, including Spain.3,4 TPP occurs in young men aged 20–40years, such as our patient, who experience transient, recurrent episodes of muscle weakness with flaccid paralysis lasting for hours. Proximal muscles are affected, sensitivity is preserved, and bone and tendon reflexes are decreased or absent. Episodes are commonly triggered by the intake of carbohydrate-rich meals, alcohol, or strenuous exercise. Clinically, TPP is similar to familial hypokalemic periodic paralysis. Differential features such as epidemiological characteristics, family history and, especially, coexistent hyperthyroidism must therefore be sought.5
Hypokalemia is the characteristic biochemical finding. The degree of hypokalemia is correlated to paralysis severity, but not to hyperthyroid clinical signs and symptoms or hormone levels. It is produced by a rapid, massive potassium entry from the extracellular to the intracellular space, mainly to myocytes because of increased activity of the Na/K-ATPase pump. Patients with TPP are predisposed to activation of the Na/K-ATPase pump, either directly through thyroid hormone excess or indirectly by adrenergic, insulin, or physical exercise stimulation.6 Two-thirds of patients are found to have increased creatine kinase levels, particularly in episodes triggered by physical exercise. Electrocardiographic findings are highly variable, including sinus tachycardia, high QRS voltage, or first-degree AV block.7 The electromyogram shows myopathy with decreased amplitude of muscle action potentials and no marked changes after epinephrine stimulation.6
Most cases of TPP detected are due to hyperthyroidism caused by Grave's disease, but other causes of thyroid hyperfunction are possible and have been reported. As regards genetic predisposition, various genes have been considered responsible. Polymorphisms have been found in the nucleotide sequence of subunit alpha-1 of voltage-dependent calcium channel Cav1.1, with differences from the mutations occurring in familial hypokalemic periodic paralysis.8
Urgent treatment consists of electrolyte replacement with potassium by either the intravenous or oral route in order to prevent cardiovascular complications.9 Oral non-selective beta-blockers are helpful for preventing paralysis attacks, and in order to avoid triggering factors. Final remission is achieved when hyperthyroidism is controlled.5
TPP is a complication associated with hyperthyroidism which is becoming increasingly common in Western countries. It should be considered in patients with episodes of muscle paralysis, for whom thyroid function tests should be part of the diagnostic process.
Please cite this article as: García-Martín A, et al. Parálisis periódica tirotóxica: complicación del hipertiroidismo cada vez más frecuente en nuestro medio. Endocrinol Nutr. 2012;59:394–6.