Anaplastic thyroid cancer (ATC) is the most aggressive solid tumour known and is a rare but highly lethal form of thyroid cancer that requires a multidisciplinary team approach. No Spanish consensus exists for management of patients with ATC.
The Thyroid Cancer Group of the Spanish Society of Endocrinology and Nutrition and the GETHI (Grupo Español de Enfermedades Huérfanas e Infrecuentes) of the Spanish Society of Oncology, in agreement with the Boards of these Societies, commissioned an independent task force to develop a wide consensus on ATC. The relevant literature was reviewed, including serial PubMed searches supplemented with additional articles.
The consensus includes the characteristics, diagnosis, initial evaluation, establishment of treatment goals, approaches to locoregional disease (surgery, radiotherapy, systemic therapy, supportive care during active treatment), approaches to advanced/metastatic disease, palliative care options, monitoring, and long-term follow-up of ATC. For operable disease, a combination of radical surgery with adjuvant radiotherapy or chemotherapy, using agents such as doxorubicin, cisplatin and paclitaxel, is the best treatment strategy. Cytotoxic drugs are poorly effective for advanced/metastatic ATC. On the other hand, targeted agents may represent a viable therapeutic option. Patients with stage IVA/IVB resectable disease have the best prognosis, particularly if a multimodal approach is used, and some stage IVB unresectable patients may respond to aggressive therapy. Patients with stage IVC disease should be considered for clinical trials or for hospice/palliative care depending on their preference.
This is the first Spanish consensus for ATC, and provides recommendations for management of this extremely aggressive malignancy. Novel systemic therapies are being tested, and more effective combinations are needed to improve patient outcomes. Although more aggressive radiotherapy has reduced locoregional recurrence, mean overall survival has not improved in the past 50 years.
El cáncer anaplásico de tiroides (CAT) es el tumour sólido más agresivo conocido y es una forma rara pero muy letal de cáncer de tiroides que requiere un enfoque multidisciplinario. No existe ningún consenso español para definir la conducta a seguir en los pacientes con CAT.
El Grupo de Cáncer de Tiroides de la Sociedad Española de Endocrinología y Nutrición y el GETHI (Grupo Español de Enfermedades Huérfanas e Infrecuentes) de la Sociedad Española de Oncología, de acuerdo con las Juntas Directivas de estas Sociedades decidieron que un grupo de trabajo independiente desarrollaran un amplio consenso sobre el CAT. Se revisó la literatura relevante, incluyendo la búsqueda en PubMed de las series más relevantes.
En el consenso se incluyen las características, el diagnóstico, la evaluación inicial, el establecimiento de los objetivos del tratamiento, la actitud a seguir ante la enfermedad locorregional (cirugía, radioterapia, terapia sistémica, la atención de apoyo durante el tratamiento activo), acerca a la enfermedad avanzada/metastásica, las opciones de cuidados paliativos, la vigilancia y el seguimiento a largo plazo del CAT. Para la enfermedad operable, la combinación de la cirugía radical con radioterapia o quimioterapia adyuvante, utilizando agentes tales como doxorrubicina, cisplatino y paclitaxel, es la mejor estrategia de tratamiento. Los fármacos citotóxicos para los casos avanzados/metastásicos de CAT son poco eficaces. Por otra parte, los agentes dirigidos a dianas específicas pueden representar una opción terapéutica viable. Los pacientes con enfermedad resecable en estadio IVA/IVB tienen el mejor pronóstico, sobre todo si se utiliza un enfoque multimodal, y algunos pacientes no resecables etapa IVB pueden responder a una terapia agresiva. En los pacientes con enfermedad en estadio IVC se debe considerar o bien si son aptos para entrar en un ensayo clínico o bien para cuidados paliativos, dependiendo de la preferencia del paciente.
Este es el primer consenso español para el CAT y ofrece recomendaciones para la conducta a seguir en este tumour maligno extremadamente agresivo. Las terapias sistémicas más recientes están siendo evaluadas, y se necesitan combinaciones más eficaces para mejorar los resultados en los pacientes tratados. Aunque la radioterapia más agresiva ha reducido las recurrencias locorregionales, la media de supervivencia global no ha mejorado en los últimos 50 años.
Anaplastic thyroid cancer (ATC) is the most aggressive solid tumour known to humans. Even when found in a localized form, the prognosis is severe. Rapid evaluation and establishment of treatment goals are imperative for optimum patient management and require a multidisciplinary team approach. Over a five decade interval, there has been little progress in the treatment of this malignancy and no such Spanish consensus exists for management of patients with ATC. The Thyroid Cancer Group of the Spanish Society of Endocrinology and Nutrition and the GETHI (Grupo Español de Enfermedades Huérfanas e Infrecuentes) of the Spanish Society of Oncology accordingly the Board of these Societies requested that an independent task force develop a more comprehensive set of consensus to assist practitioners in the management of ATC. They selected nine chairpersons to lead the task force and membership on the panel was based on expertise and previous contributions to this field. Panel members declared whether they had any potential conflict of interest during the course of deliberations. The topics about ATC were distributed to a two writers to elaborate this consensus (JMGS and PJF) and were commissioned to write the manuscript that was afterwards reviewed and approved by all the experts. They used his and her knowledge of the subject as well as a systematic PubMed search for primary references, reviews, and other materials publicly available before September 2014, to develop a set of recommendations. In preparing this consensus, we developed a list of questions covering the areas of characteristics, diagnosis, initial evaluation, establishing treatment goals, approaches to locoregional disease, approaches to advanced/metastatic disease, palliative care/hospice, and surveillance and long-term monitoring. All drafts were reviewed and edited by the members of panel for consistency and sent back to the primary writers for review. The authors include physicians who specialize in Endocrinology and Oncology. Task force deliberations took place largely through electronic communication and there were also a meeting of the authors. The medical opinions expressed in this consensus are those of the authors. The Board of Spanish Society of Endocrinology and Nutrition and the Spanish Society of Oncology approved the final document.
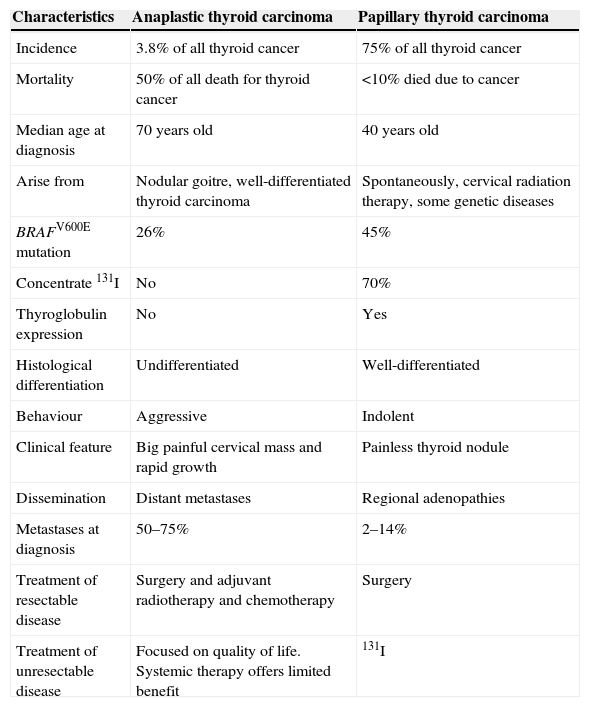
ConsensusAre there any features of anaplastic thyroid cancer that must be known and taken into account before deciding on any approach?ATC is an uncommon type of thyroid cancer, accounting for 0.6 to 9.8% of all thyroid cancers1, and has many differences with respect to other histological types (Table 1).
Differences among anaplastic carcinoma and papillary thyroid tumour.
| Characteristics | Anaplastic thyroid carcinoma | Papillary thyroid carcinoma |
|---|---|---|
| Incidence | 3.8% of all thyroid cancer | 75% of all thyroid cancer |
| Mortality | 50% of all death for thyroid cancer | <10% died due to cancer |
| Median age at diagnosis | 70 years old | 40 years old |
| Arise from | Nodular goitre, well-differentiated thyroid carcinoma | Spontaneously, cervical radiation therapy, some genetic diseases |
| BRAFV600E mutation | 26% | 45% |
| Concentrate 131I | No | 70% |
| Thyroglobulin expression | No | Yes |
| Histological differentiation | Undifferentiated | Well-differentiated |
| Behaviour | Aggressive | Indolent |
| Clinical feature | Big painful cervical mass and rapid growth | Painless thyroid nodule |
| Dissemination | Distant metastases | Regional adenopathies |
| Metastases at diagnosis | 50–75% | 2–14% |
| Treatment of resectable disease | Surgery and adjuvant radiotherapy and chemotherapy | Surgery |
| Treatment of unresectable disease | Focused on quality of life. Systemic therapy offers limited benefit | 131I |
Most cases arise from a nodular goitre or from a differentiated thyroid carcinoma; hence, the age of presentation is more advanced, with up to 80% of patients older than 50 years at diagnosis, median age from 66 to 72 years, which will influence on the therapeutic decision.
Features include being histologically undifferentiated and their aggressiveness, with a tendency towards early systemic dissemination, and thus 50% of cases have metastasis at diagnosis and 25% do so within the following months with a median survival of six months.1,2 Therefore, ATC is the thyroid cancer with the poor prognosis and highest mortality. Despite its rarity, it is responsible for half of all deaths from thyroid cancer, usually due to distant metastases rather than local progression, unlike what happens in differentiated thyroid cancers.3
What are the most common genetic abnormalities of anaplastic thyroid cancer?Some differentiated thyroid cancers usually have a single known mutation, but undifferentiated ones, such as ATC, have many mutations, genetic, epigenetic, and proteomic abnormalities (DNA methylation, posttranslational modification, and chromatin remodelling).4
The most prevalent genetic mutations are listed in Table 2 and on the other hand, several altered pathways have been identified in undifferentiated anaplastic tissues, including the cell cycle, focal adhesion, mitogen-activated protein kinase, cytoskeleton, transforming growth factor β1,5p53 gene mutation with overexpression of the p53 protein. Moreover, the p53 protein plays a major role in tumour progression by means of the cell dedifferentiation pathway, increasing tumour aggressiveness; as a result, its presence entails a worse prognosis.5
Furthermore, they present anomalies in the number of chromosomes or of their integrity that affect regions containing epidermal growth factor receptor, MET, BRAF, K-RAS, CCND1, FOSL1, UBE2C, CDKN2A and PPARγ-28; furthermore, gains in the number of copies in epidermal growth factor receptor, vascular endothelial growth factor receptor 1,2, platelet-derived growth factor receptor A, B, phosphatidylinositol-4,5-bisphosphate 3-kinase a, b, KIT, PDK1, AKT1, and MET,6,7 have been described.
Adult stem cells have been identified in ATC and it is believed that there may be a relation between this type of cell and tumour development in ATC that might derive from the remains of foetal thyroid cells.7
MicroRNA are scantly expressed in other cancers. Some researchers have found down-regulated microRNA in cancer that can be targets of the proteins involved in thyrocyte transformation. Likewise, epigenetic silencing of several thyroid-specific genes has been detected. These changes can diminish the tumour ability to concentrate 131I, which decreases treatment options4; microRNA have been used to classify thyroid tumors.4,8–10
At present no familial association with ATC has been described.
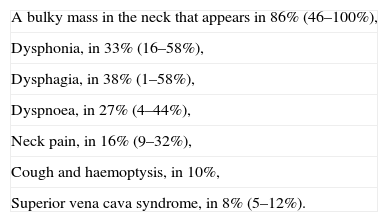
What studies should be carried out before deciding on treatment for a patient with anaplastic thyroid cancer?The clinical interview should inquire about the most common symptoms, and those are presented on Table 3. Clinicians should consider and be alert to the possibility of unusual presentations, such as respiratory obstruction, gastrointestinal symptoms or bone symptoms due to metastases.
Clinical most common symptoms in anaplastic thyroid carcinoma.
| A bulky mass in the neck that appears in 86% (46–100%), |
| Dysphonia, in 33% (16–58%), |
| Dysphagia, in 38% (1–58%), |
| Dyspnoea, in 27% (4–44%), |
| Neck pain, in 16% (9–32%), |
| Cough and haemoptysis, in 10%, |
| Superior vena cava syndrome, in 8% (5–12%). |
The patient should be asked about a history of differentiated thyroid carcinoma prior or concurrent, given that between 28% and 71% of them will have a previous positive history.
The physical examination will typically reveal palpable hard thyroid masses that tend to be multiple and bilateral; 80% of the patients present tumours measuring more than 5cm. More than 40% exhibit cervical adenopathies and some 30% will have vocal cord paralysis. Clinicians can look at how tumours double in size in a short period of days or a few weeks.
Most patients present with a preoperative cervical mass apparently limited to the neck. However general imaging studies may show extensive disease at the time of diagnosis, thus imaging studies must always be carried out to determine the state and existence of distant metastases. If computerized tomography fails to locate metastases, a positron emission tomography may be indicated, and in some cases, ultrasound and whole body magnetic resonance imaging.11–13 Distant metastases can be observed in 50% of the cases and of these, 60% will be found in the soft tissue of the neck, 27% in the trachea, and 53% will be located in other soft tissues, lungs (37.2%), mediastinum (25%), liver (10.1%), bones (6.4%), kidneys (5.3%), heart and adrenal glands (5.2%), and brain (4.4%).13
Every ATC is classified as IV stage which are subsequently divided in:
- •
IVA: intrathyroidal tumour without extracapsular extension
- •
IVB: extrathyroidal tumour with spread without distant metastases
- •
IVC: presence of distant metastases
Biopsy is essential to confirm diagnostic suspicion either with fine needle aspiration cytology or biopsy and in both cases immunohistochemical staining methods usually are positive for cytokeratins, and TP53, but negative for thyroglobulin and the Ki-67 (MIB-1) index is high.
Laboratory testing should include: hemogram, biochemistry with liver function, kidney function, calcium ion, and thyrotropin hormone.
Some authors suggest the use of positron emission tomography image/computer tomography in staging of poorly differentiated carcinoma and ATC, but more studies are needed to define its diagnostic and prognostic use in this setting.14,15
Is anaplastic thyroid tumour the same as poorly differentiated thyroid carcinoma?Poorly differentiated carcinoma is intermediate on the spectrum between well differentiated and ATC and may represent a transition form. They have some morphological and behavioural characteristics that are between the well-differentiated carcinomas and ATC but contrary to what happens in the well-differentiated tumours are multiple activating mutations. The criteria for the diagnosis of poorly differentiated thyroid carcinoma to attempt to universally define are: (1) the presence of a solid/trabecular/insular pattern of growth; (2) the absence of the conventional nuclear features of papillary carcinoma; and (3) the presence of at least one of the following features: convoluted nuclei, mitotic activity three or more per 10 high-power fields, and tumour necrosis. The most common molecular findings are the presence of mutations of TP5383 also can show BRAF mutations probably reflecting that the tumour originated in an existing papillary carcinoma; RAS mutations occur in up to 50% of the poorly differentiated thyroid carcinoma.16 ATC, as presented before, have many mutations, genetic, epigenetic, and proteomic abnormalities.4 Preservation of immunohistochemical markers of epithelial and thyroid differentiation, such as thyroglobulin and thyroid transcription factor 1, in poorly differentiated carcinoma can help to distinguish it from ATC. Other differences with ATC are that is recommend a total thyroidectomy and central node dissection, as appropriate, in patients with poorly differentiated thyroid carcinoma and a lateral neck dissection. The use of 131I therapy for patients with distant metastases that are 131I avid is also indicated. Also, the use of external radiation therapy directed at local residual disease if the surgical team suspects residual disease or if there are positive margins on histological examination.17
Are there any other prognostic factors different from the stage in anaplastic thyroid cancer?The median survival for patients with ACT is between 2.5 and 8.5 months depending upon the presence or absence of metastases3,14; the survival at six months being less than 20%.2 Therefore, the presence of metastases at diagnosis is the most decisive factor that tells of a poor prognosis, with a relative risk of mortality of 3.2 (range 2.0–51.1) compared with patients with localized disease.
A retrospective Japanese study identified age of 70 years or higher, white blood cell more than 10,000mm, extrathyroidal invasion, distant metastasis at the time of diagnosis, and incomplete or no resection as prognostic factors. Multimodal, aggressive treatment and radiation doses of 40Gy or more enhance survival in stages IVA and IVB, that is, locally confined tumours, but they are of doubtful benefit in the presence of metastases, stage IVC.18 Another large, multicentre Japanese study also identified age of 70 years or higher, presence of acute symptoms, leucocytosis, large tumour higher than 5cm, T4B tumour, and distant metastasis as significant risk factors for lower survival.19
Long-term survival is possible for selected patients with ATC. To determine the treatment strategy, classification system established by the ATC Research Consortium of Japan (disease extent) and other prognostic factors (e.g., biologic malignancy grade) should be considered.19
As a result, the aggressiveness of this tumour make variables having to do with treatment in non-metastatic stages, i.e., more extensive surgery, high doses of radiotherapy, combined therapy, and although the prognosis of most patients with ATC continues to be poor, surgery, radiotherapy, and a combination of both improved the survival of patients with ATC.
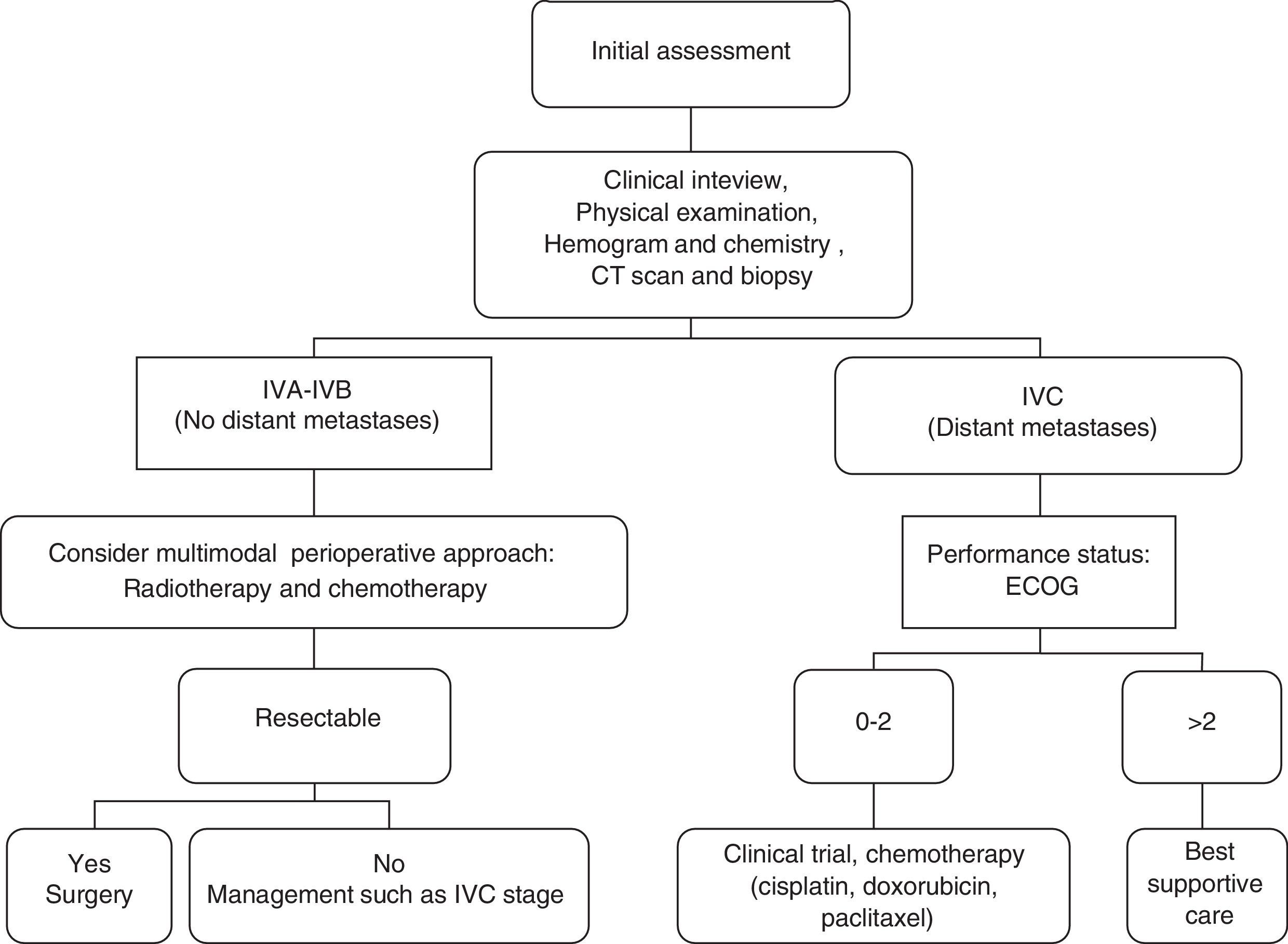
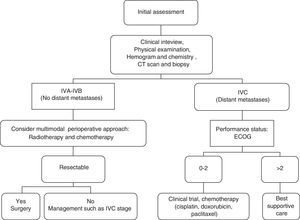
Is an aggressive therapy suitable or merely palliative/hospice care in anaplastic thyroid cancer?Once the diagnosis of ATC has been confirmed, the tumour stage should be assessed; that its extension and resectability, in addition to the patient overall health status, the presence of co-morbidities, functional status and, hence, operability, which are the most important factors in deciding how best to treat the disease.
Treatment goals and strategies, aggressive therapy, or palliative care must then be established, assessing both the risk and benefit and talking with the patient about they interests, expectations, and preferences, so that they make an informed decision.
In order to give this information, it must be remembered that regardless of the treatment strategy and stage, the median survival is less than one year and, hence, symptomatic control and quality of life take priority over any other consideration.20
If the tumour is confined to the thyroid, expedient action and management are essential, given the speed with which metastases appear. In stage IVA, radical surgery, and complementary treatment with radiotherapy and/or chemotherapy are the best alternatives when the aim is to increase survival.
In which patient a radical curative surgery and perioperative multimodal treatment is indicated?Surgical resection for patients with stage IVA disease with postoperative chemoradiation remains the standard recommendation for American Thyroid Association. The recent guidelines put forth by this Association propose these recommendations for multimodal therapy in stages IVA/IVB, that is to say, resectable disease, since they have a better prognosis and longer survival.13 If surgery is not possible, chemoradiotherapy should be offered.21,22
Additionally, some non-resectable IVB stages may respond to aggressive multimodal therapy, including the different treatment regimes described below.
The recommended surgery for localized ATC is total thyroidectomy that includes all regional structures and lymph nodes. External radiation should be started as soon as the patient is sufficiently recovered from neck surgery, usually within two to three weeks after surgery. Systemic chemotherapy can begin as soon at the patient is sufficiently recovered from surgery, potentially even within one week of surgery, depending upon treatment goals. Perioperative treatment can consist of radiotherapy, chemotherapy, or chemoradiotherapy.
External beam radiation therapy should be given with once or twice daily fractionation (1.5Gy per fraction) to a total dose of 45–66Gy. A twice-daily fractionation regimen has a trend towards longer survival. The use of conformal 3-dimensional radiotherapy or intensity modulated radiotherapy did not influence survival or toxicity.23,24
Regarding the combination of radiation and chemotherapy, the most promising results have been shown with doxorubicin with or without taxanes or cisplatin combined with external beam radiation therapy.25,26 Thus, induction chemotherapy with by weekly paclitaxel is a promising therapeutic strategy for stage IVB ATC patients since responders can be expected to achieve long-term survival after surgery.27,28
In FACT (Fosbretabulin in Anaplastic Cancer of the Thyroid) phase 2/3 trial, thyroidectomy followed by fosbretabulin combination regimen compared with carboplatin/paclitaxel without fosbretabulin showed a no significant (p=0.25) trend towards improvement in patient survival, 8.2 vs. 4.0 months and 33.3% vs. 7.7% one year survival, respectively.29
What systemic therapies should be considered for metastatic anaplastic thyroid cancer?Research into different molecular therapies (deacetylase inhibitors, tubulin binding compounds, etc.) and the pathogenesis of anaplastic carcinoma continue to evolve. Care for patients with metastatic disease, stage IVC, is focused on quality of life, due to the fact that systemic therapy offers limited benefit. For this reason, chemotherapy may be used in patients with good performance status with a controversial role in elderly people having a poor performance status and other comorbidities. Therefore, one appropriate alternative is trying to include the patient in a clinical trial.
Likewise, debulking or radiotherapy may be necessary to avoid respiratory or oesophageal obstruction.13 Motesanib, sorafenib, vandetanib, sunitinib, lenvatinib, imatinib, cabozantinib, gefitinib, everolimus, axitinib and pazopanib are targeted agents known as tyrosine kinase inhibitors that are capable of suppressing the RET oncogene, vascular endothelial growth factor receptors, as well as other kinases and have been used in advanced differentiated thyroid carcinoma.
Of all the aforementioned, sorafenib,30–32 axitinib,33 gefitinib,34 imatinib,35 and the antimicrotubule fosbretabulin and combretastatin A4 phosphate agents36 have been studied in phase II clinical trials with a small number of patients with promising results and a 33–75% rate of disease control. There are several studies underway with different drugs in monotherapy or associated with chemotherapy.
Among the cytotoxic agents, paclitaxel has been the most widely studied in recent years. This antimicrotubule agent exhibits activity both in monotherapy,37 as well as in combination with new agent targets such as efatutazone,38 and pazopanib.39 Currently, there are two ongoing studies that assess the efficacy of combretastatin or fosbretabulin in combination with carboplatin/paclitaxel (NCT00507429, NCT01701349).40–42 Although the study did not meet statistical significance in improvement in overall survival with the addition of fosbretabulin to carboplatin/paclitaxel, it represents the largest prospective randomized trial ever conducted in ATC. The regimen is well tolerated, with adverse effects and deaths primarily related to ATC and disease progression.40
Case reports have been published of maintained responses to chemotherapy regimens such as cisplatin and doxorubicin associated with radiotherapy, peplomycin, and granulocyte colony stimulating growth factor42 or with valproic acid,43 docetaxel and gefitinib,44 erlotinib in a patient with an important expression of epidermal growth factor receptor45 and vemurafenib in patients with BRAFV600E mutation.46 Also the case of a patient treated with everolimus was published and he maintained a response of the ATC during 18 months and whole-exome sequencing revealed a nonsense mutation in TSC2, a negative regulator of mTOR, suggesting a mechanism for sensitivity to everolimus.47
It is important to note that about one third of differentiated thyroid carcinomas and all ATC do not concentrate 131I, so radionuclides are not active as therapy in this cancer The effect of sodium iodide symporter gene transfection on the uptake of some β and γ-emitters in human ATC has been evaluate. The results demonstrate the possibility of imaging and therapy using sodium iodide symporter gene transfection in ATC, although the short retention time is considered the major impediment to be resolved for the successful implementation.48
In the near future, biological agents will probably be the standard treatment for this aggressive disease in the same way as they are today in 131I resistant differentiated thyroid and other refractory tumours, such as renal or liver cancer.
Patients with known bone metastases from ATC should also be deemed candidates for periodic treatment with intravenous perfusions of bisphosphonates or with subcutaneous administration of the agonists of RANKL (Receptor Activator for Nuclear Factor κβ Ligand inhibitor), activator of the NF-κβ ligand receptor, there are currently no precise recommendations regarding treatment duration or frequency.13 Treatment with radiotherapy or palliative surgery may be needed if the mass obstructs the airway.
What palliative care can improve quality of life for these patients?The palliative care service is addresses physical, emotional, social, intellectual, and spiritual needs of the patient and family. Such services typically include one or more of the following: a medical practitioner specifically trained in palliative medicine; a nurse practitioner; and trained counsellors to deal with patients and families coping with serious illness, life-limiting illnesses with no predictable endpoint, complications of therapies, or end-of-life situations. Palliative care is inclusive of life-prolonging therapies. The treatment team should include palliative care expertise at every appropriate stage of patient management to help with pain and symptom control, as well as addressing psychosocial and spiritual issues. Palliative care services are appropriate for any American Thyroid Association patient receiving treatment intended to prolong life. The treatment team should engage care for ATA patients who decline therapies intended to prolong life, yet still require symptom and pain relief spanning the remainder of their illness.13,19,20
What is the optimal frequency of imaging and follow up studies after initial therapy in patients with remission?In patients who have undergone complete tumour resection, it is suggested that brain, neck, chest, abdomen, and pelvis computed tomography be performed every one to three months during first year, and every four to six months thereafter.
In patients with no clinical evidence of disease, positron emission tomography with 18-fluorodeoxyglucose scanning should be considered every six months after treatment to identify small volume disease that usually requires a quick therapeutic decision.
Is it possible to establish a decision algorithm for the therapeutic approach?We establish this decision algorithm in Fig. 1.
ConclusionsThe absence of distant metastases, stages IVA/IVB, is the best known prognostic factor in subjects with ATC. In these patients, multimodal treatment with surgery, radiotherapy, and chemotherapy has an impact on survival.
In advanced disease, given the low survival rates and scant efficacy of systemic treatments, priority should be given to quality of life and offering the patient the possibility of participating in a clinical trial whenever possible. Doxorubicin and cisplatin are classical agents and, like paclitaxel, they are currently the most widely used; they are active alone or used in combination with radiotherapy and of the new targeting agents, the microtubule disrupting agent, combrestatin and its prodrug, fosbretabulin have demonstrated efficacy in phase II–III studies.
There is a need to go deeper in the molecular understanding of the disease in order to personalize our systemic treatment options for disseminated ATC patients.
Conflict of interestThe authors have nothing to disclose.