The aim of this study is to evaluate the sentinel lymph node mapping (SLNM) with methylene blue staining “ex vivo” in colon cancer, as well as calculate the upstaging obtained by the determination of micrometastases and its correlation with the evolution of the disease.
MethodsBetween 2008 and 2011, 101 patients with colon cancer undergoing resection were studied prospectively with SLNM and detection of micrometastases. The correlation of SLN micrometastases with the disease evolution was evaluated in patients with a follow-up of more than 1 year.
ResultsThe SLNM rate was 92 cases (91%). Only SLN was positive for micrometastases in 9 cases, with a 14% upstaging. The incidence of false negatives was 9 patients (10%). Mean follow-up of N0 patients (n=74) was 38 months. The SLN-(negative) group (65 patients) had a recurrence rate of 4 patients (7%), whereas this rate was 2 patients (22%) in the group of SLN+ (positive) (9 patients), but without significant differences. No differences in survival were observed.
ConclusionsSLNM is a reproducible technique without significant increase in time and costs. Upstaging was obtained in 14% of the patients staged as N0 by conventional technique. At follow-up of N0 patients with SLN+ there seems to be a higher rate of recurrence, which could change the guidelines of adjuvant treatment, but we must interpret the results of it with caution because the sample is small.
El objetivo de este estudio es llevar a cabo la evaluación de la técnica de detección del ganglio centinela (GC) con tinción de azul de metileno «ex vivo» en el cáncer de colon, así como calcular la supraestadificación y su correlación con la evolución de la enfermedad.
MétodosEntre 2008 y 2011, 101 pacientes fueron operados de cáncer de colon con la detección del GC, estudiándose las micrometástasis. El seguimiento de los pacientes N0 fue mayor a un año en búsqueda de recidivas y si tenían relación con la aparición de dichas micrometástasis.
ResultadosEl índice de detección del GC fue de 92 casos (91%). Fue positivo para micrometástasis en 9 casos, con una supraestadificación del 14%. La incidencia de falsos negativos fue de 9 pacientes (10%). El seguimiento medio de los 74 pacientes N0 fue de 38 meses. Se observó recurrencia en 4 pacientes (7%) del grupo de pacientes con GC− (65 pacientes) y en 2 pacientes (22%) en el grupo con GC+ (9 pacientes, sin diferencias estadísticas significativas. Tampoco se observaron diferencias en términos de supervivencia entre los 2 grupos.
ConclusionesEl estudio del GC es una práctica reproducible sin aumento significativo del tiempo y de costes. Puede llegar a supraestadificar el 14% de pacientes que habían sido clasificados como N0 con técnica convencional. En el seguimiento de los pacientes N0 con GC+ parece haber una tendencia a un porcentaje mayor de recidivas, lo que podría llevar a cambios en las pautas de tratamiento adyuvante, aunque debemos tomarlo con cautela ya que la muestra es escasa.
Colorectal cancer is the second most frequent malignant neoplasia in developed countries and the second cause of death related to neoplasia. Curative treatment is based on surgical excision, sometimes combined with adjuvant therapy, and as in most malignant tumours, tumour staging at diagnosis is the most important prognostic factor to predict survival. Although surgery is considered effective for patients with localised disease, survival decreases dramatically to 25%–35% if lymph nodes are affected. Affected lymph nodes determine the need for adjuvant chemotherapy administration, since it has been demonstrated that it improves survival in more than a third of these patients,1,2 reaching 84% survival. This benefit is not found in patients free of lymph node involvement, and therefore no adjuvant treatment is indicated, provided it is not associated with unfavourable characteristics of the primary tumour (perineural and vascular invasion, unsuitable lymph node sampling, primary tumour obstruction). However, in 10%–25%3 of patients free of lymph node involvement at the time of diagnosis, the disease will progress and they will develop distant metastasis within 5 years after curative surgery. These results raise some questions on the possible inaccuracy of current staging methods, which may lead to infra-staging. Considering that for patient staging we preferably analyse 3 aspects: tumour size (T), involved lymph nodes (N), and presence of distant metastasis (M), it is reasonable to assume that the greatest risk for error falls on the lymph node analysis and that most of these patients could have lymph node micrometastasis that goes undetected by conventional histological examination. This group of patients is used as the reference for some authors to estimate 10%–20% infra-staging in patients with colorectal cancer when performing a conventional histological analysis of the lymph node domain.4 This prompts the search for methods to help obtain proper lymph node staging5 by performing lymph node serial sections6 as well as cytokeratin immunohistochemistry techniques,7 and more recently by RT-PCR (reverse transcription polymerase chain reaction) techniques that allow for the detection of micrometastasis (less than 2mm). However, its use for all resected lymph nodes is not practical under regular clinical settings, due to financial and time restrictions, and would only be feasible on a limited group of lymph nodes. The latter, which could represent the entire lymph node domain, would be those known as sentinel lymph nodes8 (SLN) and would provide more accurate staging, possibly significant for the required adjuvant treatment.9
This study aims to assess the “ex vivo” methylene blue staining technique to determine SLN and calculate supra-staging obtained from SLN analysis and its correlation with the course of the disease.
MethodsThis is a prospective clinical study including patients diagnosed with colon neoplasia (above the sacrum promontory by radio-opaque enema or more than 15cm from anal margin by fibronoscopy) without distant dissemination in the extension study, who underwent surgery at our centre between January 2008 and February 2011. All surgeries followed established oncological criteria.
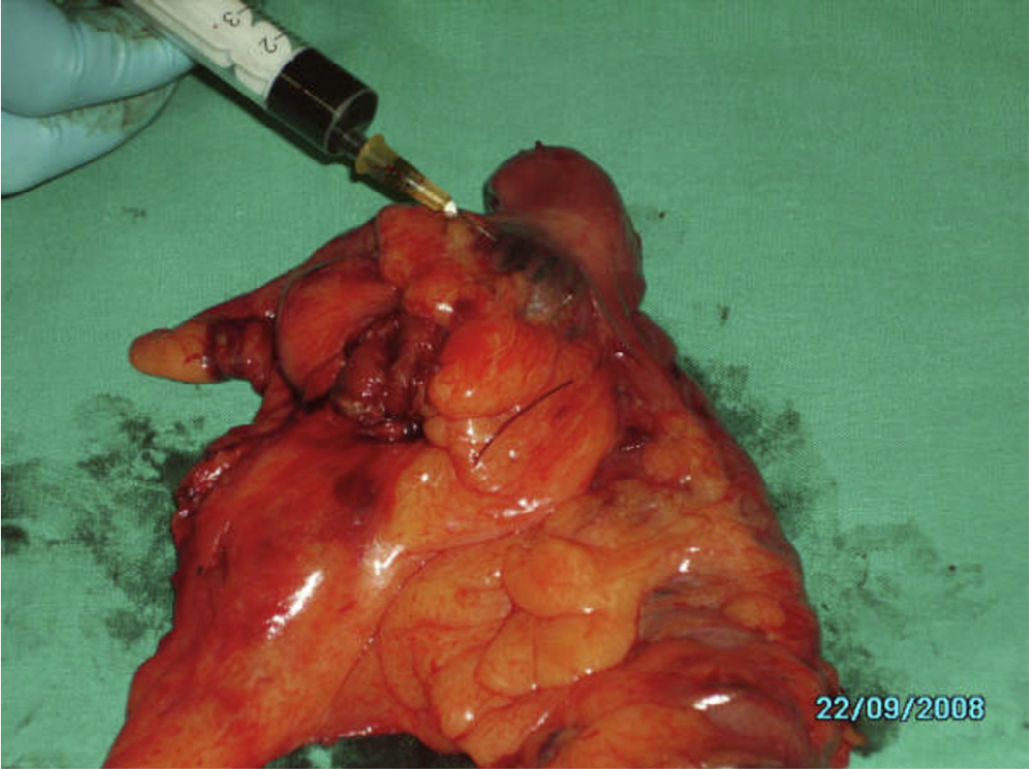
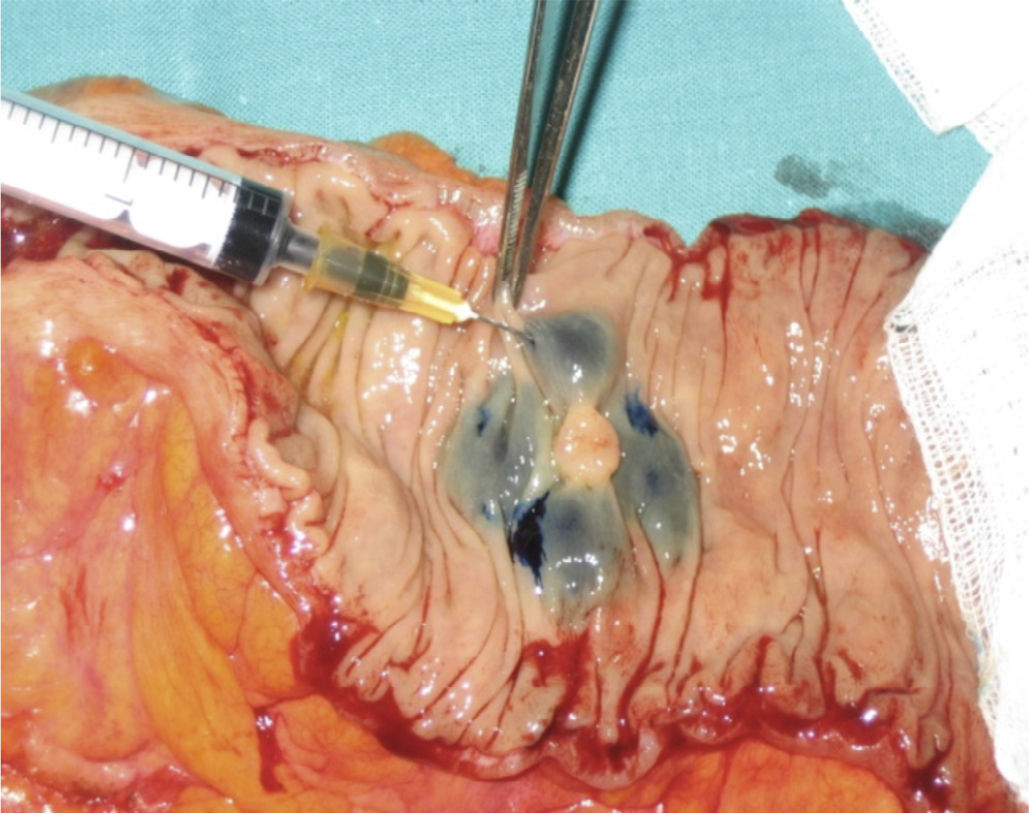
After surgery, “ex vivo” marking of the lesion was performed by peritumoural inoculation below the subserosa layer, with 2ml methylene blue distributed along 0.5ml in each quadrant (Fig. 1). After inoculation, infiltration points were massaged for 5min until the first lymph nodes were seen, ranging from 1 to 4, and they were considered SLN (Fig. 2). When the tumour lesion was not palpable, we conducted an incision through the antimesenteric border and the submucosa inoculation following the process described above (Fig. 3).
Marker-stained lymph nodes were considered as SLN.
An anatomopathological study was conducted on the sample. All SLN measuring 0.5cm or less were included, and when their size was larger, they were cut into sections of approximately 3mm. Each SLN or sections thereof were included in paraffin blocks and marked according to standard procedure. Sections measuring 5μs were cut from each block at 3 levels; for each level, 2 sections were stained with haematoxylin and eosin, and a third one was kept for the immunohistochemical analysis, which was performed if the conventional analysis turned out negative. The immunohistochemical analysis included identifying Cytokeratin CAM 5.2 (Becton-Dickinson) diluted at 1/5, and pepsin treatment, using a Horizon autostainer, and the technique, equipment, and instruments of standard use at the immunohistochemistry laboratory of the Pathology Department. Micrometastasis was considered as tumour cell deposits less than 2mm (Fig. 4).
Patient follow-up was performed according to the protocol at our centre, in line with quarterly checks for the first 2 years, and every 6 months in the years thereafter.
Statistical AnalysisAbsolute and percentage numbers were used to describe categorical variables. Quantitative variables were given in median and mean values. Univariate and multivariate studies were conducted on factors predicting relapse; a mortality analysis was also performed. Cox regression was used to evaluate the different influence of predictive factors (surgical procedure, presence or absence of micrometastasis). A P<.05 was considered statistically significant. Statistical Package for the Social Sciences (SPSS, version 17.0) was used to conduct the analysis.
ResultsBetween January 2007 and February 2011, 101 patients diagnosed with colon adenocarcinoma underwent surgery (56 males and 45 females); mean age was 73 years, and BMI was 27.8. We performed 39 right colectomies, 13 left colectomies, 37 sigmoidectomies, and 7 high anterior resections. A laparoscopic approach was used for 85% of cases; the conversion rate was 7.7%. Morbidity rate was 34%; mean stay was 7 days (median). Resurgery rate was 5.8% (6 cases: 1 hemoperitoneum and 5 cases of anastomotic leak); mortality was 2.3% (2 patients).
Average tumour size was 5.17cm, and resection margins were free of disease for all of them. Lesion staging and conventional lymph node analysis are reflected in Table 1.
1482 lymph nodes were obtained from the lymph node analysis. An average of 15 lymph nodes were obtained per surgery. SLN total was 198, for an average of 2 SLN per sample. SLN detection rate was 91% (92 cases).
Table 2 shows the correlation between lymph node conventional anatomopathological diagnosis and SLN analysis results.
For patients whose conventional lymph node analysis yielded no lymph node involvement (N0), the SLN analysis was positive for micrometastasis in 9 cases, resulting in a 14% SLN detection and overstaging rate.
In 8 of the 9 cases, positive detection was performed using haematoxylin and eosin staining on a greater number of lymph node sections. Immunohistochemical analysis was used for the remaining case.10
Furthermore, incidence of false negatives (negative SLN with other lymph nodes involved as per conventional analysis) was 9 patients (10%).
These results yielded a negative predictive value of 86% (59/9+59) and a positive predictive value of 62.5%.
Clinical Follow-upA total of 97% of the 101 patients included in the study were followed up for an average of 38 months (3 patients were lost for follow-up due to address change). Mortality during follow-up was 5% (5 patients), all due to relapse.
We have analysed the follow-up on patients without lymph node involvement according to the conventional analysis (74 patients), in order to compare results based on whether they showed SLN involvement or did not (SLN+ vs SLN−). All of them were followed up for more than 20 months and the average follow-up was 38 months. The N0 patient group according to the conventional analysis and SLN− (65 patients) had 7% recurrence (4 patients), while this rate was 22.2% (2 patients) in the GC+ patient group (9 patients). The relapses that occurred were 4 local and 2 distant at 20 months after surgery.
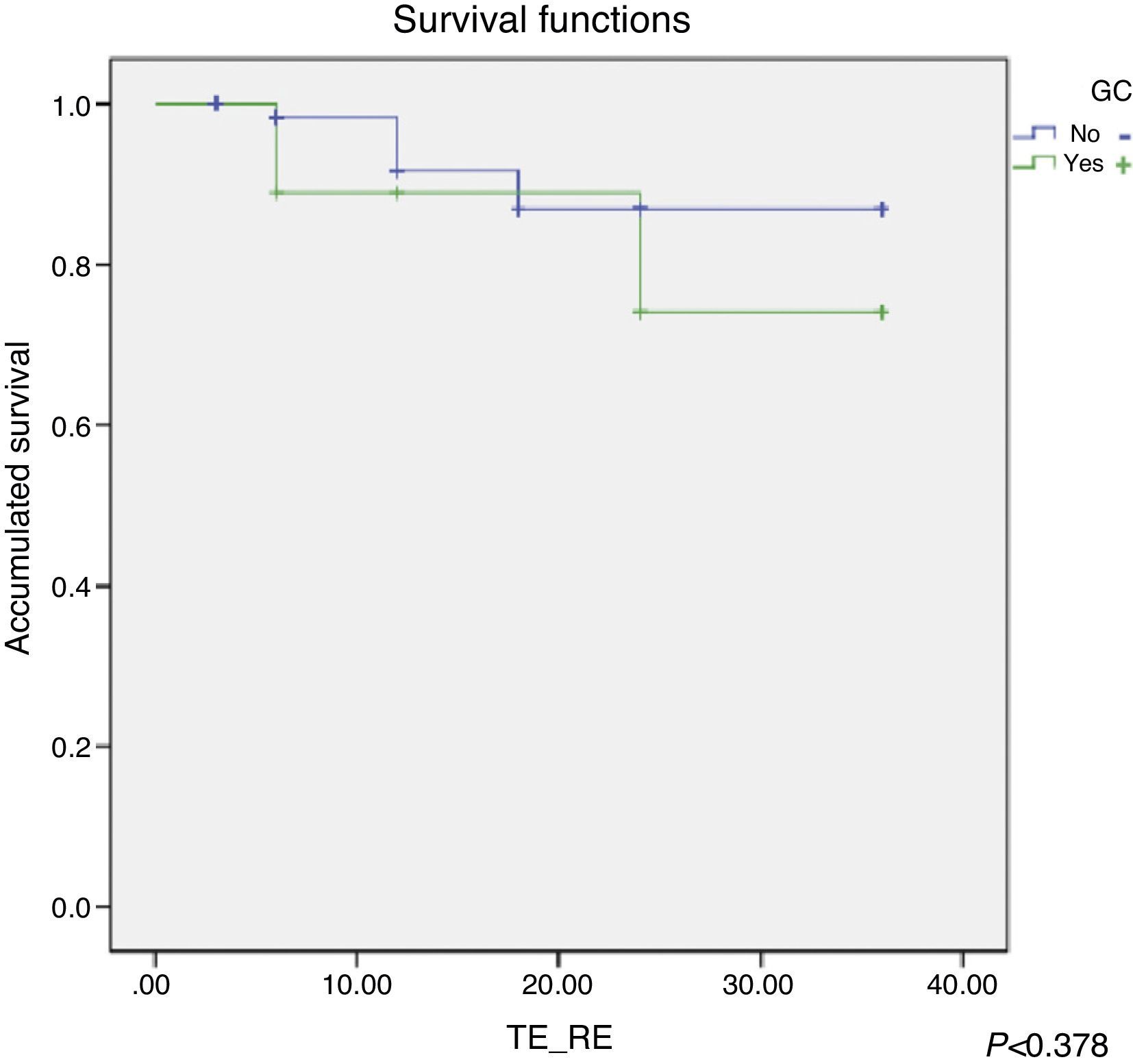
Patient survival was analysed applying the Cox regression which showed greater survival rates for SLN− patients (Fig. 5); however, it was not statistically significant (P<.378).
DiscussionEver since Saha introduced SLN analysis in colorectal cancer, a few authors have applied it. Several published studies report SLN identification in ranges between 58% and 100%,11,12 and the greatest percentage is reached in case series with the largest number of patients. Likewise, supra-staging obtained with SLN analysis varies greatly, ranging from 6% to 60%, yielding non-conclusive results.
Variability in the results obtained may be due to the heterogeneous aspect of the procedures used for staining (dye, tracer) and for determining SLN positivity (haematoxylin–eosin, IH, PCR).10–14
The use of different substances for peritumoural staining has been reported; even though isosulfan blue is the most frequently used, due to its high cost, some centres such as ours have used methylene blue instead with similar results. Furthermore, some authors advocate using a tracer and a probe for detection.15 These two detection systems can be complementary.
Some authors16 consider that, in patients with high BMI and a larger mesocolon, stained lymph node identification can be difficult while the probe can help detecting the tracer and guide SLN dissection. In our case series, we found no significant differences based on the patient's BMI when staining was used as the only detection method.
While most authors perform “in vivo” lymph node determination, other authors use “ex vivo” for the same purpose, after excising the sample. Several published case series report comparable results in terms of lymph node determination percentage. In a case series of 240 cases, Saha et al.4 described the “in vivo” technique by inoculating 1–2ml of isosulfan blue (Lymphazurin®) at 1%, yielding SLN determination in 100% of cases with 89% sensitivity, 100% specificity and 93.5% negative predictive value. Regarding the “ex vivo” method, Wong et al.17 reported inoculation of isosulfan blue 1ml at 1%, reaching SLN identification in 92.3% of patients; the mean per patient was 3 SLN, with 29% overstaging. The main reasons supporting “ex vivo” analyses is that the tumour can be manipulated and the lymph node can be dissected to identify SLN without risking tumour cell dissemination; however, “in vivo” examination can be used to detect aberrant lymph nodes outside the expected lymph node domain, and therefore, allow to expand the resection area. Other studies have detected SLN using both methods,18,19 obtaining identification in 94%–100% of cases.
In our study, we opted for methylene blue by “ex vivo” technique because, at our centre, we use laparoscopy in 85% of oncological colon surgery cases. Although there are studies reporting “in vivo” SLN detection during laparoscopy surgery,20 the puncture by this route seems excessively complex to us, as it involves greater tumour manipulation; therefore, we have it ruled out so far. Other options suggested include pre-operative endoscopic inoculation of the tracer. We considered that it would be the most adequate technique in patients who underwent laparoscopy; however the “fast-track” protocol used in our centre involves no pre-operative mechanical preparation of the colon, which renders endoscopic marking impossible. Our detection rate is 90%, similar to the rate published in literature.
Furthermore, our false-negative rate is 10%. This fact can be due to several factors. In our experience, the learning curve was the determining factor, since most non-detected cases happened amongst those initially included in the study. Likewise, we have not found that tumour stage would indicate a greater percentage of error in detecting SLN (P<.8842).
Regarding how to identify micrometastasis in SLN from the anatomopathological point of view, in our case, most (8/9) have occurred by increasing lymph nodes sections, reducing the importance of IH or PCR that other studies highlight.10–13
The fundamental aspect to be considered is the clinical relevance of SLN analysis. In a retrospective multi-centre study of 868 CRC patients followed up for a minimum of 2 years, Saha et al.4 claimed that patients stratified with the SLN procedure had a lower neoplastic recurrence rate (7% vs 25%). The authors attribute these results to chemotherapy being administered for SLN positive cases. In our case series, we obtained 9 positive SLN (with native conventional analysis), which indicates 14% overstaging. By analysing follow-up results, we found that recurrence rates for the SLN+ patient group were very close to those of patients with positive lymph nodes in the conventional analysis, which was not the case for results obtained from the SLN− group. However, the sample quantity was not sufficient to be able to obtain statistically significant differences. Currently, our protocol does not indicate chemotherapy as a treatment in the event of positive SLN, although in recent years, it is starting to be considered as the adjuvant option for these patients.21
After applying the “ex vivo” SNL procedure on our first 100 patients, we were able to conclude that it is a reproducible practice for lymph node analysis, without significant time or cost increase. This is a technique that may be able to overstage 14% of patients who were classified as N0 under the conventional technique, which could lead to changes in adjuvant treatment guidelines. We believe that studies should be conducted on a greater number of patients, thus multicentre, and standardising the technique applied, as much as possible, regarding the marker used, surgical approach (laparoscopy vs laparotomy), and “in vivo” or “ex vivo” lymph node dissection.
FundingBeca MIA (Moult il·lustre administració) Fundación privada Hospital de la Santa Creu i Sant Pau [Santa Creu i Sant Pau Private Foundation]. 2nd edition of research grants (2010). 2nd Medical Staff award. 2010 CTO Group Ulysses Research Award.
Conflict of InterestAuthors declare having no conflict of interest.
Please cite this article as: Pallarés-Segura JL, Balague-Pons C, Dominguez-Agustin N, Martinez C, Hernandez P, Bollo J, et al. El papel del ganglio centinela en la evolución del cáncer de colon. Cir Esp. 2014;92:670–675.