Minimally invasive surgery provides for the treatment of esophagogastric junction tumors under safe conditions, reducing respiratory and abdominal wall complications. Recovery is improved, while maintaining the oncological principles of surgery to obtain an optimal long-term outcome. It is important to have a sufficient volume of activity to progress along the learning curve with close expert supervision in order to guarantee R0 resection and adequate lymphadenectomy. Minimal invasiveness ought not become an objective in itself.
Should total gastrectomy be performed, the risk of a positive proximal margin makes intraoperative biopsy compulsory, without ruling out a primary open approach. Meanwhile, minimally invasive esophagectomy has been gaining ground. Its main difficulty, the intrathoracic anastomosis, can be safely carried out either with a mechanical side-to-side suture or a robot-assisted manual suture, thanks to the 3-D vision and versatility of the instruments.
La cirugía mínimamente invasiva permite el tratamiento de los tumores de la unión esofagogástrica en condiciones de seguridad, reduciendo las complicaciones respiratorias y parietales y mejorando la recuperación postoperatoria, manteniendo además los principios de la cirugía oncológica que permitan obtener unos resultados óptimos de efectividad a largo plazo. Para ello, es necesario un volumen de actividad suficiente y avanzar en la curva de aprendizaje de forma tutelada, para poder garantizar una resección R0 y una linfadenectomía adecuada. La mínima invasión no puede ser un objetivo en sí misma.
En caso de gastrectomía total, el riesgo de afectación del margen proximal obliga a verificarlo mediante biopsia intraoperatoria, sin descartar la cirugía abierta de entrada. Por su parte, la esofagectomía mínimamente invasiva se ha ido imponiendo progresivamente. Su principal dificultad, la anastomosis intratorácica, puede realizarse mediante una sutura laterolateral mecánica o manualmente asistida por robot, gracias a la visión tridimensional y a la versatilidad del instrumental.
Determining the approach for the surgical treatment of esophagogastric junction (EGJ) tumors requires a combination of several preoperative studies, such as:
- •
Endoscopy with vision of the junction in retroflexion
- •
The histologic type and the presence of Barrett's esophagus, in which case the tumor should be classified as esophageal.
- •
PET/CT
- •
Endoscopic ultrasound and barium swallow, if necessary
The purposes of testing are to determine whether the lesion is predominantly esophageal or gastric (despite the additional difficulties of a bulky lesion or the presence of a hiatal hernia) and to determine the lymph node dissemination pattern.
The objective of surgical treatment is to achieve R0 resection with a proximal and distal margin of about 5cm and a circumferential margin greater than 1mm,1 adding a lymphadenectomy that provides for adequate staging (at least 15 lymph nodes) and a potential therapeutic benefit.2–4 Thus, the approach must be adapted to each patient.5
The transthoracic approach seems unavoidable in case of esophageal invasion greater than 2–3cm,6,7 mediastinal lymph node involvement,4,8 advanced or bulky lesions,7,9 or difficulty in obtaining an adequate proximal margin.10
The greater morbidity and mortality and the impact on the quality of life associated with a more extensive dissection must also be contemplated, which is why some authors propose the management of suspicious lesions or those in elderly patients as gastric cancer,11 or the transhiatal approach for patients with impaired lung function.
In experienced hospital units, pulmonary complications of the thoracic approach are similar,7,12 as is the quality of life based on respiratory and reflux symptoms.
Once the most appropriate surgical resection strategy has been decided for each patient (based on the tumor location and extension, patient characteristics and the experience of the surgical team), minimally invasive surgery (MIS) can provide additional benefits.
It is well known that MIS involves less pain, fewer parietal complications, less blood loss, better preservation of postoperative lung function and shorter hospital stay.13–15 Since it minimizes morbidity, it has been shown to be a safe approach in senior patients. Additionally, due to the magnification it provides, MIS is able to provide a more accurate assessment of the surgical anatomy. These advantages are also observed in the hybrid approach,16,17 where only the abdominal phase is performed laparoscopically. Furthermore, by promoting a speedy recovery, minimally invasive surgery increases the chances of being able to administer adjuvant therapy.18
Nevertheless, MIS implies certain uncertainties, including the possibility to perform a lymphadenectomy comparable to open surgery, complications in the initial stages of the experience with a higher number of reoperations,15,19,20 and the ability to obtain adequate resection margins and oncological results in the long term. Over time, however, the possible differences seem to decrease as the volume of activity increases.
In any case, gastric or esophageal cancer surgery must respect the oncological principles for resection, and the goal is long-term survival. Perioperative well-being cannot take precedence over the quality of surgery. As Sasako points out, the first operation decides the fate of the patient, and surgical imperfections cannot be compensated by radiotherapy or chemotherapy.21
This study reviews the available experience and evidence, and recommendations are provided for the minimally invasive approach and robotic surgery in EGJ cancer.
Utility of Minimally Invasive SurgeryIn addition to the general benefits of the minimally invasive approach in digestive surgery, one recognized utility of laparoscopy is the ability to perform a diagnostic procedure prior to the initiation of neoadjuvant therapy in transmural tumors of the EGJ that present risk of carcinomatosis.
Laparoscopic GastrectomyIn gastrectomy, the laparoscopic approach is often applied in earlier presentations of the disease, while open surgery is used for higher TNM stages.22 In Asian societies, it is accepted that there are no differences in recurrence or survival patterns between total open or laparoscopic gastrectomy in early-stage gastric cancer. However, in the Lee series analyzing 753 patients, there were significantly more anastomotic complications and greater postoperative mortality in minimally invasive surgery, versus more parietal complications in open surgery.23 The anastomosis in the mediastinum is considered the most difficult step in laparoscopic total extended gastrectomy, although the evolution of the technology, ample exposure of the hiatus and transoral introduction of the head of the circular stapler have improved resection margins and increased safety.
The use of laparoscopic surgery in the treatment of advanced gastric cancer is not widely accepted, mainly due to the technical difficulty of performing adequate D2 lymphadenectomy. Its use seems advisable only at high-volume hospitals with experience in complex minimally invasive surgery and in a clinical research setting. The evidence is even more uncertain in total than in subtotal gastrectomy,24 although perhaps the STOMACH trial, with the recruitment of recently closed patients, will provide further insight.25 A recently published large Asian series26 has compared two groups of patients undergoing open or laparoscopic total gastrectomy in type II and III tumors (87+84 in each arm, respectively), finding better short and long-term results with the MIS route, especially in terms of survival in type II. In the Shi series,27 with 132 laparoscopic gastrectomies versus 264 open procedures, no differences in complications or 2-year survival were observed. Since both are retrospective studies with matched patients, there may be a selection bias that calls into question the conclusions.
When open surgery is compared with MIS, lymphadenectomy is frequently more extensive in the former. According to the Viñuela review,28 D2 lymphadenectomy is performed in 39% of laparoscopic gastrectomies and 69% of open procedures. On the other hand, macroscopic involvement of the proximal margin occurs more often in laparoscopic surgery (9% vs 1% in the Kelly series18), particularly in the Lauren diffuse type, so intraoperative confirmation by biopsy of the negative proximal margin is essential. These findings suggest that, in certain cases of advanced tumors (bulky or with questionable resectability), especially diffuse, an open approach may be recommendable as it allows for palpation of the tumor.
Table 1 shows the main limitations of comparative studies between open and minimally invasive esophagogastric surgery.
Reasons for Heterogeneity of the Comparative Studies.
| Differences in laparoscopic or robotic proceduresSmall number of patientsSeveral retrospective studiesVariable percentage of total or subtotal gastrectomiesDifferent phases of the learning curveExtension of the lymphadenectomyNumber of lymph nodes often inferior in laparoscopic surgerySelection bias (no randomization), with frequent assignation of more advanced disease to open surgeryPredominance of Asian seriesUsual absence of long-term results (short-term follow-up) |
Adapted from Caruso et al.24
Numerous studies find similar oncological results between minimally invasive and open esophagectomy.29 Moreover, some indicate with the MIS approach an increase in the number of lymph nodes obtained in certain cases, and even better survival rates.14 The quality of life is also significantly better. The 3-year results of the TIME trial30 do not observe differences between overall and disease-free survival between open esophagectomy and MIS.
In a meta-analysis with 5235 MIS esophagectomies and 10555 open surgeries, general, pulmonary and cardiovascular complications as well as mortality are significantly reduced with MIS.31 However, in the Dutch national registry (Dutch Upper Gastrointestinal Cancer Audit, or DUCA), the rate of dehiscence is significantly higher in MIS than in open surgery.20
In the US, as reflected in the National Cancer Data Base in 2015, the number of minimally invasive esophagectomies exceeded the number of open procedures.32 In the Netherlands, the percentage of minimally invasive transthoracic esophagectomies rose from 42% in 2011 to 84% in 2015.20
The main problem for performing minimally invasive esophagectomy lies in the difficulties for creating anastomoses in the thoracic apex, which is unavoidable, both in terms of guaranteeing the proximal resection margin and minimizing posterior reflux. Several teams use mechanical circular anastomosis, particularly in lateral decubitus, introducing the 25–28mm head transorally or transthoracically33 in order to perform end-to-side anastomosis. This sometimes requires an assistance mini-thoracotomy, in addition to the one used to insert the stapler.
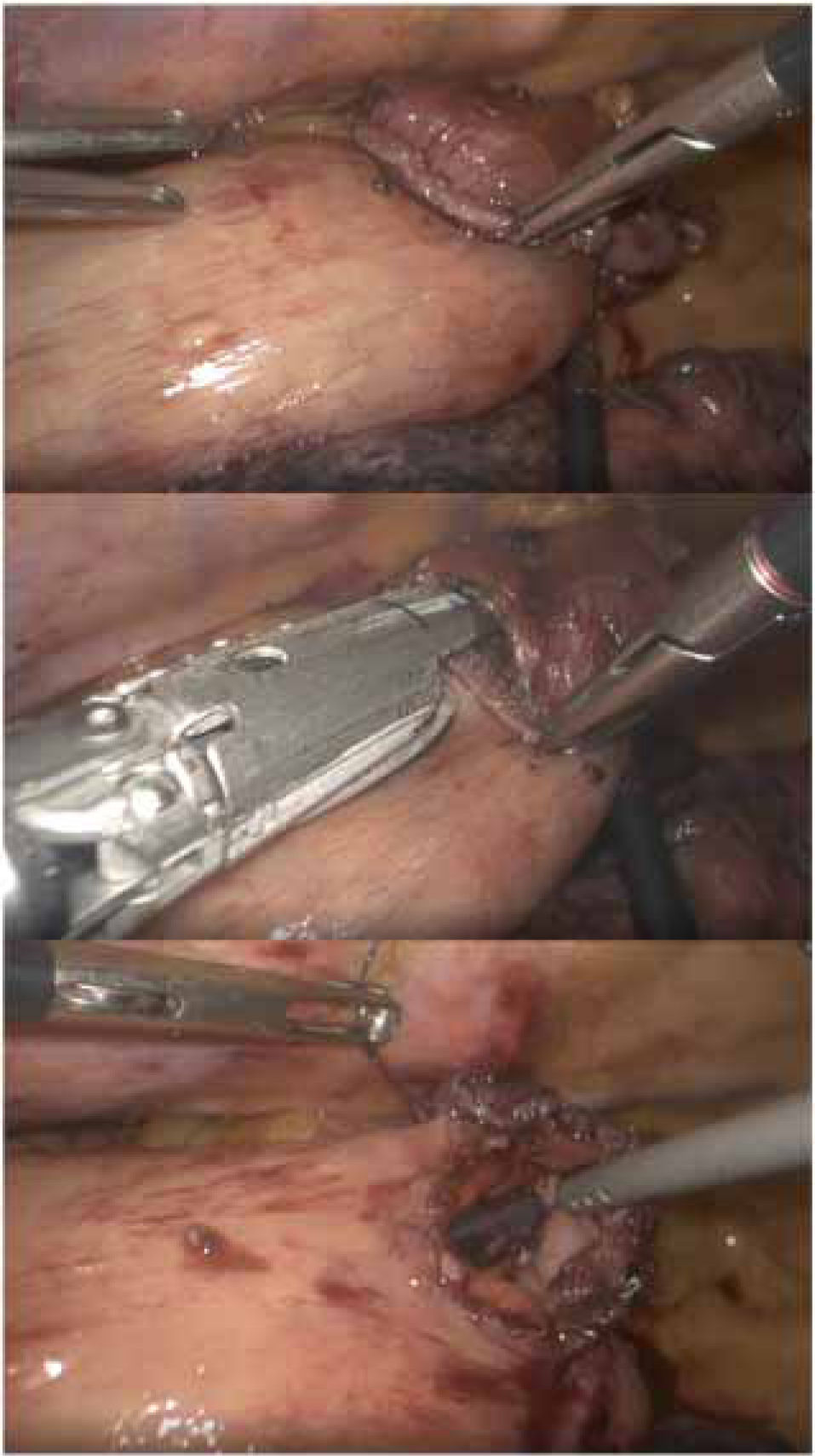
Other authors propose performing a ‘simplified’ side-to-side anastomosis in prone position34,35 (Fig. 1), with a low rate of dehiscence and apparently lower percentage of stenosis. This is the technique usually performed at the Hospital Universitario Donostia.
Contributions of Robotic SurgeryThe da Vinci surgical system was developed as a promising technological innovation that aims to offer more intuitive potential solutions to the limitations of laparoscopic surgery, particularly the increase in physiological tremor at the tip of the instruments, the restriction of movements due to the linear nature of laparoscopy forceps, or loss of depth perception due to two-dimensional vision. The robot offers three-dimensional vision, reduction of tremor, magnification of the image, scaled movements with greater freedom (7 degrees of freedom, compared to 5 in laparoscopy), camera control by the surgeon and better ergonomics (Table 2). The issue of ergonomics is crucial, because surgeon fatigue is a potential source of inaccuracy, and therefore compromised safety and complications, particularly in long interventions that require delicate sutures after several hours of complex dissection. As Coratti indicates, this set of advantages is able to improve the quality of minimally invasive surgery.36
Advantages of Robotic Versus Laparoscopic Surgery.
| Precise dissections in small fieldsAbsence of tremorGreater maneuverability of the instruments, 7 degrees of freedom with 180° of articulation and 540° of rotationScale of movements at a ratio of 3:1 or 5:1High-resolution 3D imageImage stabilityManagement if the camera by the head surgeonFacilitates manual anastomosisShorter learning curveReduces surgeon fatigue due to ergonomics |
In addition, the robotic approach seems to reduce the learning curve of complex minimally invasive procedures, particularly among surgeons with experience in laparoscopic surgery.
Its limitations include the absence of tactile sensation, which must be compensated visually, and cost associated with the initial investment and maintenance costs (Table 3).
Disadvantages of Robotic Surgery.
| Absence of tactile sensationPredetermined tension of forcepsPotential risk of tissue injury, particularly during intestinal manipulationField of vision restricted compared to laparoscopyVoluminous equipmentImpossibility for changes in position of the patient once the arms are assembledCost |
Since the publication of the first consensus document in robotic gastrointestinal surgery, gastrectomy and esophagectomy have been among the procedures that could benefit from the robotic approach due to their difficult access, limitation within a single quadrant, and the need for fine dissection and difficult sutures.37
Robotic gastrectomy has been shown to be a safe technique that reduces blood loss compared to the open or laparoscopic approach, although it prolongs the surgical time.38,39 The complication rate seems comparable, although some authors have reported a lower dehiscence rate with open surgery,22 and the number of lymph nodes collected is similar. Other authors find a significant reduction in postoperative complications with robotic surgery versus conventional laparoscopy, which could at least partially compensate for the high cost.39 Its advantages derive from the robot's nature, especially the stability, instrument length and visual definition, which facilitate access to certain lymph node groups, such as the suprapancreatic or splenic hilar nodes.24 The benefits of robotic surgery seem more significant in obese patients, where the number of resected lymphadenopathies is greater than with laparoscopy.23
The possible interest of manual anastomosis is controversial,36 and only certain Eastern authors have communicated their preliminary experience with satisfactory results. Although feasible, it means converting a standardized and theoretically ‘resolved’ surgical method into a more complex one and, therefore, subject to potential complications, especially in environments with limited robot availability where it is shared with other specialties.
Robotic EsophagectomyThe systematic review by Ruurda reflects the initial experience in adapting robots by several groups and the logical results of the learning curve, as well as the potential benefits.40
The recently published ROBOT trial shows a reduction in pain and global complications, particularly pulmonary and cardiac, with the robotic approach versus the open technique.41 The quality of life is superior and the long-term cancer outcomes are similar.
Although there are still doubts about the ideal esophagogastric reconstruction technique,42 robot-assisted manual intrathoracic anastomosis offers the benefits of minimally invasive surgery and a correct lymphadenectomy. It also allows surgeons to take advantage of the benefits of thoracic anastomosis versus cervical43 and manual sutures versus mechanical,44 without forgetting the relative simplicity of the technique thanks to the versatility of the instruments.45,46 Several authors, however, perform mechanical circular anastomosis after using the robot to make a tobacco pouch at the proximal esophageal end. Due to the lack of tactile sensation, there is a risk of injuring the gastroplasty during traction with robotic instruments from the thorax.45 For this reason, it may be useful to introduce the plasty in the right hemithorax at the end of the abdominal phase, subsequently proceeding to the hiatal closure.46
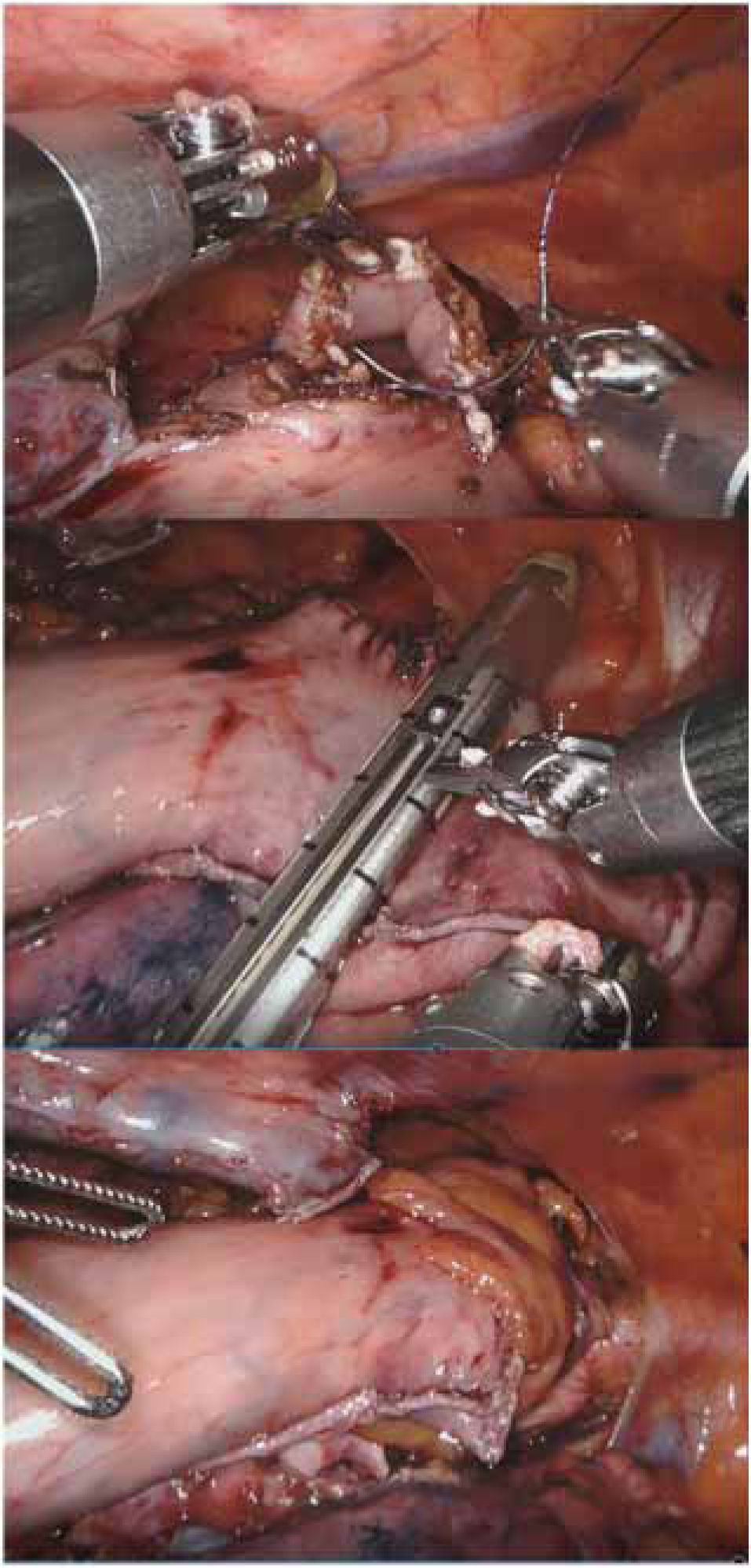
At Basurto University Hospital, the robot has been shown to be especially useful in deep-plane surgery in a single quadrant with little visceral manipulation, no mechanical anastomosis, ‘poorly oriented’ manual sutures and moderate sealing needs. Over time, Heller myotomy, redo due to failure or complications of prior antireflux surgery, and prone manual anastomosis in Ivor-Lewis esophagectomy (Fig. 2) have become the main indications in robotic esophagogastric surgery.46 Also, robotic transhiatal esophagectomy, although rarely indicated, can be performed with direct vision until the carina is surpassed.
The utility of fluorescence with indocyanine green associated with the robot remains to be determined, particularly in the visualization of the vascular network of the gastroplasty. If used to select the location of the anastomosis, it could reduce the risk of leakage by up to 69%.47 Zehetner observed a difference in the dehiscence rate between 2% and 45% (P<.0001) according to the quality of the perfusion at the site of the anastomosis.48
DiscussionThe optimal treatment of EGJ tumors requires an accurate diagnosis and decision-making within a multidisciplinary committee. This must take into account tumor characteristics (epicenter, size, presence of Barrett's metaplasia, esophageal invasion, distribution of possible metastatic lymphadenopathies and involvement of neighboring organs) as well as patient age, comorbidity, nutritional status and functional status in order to decide the most appropriate treatment regimen, including the indication of neoadjuvant chemo- or radiochemotherapy10,49 and surgical approach. The Siewert classification, which analyzes epidemiological/morphological characteristics and lymph node dissemination, helps determine the best surgical treatment for each patient.
In the case of tumors with uncertain behavior (esophageal or gastric), the decision to perform a gastrectomy or an esophagectomy should include starting the procedure through the abdominal route. In addition, as a general rule, the treatment of EGJ tumors should be performed by a unit with experience in both gastric and esophageal surgery.
Fig. 3 shows the therapeutic management algorithm in EGJ tumors at Hospital Universitario Basurto.
It is well known that the surgical results in terms of safety depend not only on a refined technique, but also on the optimization of comorbidities and adequate preparation of patients physically, psychologically and nutritionally.
The standard treatment of Siewert II tumors should be Ivor-Lewis esophagectomy,7,9,12 which enables the resection of the perihiatal and mediastinal lymph nodes as well as around the lesser curvature, also guaranteeing the proximal, distal and circumferential margins. As for the cervical anastomosis, it guarantees better vascularization in the area of the anastomosis, fewer recurring lesions and less stenosis.43 There seems to be no benefit to lymphadenectomy of groups 4, 5 and 6.3
The safety and effectiveness results of the left thoracoabdominal approach currently make its use inadvisable.50 Distal esophagectomy with proximal gastrectomy seems to be an exception.
The MIS route significantly reduces respiratory complications (as seen in the TIME13 and MIRO17 trials) and even mortality. Of course, elderly and/or fragile patients, including ‘pure’ tumors of the cardia that barely penetrate the esophagus, can be adequately treated with a total extended gastrectomy, respecting the proximal margin of about 5cm, although some authors argue that 2–3cm may be sufficient.51 In any case, after gastrectomy, intraoperative confirmation that the proximal margin is free is required – particularly in MIS,18 where tactile sensation is reduced – in case it could require proximal extension, even in the form of transhiatal esophagectomy. In some cases of bulky tumors, difficult manipulation or questionable resectability, open surgery can provide advantages over laparoscopy.
Minimally invasive surgery improves postoperative well-being (including pain), hospital stay, lung function and even blood loss. However, in the initial phases of the experience, the rate of dehiscence, including mortality, may increase, and the quality of the resection (free margins, R0 rate and number of resected lymph nodes) can be affected.
In order to ameliorate these risks of a complex surgery with serious potential complications, patients must be managed at high-volume hospitals with experienced surgeons and multidisciplinary teams,52 which provides approximate 90-day hospital mortality figures below 5%. Likewise, training programs must be established53 in order for the use of minimally invasive surgery to be extended without significantly increasing complications. Such programs would also support expert surgeons in esophagogastric surgery so that they are aware of new approaches as well as during the implementation phase in their own units.
Robotic surgery offers the possibility of overcoming certain limitations of conventional MIS. In the treatment of EGJ tumors, its main contributions are abdominal lymphadenectomy and intrathoracic anastomosis. Its main drawback (i.e. its high cost) could be partially compensated by a potential reduction in complications, thanks mainly to the vision quality and the versatility of the instruments.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Díez del Val I, Loureiro González C, Asensio Gallego JI, Bettonica Larrañaga C, Leturio Fernández S, Eizaguirre Letamendia E, et al. Cirugía mínimamente invasiva y robótica en el tratamiento quirúrgico de las neoplasias de la unión esofagogástrica. Cir Esp. 2019;97:451–458.